Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Tejaswi Tanaji Salunkhe | -- | 4736 | 2023-11-15 03:58:15 | | | |
| 2 | Catherine Yang | + 3 word(s) | 4739 | 2023-11-15 04:35:40 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Salunkhe, T.T.; Gurugubelli, T.R.; Babu, B.; Yoo, K. Metal Oxide Quantum-Dot-g-C3N4 Nanocomposites. Encyclopedia. Available online: https://encyclopedia.pub/entry/51574 (accessed on 07 February 2026).
Salunkhe TT, Gurugubelli TR, Babu B, Yoo K. Metal Oxide Quantum-Dot-g-C3N4 Nanocomposites. Encyclopedia. Available at: https://encyclopedia.pub/entry/51574. Accessed February 07, 2026.
Salunkhe, Tejaswi Tanaji, Thirumala Rao Gurugubelli, Bathula Babu, Kisoo Yoo. "Metal Oxide Quantum-Dot-g-C3N4 Nanocomposites" Encyclopedia, https://encyclopedia.pub/entry/51574 (accessed February 07, 2026).
Salunkhe, T.T., Gurugubelli, T.R., Babu, B., & Yoo, K. (2023, November 15). Metal Oxide Quantum-Dot-g-C3N4 Nanocomposites. In Encyclopedia. https://encyclopedia.pub/entry/51574
Salunkhe, Tejaswi Tanaji, et al. "Metal Oxide Quantum-Dot-g-C3N4 Nanocomposites." Encyclopedia. Web. 15 November, 2023.
Copy Citation
Quantum dots and graphitic carbon nitride (g-C3N4) are essential elements in this science. In contrast to quantum dots, which have enormous potential due to their size-dependent bandgap tunability and effective charge carrier production, g-C3N4 has properties like chemical stability and a configurable bandgap that make it a versatile material for photocatalysis.
quantum dots
g-C3N4
photocatalytic
nanocomposite
1. Introduction
Size and Quantum Confinement: the primary distinction lies in the size. Quantum dots are typically smaller than nanoparticles and are in the range of 1–10 nanometers (approximately 10–50 atoms in diameter). At this scale, quantum effects significantly influence the material’s properties, leading to phenomena like quantum confinement in semiconductor QDs, which is not observed in larger nanoparticles. This results in unique optical, electronic, and catalytic properties for QDs.
Optical Properties: due to quantum confinement, QDs exhibit size-dependent tunable photoluminescence, allowing them to absorb and emit light over a wide spectrum. This property is crucial for various applications, including photocatalysis, and is not prominently observed in larger nanoparticles.
Surface Properties: the high surface-to-volume ratio of QDs leads to a significant proportion of atoms being at the surface, which profoundly impacts their chemical reactivity and catalytic activity. While nanoparticles also have a high surface-to-volume ratio, the quantum effects in QDs enhance these surface-related properties.
Energy Band Structures: the discrete energy levels in QDs, a consequence of quantum confinement, differ substantially from the continuous band structure of bulk materials or larger nanoparticles. This affects their interaction with light, charge carrier generation, and transfer—critical factors in photocatalytic processes.
2. Wide-Bandgap Metal Oxide QD-g-C3N4 Nanocomposites
In recent half-decade research, TiO2 quantum dots (QDs) have solidified their position as stalwarts in the realm of nanotechnology, largely attributed to their exceptional photocatalytic proficiencies [1]. These quantum entities hold immense promise in efficiently absorbing solar energy, paving the way for their integration into an array of environmental and energy-focused applications. Properties intrinsic to TiO2 QDs set them apart in the vast quantum landscape. The quantum confinement effect empowers them with a modifiable bandgap, a boon for diversifying photocatalytic ventures. Their magnified surface-to-volume ratio augments their inherent reactivity, and their commendable photostability ensures longevity in demanding applications [2]. As for their real-world implications, these QDs shine in water purification, adeptly obliterating organic contaminants. Their prowess extends to hydrogen production, where they serve as linchpins in photoelectrochemical water splitting. Furthermore, their capabilities in air purification, specifically in annihilating noxious air pollutants, have been documented. In essence, the ongoing research narrative accentuates the transformative potential of TiO2 QDs, suggesting a luminous path ahead in environmental rejuvenation and sustainable energy paradigms. According to Wang et al., creating a p-TiO2 QDs@g-C3N4 p-n junction results in better photocatalytic performance than using pure g-C3N4. The improved performance is a result of the p-n heterojunction, strong interface interaction, and quantum-size impact [3]. Wang (2021) synthesized an F-doped TiO2 quantum dot/g-C3N4 nanosheet Z-scheme photocatalyst through chemical bonding, resulting in improved oxidizability, reducibility, and interfacial charge transfer ability [4]. Lee (2023) created a 0D/2D heterojunction nanocomposite with TiO2 quantum dots anchored on g-C3N4 nanosheets (Figure 1) that demonstrated accelerated solar-driven photocatalysis [5]. The integration of anatase/rutile homojunction quantum dots onto g-C3N4 nanosheets, which is intended to target the breakdown of antibiotics in saltwater matrices, was documented by Hu and colleagues. Their study delves into the combined mechanism of adsorption and photocatalysis, shedding light on its underlying intricacies. They further evaluated the ternary heterojunctions formed between anatase/rutile quantum dots (QDs) and g-C3N4, emphasizing their effectiveness in the removal of Oxytetracycline (OTC). Additionally, the research gauges the toxicity levels of the resultant intermediates detected post-process [6].
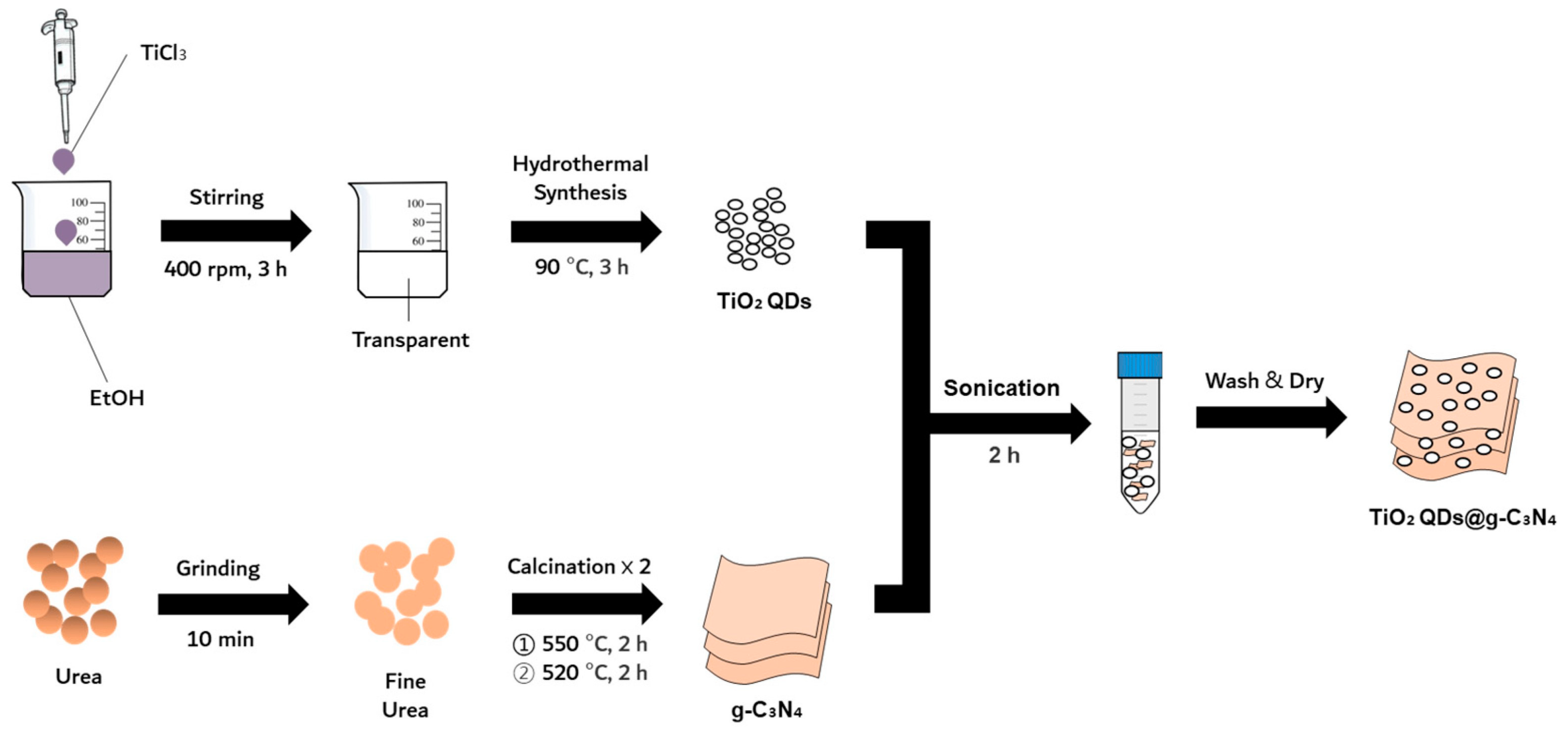
Figure 1. Synthetic process for fabricating TiO2 QDs@g-C3N4 nanocomposite [5].
In recent years, the world of nanotechnology has seen an upswing in interest towards SnO2 quantum dots (QDs), primarily owing to their potent capabilities in photocatalysis [7]. These quantum dots excel in efficiently harnessing light, thereby driving effective charge separation and curbing recombination—traits indispensable for successful photocatalysis. Delving into their synthesis, several cutting-edge methods have emerged over the past half-decade. The hydrothermal method, which revolves around reacting tin salts in water under specific temperature and pressure conditions, remains a favored choice [8]. However, the sol-gel approach, where a precursor solution transitions from a gel-like consistency to the desired quantum dots upon drying and calcination, is also prevalent [7]. Not to be overshadowed, the microwave-assisted synthesis leverages the power of uniform and rapid microwave heating, often resulting in SnO2 QDs of superior crystallinity in a fraction of the conventional synthesis time [9]. What truly sets SnO2 QDs apart are their intrinsic properties. The quantum confinement effect grants researchers the liberty to tweak their bandgap, ensuring adaptability for a range of light-driven reactions. Their nanoscale stature bestows upon them a vast surface area, ideal for fostering enhanced reactant interactions. Furthermore, they stand out in the quantum dot family for their remarkable chemical and thermal stability. On the application front, these QDs have been instrumental in several arenas, from water splitting, where they play a role in converting water into hydrogen fuel using sunlight, to the degradation of persistent organic pollutants in water [10]. Another noteworthy application is their potential in reducing CO2, where they can transform atmospheric carbon dioxide into valuable fuels, presenting a promising avenue to combat escalating CO2 levels [11]. Recent studies and trends hint that the true potential of SnO2 QDs, especially when amalgamated with complementary materials, is yet to be fully unlocked, holding promises for advances in sustainable energy and environmental solutions.
SnO2 quantum dots (QDs) with graphitic carbon nitride (g-C3N4) can improve photocatalytic activity. In 2018, Babu found that when exposed to sunlight, the mixture of SnO2 QDs and g-C3N4 nanolayers displayed increased photocatalytic performance, effectively breaking down methyl orange. This increase in sunlight-driven photocatalytic activity is attributed to the cooperative interaction between the g-C3N4 nanolayers and SnO2 quantum dots. These results highlight the potential of g-C3N4 nanolayers and SnO2 QDs as powerful sunlight-responsive photocatalysts, particularly for the degradation of pollutants like methyl orange [12]. In 2019, Yousaf noted a marked increase in photocatalytic performance upon the embellishment of g-C3N4 with SnO2 QDs, which led to the successful decomposition of Rhodamine B. The relative proportion of SnO2 to g-C3N4 in these nanohybrids plays a pivotal role in determining their photocatalytic efficacy (Figure 2). Such findings highlight the potency of SnO2/g-C3N4 nanocomposites, particularly in the domain of degrading contaminants like Rhodamine B (RhB) in solutions [13]. In 2017, Ji pioneered the synthesis of a composite photocatalyst combining SnO2 with graphene-like g-C3N4. This composite exhibited superior visible-light-driven activities in degrading organic pollutants. Remarkably, its optimal photocatalytic efficiency under visible light exposure surpassed that of SnO2 and g-C3N4 by almost 9 and 2.5 times, respectively [14]. The synergy between SnO2 and graphene-like g-C3N4 is highlighted in this composite, which is represented as SnO2/graphene-like g-C3N4, underlining its potential in photocatalytic processes. Its process in the degradation of Rhodamine B (RhB) with visible light in particular provides encouraging insights into its functional possibilities. All of these results point to the possibility that SnO2 quantum dots and g-C3N4 work better together to accelerate photocatalytic breakdown of organic contaminants in water.
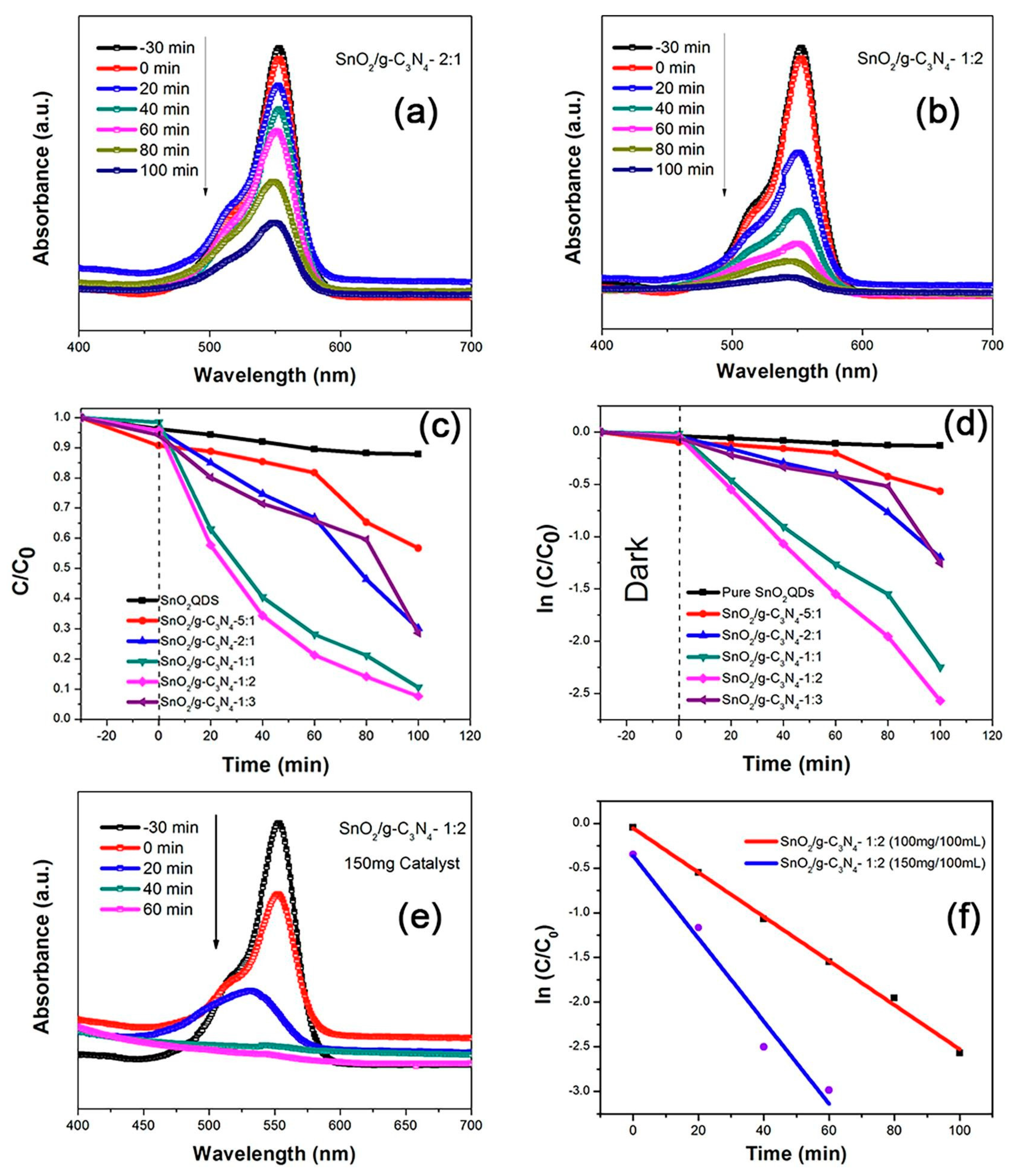
Figure 2. (a–f) Photocatalytic application of SnO2 QD/g-C3N4 nanocomposite [13].
Zinc oxide (ZnO) quantum dots have shown significant promise for photocatalytic applications, driven by their unique physicochemical properties. ZnO quantum dots exhibit enhanced photocatalytic efficiency owing to their high surface area and quantum confinement effects [15]. Their ability to generate reactive oxygen species upon light irradiation makes them potent catalysts for degrading organic pollutants. Researchers have delved into surface modifications to improve the photocatalytic performance of ZnO quantum dots [16]. Techniques like doping, coating, or hybridizing with other materials have been shown to enhance their stability and photocatalytic activity. The advent of black ZnO quantum dots has opened up the possibility of utilizing visible light, significantly broadening the spectrum of light that can be used for photocatalytic applications. Innovations in the design of heterostructures with ZnO quantum dots have shown promise in promoting charge separation, which is crucial for efficient photocatalysis. ZnO quantum dots have found real-world applications in water treatment, air purification, and energy conversion, embodying the translation of academic research to practical solutions [17]. Studies have showcased the robustness of ZnO quantum dots in diverse environmental conditions, highlighting their potential for outdoor applications. The integration of ZnO quantum dots with other nanomaterials like graphitic carbon nitride has led to the creation of novel nanocomposites with superior photocatalytic properties [18]. Ren et al. created a composite by mixing graphitic carbon nitride (g-C3N4) and ZnO quantum dots (QDs) with the goal of enhancing the material’s photocatalytic properties. It was impressive to see how well the composite degraded Rhodamine B when exposed to visible light. A 96.8% degradation rate of Rhodamine B was attained under visible light within a 40 min window, which was a startling achievement. This heightened photocatalytic efficacy is believed to emanate from the synergistic interplay between ZnO QDs, GO, and g-C3N4. The separation of photogenerated electron–hole pairs was accelerated with this combination [19]. In conclusion, the ZnO QD/GO/g-C3N4 composite emerges as a potent contender for the remediation of organic pollutants in wastewater, presenting a workable solution for real-world wastewater treatment scenarios. This is due to its potent photocatalytic performance under visible light and its impressive durability. Investigating the visible-light-induced photocatalytic behavior of SnO2-ZnO quantum dots attached to g-C3N4 nanosheets was the goal of Vattikuti et al. The two main areas of concern were the degradation of contaminants and the creation of H2. They successfully anchored SnO2-ZnO quantum dots onto g-C3N4 nanosheets with their efforts. The resultant composite manifested heightened photocatalytic prowess when exposed to visible light, especially evident in its commendable degradation rates for contaminants like RhB and phenol. When the data were analyzed, it was discovered that the composite’s RhB degradation rate was 3.5 times greater than that of pure g-C3N4. Similar to this, phenol’s rate of degradation was 2.8 times more rapid than that of g-C3N4. Additionally, it was found that the composite’s capacity to produce H2 was astonishingly 4.6 times greater than that of pure g-C3N4. From these results, it is clear that the SnO2-ZnO quantum dots, when attached to g-C3N4 nanosheets, significantly improve photocatalytic activity when exposed to visible light. This composite not only excels at degrading pollutants but also evinces significant potential in H2 production. Such attributes earmark it as a viable solution for tasks ranging from environmental purification to fostering sustainable energy methodologies [20].
3. Bi-Based QD-g-C3N4 Nanocomposites
Bismuth-based quantum dots (QDs) have emerged as a captivating class of nanostructured semiconductors, drawing substantial interest because of their unique electronic, optical, and photocatalytic characteristics. Their size-dependent bandgaps offer specific tunability for photocatalytic reactions. BiVO4 QDs [21], for instance, display improved light absorption due to quantum confinement effects, and they are recognized for their proficiency in visible-light-driven water splitting and pollutant degradation. Bismuth oxide (Bi2O3) QDs exhibit enhanced optical attributes and charge transfer characteristics, positioning them as formidable catalysts for UV and visible light pollutant degradation [22][23]. Bi2WO6 QDs, with their increased electron–hole separation at the quantum level, stand out in degrading diverse organic pollutants under visible light [24]. While Bi2O4 QDs are relatively less explored, they have demonstrated potential with amplified light interaction at the nanoscale and offer efficient photocatalytic reactions. Lastly, Bi2MoO6 quantum dots, known for their extended photogenerated charge carrier lifetimes, are potential frontrunners for organic compound degradation and hydrogen evolution tasks [25]. In a nutshell, the nano-dimensionality of bismuth-based QDs accentuates their photocatalytic performance by amplifying light absorption, optimizing charge transfer, and minimizing recombination, making them versatile contenders for an array of photocatalytic applications. The scientists wanted to create a ternary heterostructure comprising C60, g-C3N4, and BiVO4 quantum dots as a photocatalyst. This was carried out to increase the photocatalytic activity when exposed to visible light. Under visible light irradiation, the synthesized ternary heterostructure shown improved photocatalytic activity. Compared to binary heterostructures and pure g-C3N4, the ternary heterostructure showed a greater photocatalytic degradation rate of Rhodamine B (RhB) [21]. The higher charge separation efficiency and expanded light absorption range were credited with the better photocatalytic performance. The potential mechanisms underlying the ternary heterostructure’s improved photocatalytic activity were discussed by the researchers. They emphasized how C60 and BiVO4 quantum dots worked together to promote charge separation and lessen recombination. The enhanced photocatalytic efficiency of the ternary heterostructure was also attributed to a wider light absorption range. In comparison to other structures, the ternary heterostructure of BiVO4 quantum dots/C60/g-C3N4 was effectively constructed and showed higher photocatalytic activity. The study offers suggestions for creating effective photocatalysts for a variety of uses, including environmental cleanup.
Liang et al. looked at how well Bi2O3 QDs/g-C3N4 performed as a photocatalyst for both organic and inorganic contaminants. Tetracycline (TC) and Cr (VI) were chosen as representative environmental pollutants to assess the effectiveness of the samples’ photocatalytic reduction and oxidation [23]. Under light illumination, the photocurrent density of the Bi2O3 QDs/g-C3N4 (ii) was noticeably higher than that of the g-C3N4, E-g-C3N4, and Bi2O3 QDs/g-C3N4, showing better charge transfer efficiency. Bi2O3 QDs/g-C3N4 had the shortest semicircular arc diameter, according to EIS Nyquist plots, indicating the lowest charge transfer resistance and quickest interfacial charge transport. Scavengers had an impact on the effectiveness of TC’s degradation, proving that certain radicals were involved in the process. Byproducts of TC were produced using photocatalytic mineralization processes, with some intermediates demonstrating decreased toxicity after photocatalytic degradation. Bi2O3 QDs/g-C3N4 underwent photoinduction to improve the separation and transfer of photogenerated charges. As model environmental pollutants, TC and Cr (VI) were used to assess the photocatalytic performance. For Bi2O3 QDs/g-C3N4, the results showed increased charge transfer effectiveness and quicker interfacial charge transport. The research showed that Bi2O3 QDs/g-C3N4 had the potential to be an efficient photocatalyst for the oxidation of both organic and inorganic contaminants. Its better photocatalytic performance was aided by its improved charge transfer efficiency and decreased charge transfer resistance. The research offers a potential method for creating highly scattered metal oxides on 2D lamella semiconductors, expanding the photocatalyst’s usefulness for removing a variety of environmental pollutants. Zeng et al. concentrated on the logical application of quantum dots (QDs) and graphitic carbon nitride (g-C3N4) semiconductors to increase their effectiveness as photocatalysts. The integration of g-C3N4 with QDs was intended to increase photogenerated electron transfer efficiency and produce significant photocatalytic activity. The researchers created brand-new Bi2WO6 QD/g-C3N4 nanocomposites with attapulgite (ATP) penetration [24]. The outcomes demonstrated that the ATP with a nanorod shape served as bridges to intercalate into the interlayers, thus enlarging the g-C3N4 inner space. With the use of an HPLC-MS system, the samples’ photocatalytic degradation activities were examined. Using a specified formula, the degrading effectiveness of MBT in the solution was determined. The MBT solution was broken down while being exposed to radiation to gauge the photocatalytic performance. The BCA5 sample exhibited favorable photoelectric characteristics, which indicated quick interfacial charge movement and low resistance for the production of charge carriers. As a potential strategy for future photocatalytic applications, the integration of g-C3N4 and QDs with the interpenetration of ATP led to improved photocatalytic performance. In order to create a 2D-0D g-C3N4/Bi2WO6-OV composite catalyst, Cheng et al. combined two-dimensional (2D) graphite carbon nitride (g-C3N4) nanosheets with oxygen-containing vacancy zero-dimensional (0D) Bi2WO6 (BWO-OV) quantum dots [22]. The goal was to improve the catalyst’s catalytic activity, increase the formation of photogenerated carriers, and improve light absorption. Utilizing Bi2WO6 with oxygen vacancies, which improved light absorption while simultaneously increasing the production of photogenerated carriers, was the novel method. The vacancy structure of Bi2WO6 and the heterojunction’s creation both contributed to the photogenerated carriers’ longer longevity. The composite of CN/BWO-OV-10 displayed the maximum intensity, indicating a greater capacity for NO degradation. The outcomes of the trapping tests revealed that superoxide radicals, holes, and electrons all contribute significantly to the photocatalytic reaction. Furthermore, hydroxyl is recognized as a less potent active free radical. By eliminating NO, the photocatalytic effectiveness was evaluated. The efficiency peaked at 61.2% when BWO-OV was 10% by mass of the total amount of CN. The CN/BWO composite demonstrated the superiority of CN/BWO-OV-10 with a rise in efficiency of 3.2%, achieving a degradation efficiency of 58%. At room temperature, the composite g-C3N4/Bi2WO6-OV structure removed nitric oxide (NO) at a rapid rate despite its low concentration. The composite catalyst’s efficiency was higher than that of g-C3N4 or BWO-OV and superior to that of g-C3N4/Bi2WO6 without oxygen vacancies. The best catalytic activity was demonstrated with the composite g-C3N4/Bi2WO6-OV-10, reaching up to 61.2%. The substance also demonstrated outstanding stability throughout several iterations of experimentation.
Ding and co. investigated Bi2MoO6 QDs/g-C3N4 with heterojunctions for their potential in the selective oxidation of aromatic alkanes into aldehydes under visible-light-driven catalysis [25]. Outstanding visible-light-driven catalytic performance was shown with the Bi2MoO6 QD/g-C3N4 heterojunction in the selective oxidation of aromatic alkanes into aldehydes. The heterojunction’s special structure, which facilitates the effective separation of charge carriers, is said to be responsible for the increased photocatalytic activity. Superior photocatalytic activity was demonstrated with the heterojunction of Bi2MoO6 QDs/g-C3N4, particularly in the selective oxidation of aromatic alkanes. The distinctive structure that enables greater charge carrier separation is responsible for this performance. Under visible light, the Bi2MoO6 QD/g-C3N4 heterojunction exhibits outstanding photocatalytic activity in the selective oxidation of aromatic alkanes to aldehydes. The distinctive structure, which improves charge carrier separation, is credited with the efficiency. The potential of 0D/2D photocatalysts with heterojunctions for the selective oxidation of C(sp3)-H bonds is crucial information provided in this study.
3. Other Metal Oxide QD-g-C3N4 Nanocomposites
Sun et al. sought to create CeO2 quantum dots anchored on g-C3N4 (CeO2/g-C3N4) and examine the photocatalytic performance of the material [26]. The creation of CeO2 quantum dots anchored on g-C3N4—which are anticipated to have improved photocatalytic properties—represents the work’s originality. It is anticipated that the combination of these materials will enhance charge separation and increase the light absorption range. XRD, FTIR, SEM, TEM, XPS, and PL were used to characterize the synthesized CeO2/g-C3N4. The outcomes demonstrated a homogeneous distribution of CeO2 quantum dots on the g-C3N4 nanosheets. Better light absorption was discovered to be indicated with the bandgap of CeO2/g-C3N4 being narrower than that of pure g-C3N4. By observing the degradation of Rhodamine B (RhB) under visible light irradiation, the photocatalytic performance was assessed. Compared to pure g-C3N4, the CeO2/g-C3N4 demonstrated improved photocatalytic activity. The better charge separation and expanded light absorption range brought on with the presence of CeO2 quantum dots are credited with the improved performance. Rhodamine B degradation under visible light showed higher photocatalytic performance from the CeO2/g-C3N4 combination. This improved performance is a result of the cooperative action of g-C3N4 and CeO2 quantum dots, which improves charge separation and light absorption. Although the exact findings section was not directly extracted, it may be deduced from the information given that the researchers were successful in creating CeO2 quantum dots anchored on g-C3N4 with enhanced photocatalytic characteristics. Its improved Rhodamine B degradation under visible light made the composite material an attractive option for photocatalytic applications.
In order to create a novel photocatalyst, Zhu et al. combined g-C3N4 nanosheets and MoO3 quantum dots (QDs). This mixture was created with the goal of improving photocatalytic activity for the reduction in U(VI) under visible light. The addition of MoO3 QDs to g-C3N4 nanosheets is what makes this work novel [27]. Following their easy hydrothermal synthesis, the MoO3 QDs were loaded onto g-C3N4 nanosheets using a straightforward ultrasonic dispersion technique. This mixture was predicted to facilitate photogenerated electron–hole pair separation and improve photocatalytic activity. The MoO3 QD/g-C3N4 nanosheets showed remarkable photocatalytic performance for U(VI) reduction when exposed to visible light in their as-prepared state. It was discovered that MoO3 QDs should be loaded at a rate of 2%. The efficient separation of photogenerated electron–hole pairs and the expanded light absorption range were credited with the improved photocatalytic activity. Various characterization approaches were used to support the postulated photocatalytic mechanism. By measuring the concentration of U(VI) in aqueous solutions while they were exposed to visible light, the photocatalytic performance was assessed. The outcomes demonstrated that the photocatalytic reduction rate for U(VI) was greater in the MoO3 QD/g-C3N4 nanosheets than in pure g-C3N4. It can be concluded that g-C3N4 nanosheets in combination with MoO3 QDs present a viable method for improving the photocatalytic reduction in U(VI) under visible light. The proposed mechanism and the innovative photocatalyst design offer important new perspectives for this field’s future study. The detailed description of recent research on the photocatalytic abilities of nanocomposites based on metal oxide QD’s-g-C3N4 were provided in Table 1.
Table 1. Detailed description of recent research on the photocatalytic abilities of nanocomposites based on metal oxide QDs-g-C3N4.
| Photocatalyst | Pollutant | Dosage | Light Source | Efficiency | Ref. |
|---|---|---|---|---|---|
| SnO2 QDs/g-C3N4 | RhB | 50 mg/L | Visible light | 95% in 60 min | [13] |
| TiO2 QDs/g-C3N4 | RhB | 10 mg/L | Visible light | 99% in 75 min | [4] |
| TiO2 QDs/g-C3N4 | Phenol | 10 mg/L | Visible light | 98% in 100 min | [4] |
| TiO2 QDs/g-C3N4 | Cr (VI) | 20 mg/L | Visible light | 99% in 60 min | [4] |
| TiO2 QDs/g-C3N4 | MO | 10 mg/L | Solar light | 98% in 120 min | [28] |
| CeO2 QDs/g-C3N4 | RhB | 10 mg/L | Visible light | 80% in 180 min | [26] |
| CeO2 QDs/g-C3N4 | MO | 10 mg/L | Visible light | 82% in 180 min | [26] |
| CeO2 QDs/g-C3N4 | MB | 10 mg/L | Visible light | 74% in 180 min | [26] |
| BiVO4 QDs-g-C3N4 | RhB | 10 mg/L | Visible light | 81% in 90 min | [21] |
| Bi2O3 QDs/g-C3N4 | TC | 10 mg/L | Visible light | 83% in 120 min | [23] |
| Bi2O3 QDs/g-C3N4 | Cr (VI) | 10 mg/L | Visible light | 88% in 60 min | [23] |
| Bi2O4 QDs/g-C3N4 | RhB | 10 mg/L | Visible light | 78% in 160 min | [29] |
| Bi2WO6 QDs/g-C3N4 | MBT | 20 mg/L | Visible light | 99% in 80 min | [24] |
| MoO3 QDs/g-C3N4 | U (VI) | 50 mg/L | Visible light | 96% in 150 min | [27] |
| Co3O4 QDs-g-C3N4 | MTZ | 10 mg/L | Visible light | 77% in 180 min | [30] |
| Bi2WO6 QDs/g-C3N4 | NO | 0.5 ppm | Visible light | 61% | [22] |
| SnO2 QDs/g-C3N4 | NO | 600 ppb | Visible light | 32% | [31] |
| SnO2 NPs/g-C3N4 | RhB | 10 ppm | Visible light | 97% in 50 min | [32] |
| SnO2 NPs/g-C3N4 | MB | 10 ppm | Visible light | 99% in 90 min | [33] |
| SnO2 NPs/g-C3N4 | CR | 10 ppm | Visible light | 96% in 90 min | [33] |
| SnO2 NPs/g-C3N4 | NO | 500 ppb | Visible light | 40% | [34] |
| SnO2 NPs/g-C3N4 | RhB | 10 ppm | Solar light | 86% in 240 min | [35] |
| TiO2 NPs/g-C3N4 | TC | 20 ppm | Visible light | 99% in 120 min | [36] |
| TiO2 NPs/g-C3N4 | TC | 20 ppm | UV light | 96% in 90 min | [37] |
| TiO2 NPs/g-C3N4 | TC | 20 ppm | Visible light | 90% in 120 min | [38] |
| TiO2 NPs/g-C3N4 | MB | 20 ppm | Solar light | 80% in 180 min | [39] |
| TiO2 NPs/g-C3N4 | TC | 100 ppm | Visible light | 80% in 100 min | [40] |
| ZnO NPs/g-C3N4 | MB | 10 ppm | Visible light | 98% in 150 min | [41] |
| ZnO NPs/g-C3N4 | MB | 50 ppm | Visible light | 98% in 180 min | [42] |
| ZnO NPs/g-C3N4 | MB | 10 ppm | Visible light | 60% in 120 min | [43] |
| ZnO NPs/g-C3N4 | MB | 10 ppm | Visible light | 92% in 120 min | [44] |
| ZnO NPs/g-C3N4 | CR | 10 ppm | Visible light | 70% in 45 min | [45] |
| CeO2 NPs/g-C3N4 | MO | 10 ppm | Visible light | 96% in 100 min | [46] |
| CeO2 NPs/g-C3N4 | RhB | 10 ppm | Visible light | 96% in 60 min | [47] |
| CeO2 NPs/g-C3N4 | Cr | 20 ppm | Visible light | 96% in 100 min | [48] |
| CeO2 QDs/g-C3N4 | TC | 10 ppm | Visible light | 78% in 160 min | [49] |
| CeO2 NPs/g-C3N4 | MB | 10 ppm | Visible light | 70% in 180 min | [50] |
| BiVO4 NPs/g-C3N4 | 4-CP | 20 ppm | Visible light | 95% in 100 min | [51] |
| BiVO4 NPs/g-C3N4 | MO | 20 ppm | Visible light | 82% in 60 min | [52] |
| BiVO4 NPs/g-C3N4 | MB | 10 ppm | Visible light | 88% in 120 min | [53] |
| BiVO4 NPs/g-C3N4 | TC | 10 ppm | Visible light | 89% in 120 min | [53] |
| Bi2WO6 NPs/g-C3N4 | CIP | 15 ppm | Visible light | 98% in 120 min | [54] |
| Bi2WO6 NPs/g-C3N4 | Diuron | 20 ppm | Visible light | 75% in 120 min | [55] |
| Bi2WO6 NPs/g-C3N4 | ADN | 10 ppm | Visible light | 98% in 80 min | [56] |
| Co3O4 NPs/g-C3N4 | Atrazine | - | Visible light | 78% in 35 min | [57] |
| MoO3 NPs/g-C3N4 | TC | 10 ppm | Visible light | 86% in 100 min | [58] |
| MoO3 NPs/g-C3N4 | RhB | 10 ppm | Visible light | 99% in 25 min | [59] |
Zhao et al. set out to create phosphorus-doped g-C3N4/Co3O4 quantum dots in a single step using vitamin B12. They sought to increase the synthesized substance’s visible-light photocatalytic activity for the destruction of metronidazole (MTZ) [30]. The innovative aspect of the process is the one-step production of phosphorus-doped g-C3N4/Co3O4 quantum dots using vitamin B12. This technique is distinctive because it enhances the photocatalytic performance of g-C3N4 by combining the traits of both phosphorus doping and Co3O4 quantum dots. Several approaches were used to characterize the synthesized composites. The photodegradation of MTZ under visible light irradiation was used to assess the photocatalytic activities of the composites. The explanation for the increased photocatalytic activity was determined to be the synergistic interaction between the produced Co3O4 quantum dots and P-doped g-C3N4. This interaction improved photo-induced electron and hole separation efficiency, prevented their recombination, and reduced band gap energy. The generated Co3O4 quantum dots and P-doped g-C3N4 worked together to enhance the photocatalytic performance of the created material. As a result of this synergy, photoinduced electrons and holes were separated and transferred more effectively, which increased the photocatalytic degradation of MTZ. The study successfully demonstrated the synthesis of g-C3N4/Co3O4 quantum dots doped with phosphorus utilizing a one-step procedure and vitamin B12. The created substance demonstrated improved visible-light photocatalytic activity for the breakdown of MTZ. The fundamental causes of the enhanced photocatalytic performance were determined to be the synergistic interactions between the produced Co3O4 quantum dots and P-doped g-C3N4.
The heterojunctions formed between traditional QDs like CdS/CdSe and g-C3N4 have demonstrated effective charge separation due to their staggered band alignment, which minimizes recombination and enhances photocatalytic performance. The researchers discussed how these strategies of interface engineering can be applied to metal oxide QDs to optimize their interactions with g-C3N4, focusing on creating synergistic band alignments that facilitate charge transfer and extend light absorption. In-depth analyses of the photocatalytic mechanisms in CdS/CdSe and Ag-In-Zn-S QDs combined with g-C3N4 have revealed critical factors such as quantum confinement effects, surface states, and the role of co-catalysts in improving photocatalytic activity. By integrating these insights, the researchers will elaborate on how similar principles might govern the activity of metal oxide QDs, and how understanding these mechanisms can guide the optimization of their photocatalytic performance. Drawing parallels between the successes of these traditional QDs and the subject metal oxide QDs, the researchers will discuss how strategies like precise size control, doping, or the introduction of defects, successful in traditional QDs, can be mirrored in metal oxides to modulate band structure, enhance light absorption, and improve charge carrier dynamics. While acknowledging the efficiency of traditional QDs, the researchers also recognize concerns regarding their toxicity and environmental impact, particularly for Cd-based QDs. This contrast presents an opportunity to highlight the relative environmental friendliness of metal oxide QDs and the importance of pursuing these materials for sustainable photocatalysis.
Carbon quantum dots (CQDs) have emerged as a captivating class of carbon nanomaterials, characterized by their unique optical, electrical, and physicochemical properties. These properties, combined with their aqueous stability, low toxicity, high surface area, economic feasibility, and tunable photoluminescence behavior, make them promising candidates for photocatalytic applications. On the other hand, graphitic carbon nitride (g-C3N4) has gained attention as a stable carbon-based polymer with potential applications in various fields. The combination of CQDs and g-C3N4 offers a synergistic effect, enhancing the adsorptive and photocatalytic activity of the resulting nanocomposite. This is attributed to the broader visible-light absorption, increased specific surface area, and enhanced electron–hole pair migration and separation efficiency of the composite. Comparatively, while metal oxide quantum dots also present potential in photocatalytic applications, the CQDs and g-C3N4 combination stands out due to its non-toxic nature, economic feasibility, and enhanced photoluminescence properties. The interaction within this multicomponent photocatalyst promotes photocatalytic performance, making it a superior choice for wastewater treatment and other environmental applications. In essence, the amalgamation of CQDs and g-C3N4 presents a novel and efficient approach to address the challenges of wastewater treatment, emphasizing the importance of continued research in this domain [60].
References
- Zhou, L.; Shen, Z.; Wang, S.; Gao, J.; Tang, L.; Li, J.; Dong, Y.; Wang, Z.; Lyu, J. Construction of quantum-scale catalytic regions on anatase TiO2 nanoparticles by loading TiO2 quantum dots for the photocatalytic degradation of VOCs. Ceram. Int. 2021, 47, 21090–21098.
- Javed, S.; Islam, M.; Mujahid, M. Synthesis and characterization of TiO2 quantum dots by sol gel reflux condensation method. Ceram. Int. 2019, 45, 2676–2679.
- Wang, S.; Wang, F.; Su, Z.; Wang, X.; Han, Y.; Zhang, L.; Xiang, J.; Du, W.; Tang, N. Controllable Fabrication of Heterogeneous p-TiO2 QDs@g-C3N4 p-n Junction for Efficient Photocatalysis. Catalysts 2019, 9, 439.
- Wang, J.; Lin, W.; Hu, H.; Liu, C.; Cai, Q.; Zhou, S.; Kong, Y. Engineering Z-system hybrids of 0D/2D F-TiO2 quantum dots/g-C3N4 heterostructures through chemical bonds with enhanced visible-light photocatalytic performance. New J. Chem. 2021, 45, 3067–3078.
- Lee, J.-H.; Jeong, S.-Y.; Son, Y.-D.; Lee, S.-W. Facile Fabrication of TiO2 Quantum Dots-Anchored g-C3N4 Nanosheets as 0D/2D Heterojunction Nanocomposite for Accelerating Solar-Driven Photocatalysis. Nanomaterials 2023, 13, 1565.
- Hu, X.Y.; Yu, Y.T.; Chen, D.D.; Xu, W.C.; Fang, J.Z.; Liu, Z.; Li, R.Q.; Yao, L.; Qin, J.J.; Fang, Z.Q. Anatase/Rutile homojunction quantum dots anchored on g-C3N4 nanosheets for antibiotics degradation in seawater matrice via coupled adsorption-photocatalysis: Mechanism insight and toxicity evaluation. Chem. Eng. J. 2022, 432, 15.
- Kumari, K.; Ahmaruzzaman, M. SnO2 quantum dots (QDs): Synthesis and potential applications in energy storage and environmental remediation. Mater. Res. Bull. 2023, 168, 112446.
- Yu, B.; Li, Y.; Wang, Y.; Li, H.; Zhang, R. Facile hydrothermal synthesis of SnO2 quantum dots with enhanced photocatalytic degradation activity: Role of surface modification with chloroacetic acid. J. Environ. Chem. Eng. 2021, 9, 105618.
- Xu, Z.; Jiang, Y.; Li, Z.; Chen, C.; Kong, X.; Chen, Y.; Zhou, G.; Liu, J.-M.; Kempa, K.; Gao, J. Rapid Microwave-Assisted Synthesis of SnO2 Quantum Dots for Efficient Planar Perovskite Solar Cells. ACS Appl. Energy Mater. 2021, 4, 1887–1893.
- Bathula, B.; Gurugubelli, T.R.; Yoo, J.; Yoo, K. Recent Progress in the Use of SnO2 Quantum Dots: From Synthesis to Photocatalytic Applications. Catalysts 2023, 13, 765.
- Wu, Z.; Jing, H.; Zhao, Y.; Lu, K.; Liu, B.; Yu, J.; Xia, X.; Lei, W.; Hao, Q. Grain boundary and interface interaction Co-regulation promotes SnO2 quantum dots for efficient CO2 reduction. Chem. Eng. J. 2023, 451, 138477.
- Babu, B.; Cho, M.; Byon, C.; Shim, J. Sunlight-driven photocatalytic activity of SnO2 QDs-g-C3N4 nanolayers. Mater. Lett. 2018, 212, 327–331.
- Yousaf, M.U.; Pervaiz, E.; Minallah, S.; Afzal, M.J.; Honghong, L.; Yang, M. Tin oxide quantum dots decorated graphitic carbon nitride for enhanced removal of organic components from water: Green process. Results Phys. 2019, 14, 102455.
- Ji, H.; Fan, Y.; Yan, J.; Xu, Y.; She, X.; Gu, J.; Fei, T.; Xu, H.; Li, H. Construction of SnO2/graphene-like g-C3N4 with enhanced visible light photocatalytic activity. RSC Adv. 2017, 7, 36101–36111.
- Mohamed, K.M.; Benitto, J.J.; Vijaya, J.J.; Bououdina, M. Recent Advances in ZnO-Based Nanostructures for the Photocatalytic Degradation of Hazardous, Non-Biodegradable Medicines. Crystals 2023, 13, 329.
- Mohamed, W.A.A.; Handal, H.T.; Ibrahem, I.A.; Galal, H.R.; Mousa, H.A.; Labib, A.A. Recycling for solar photocatalytic activity of Dianix blue dye and real industrial wastewater treatment process by zinc oxide quantum dots synthesized by solvothermal method. J. Hazard. Mater. 2021, 404, 123962.
- Mohamed, W.A.A.; Ibrahem, I.A.; El-Sayed, A.M.; Galal, H.R.; Handal, H.; Mousa, H.A.; Labib, A.A. Zinc oxide quantum dots for textile dyes and real industrial wastewater treatment: Solar photocatalytic activity, photoluminescence properties and recycling process. Adv. Powder Technol. 2020, 31, 2555–2565.
- Ye, Q.; Xu, L.; Xia, Y.; Gang, R.; Xie, C. Zinc oxide quantum dots/graphitic carbon nitride nanosheets based visible-light photocatalyst for efficient tetracycline hydrochloride degradation. J. Porous Mat. 2022, 29, 571–581.
- Ren, Z.; Ma, H.; Geng, J.; Liu, C.; Song, C.; Lv, Y. ZnO QDs/GO/g-C3N4 Preparation and Photocatalytic Properties of Composites. Micromachines 2023, 14, 1501.
- Vattikuti, S.V.P.; Reddy, P.A.K.; Shim, J.; Byon, C. Visible-Light-Driven Photocatalytic Activity of SnO2–ZnO Quantum Dots Anchored on g-C3N4 Nanosheets for Photocatalytic Pollutant Degradation and H2 Production. ACS Omega 2018, 3, 7587–7602.
- Wang, J.B.; Liu, C.; Yang, S.; Lin, X.; Shi, W.L. Fabrication of a ternary heterostructure BiVO4 quantum dots/C-60/g-C3N4 photocatalyst with enhanced photocatalytic activity. J. Phys. Chem. Solids 2020, 136, 7.
- Cheng, C.; Chen, D.Y.; Li, N.J.; Li, H.; Xu, Q.F.; He, J.H.; Lu, J.M. Bi2WO6 quantum dots with oxygen vacancies combined with g-C3N4 for NO removal. J. Colloid Interface Sci. 2022, 609, 447–455.
- Liang, Y.; Xu, W.; Fang, J.; Liu, Z.; Chen, D.; Pan, T.; Yu, Y.; Fang, Z. Highly dispersed bismuth oxide quantum dots/graphite carbon nitride nanosheets heterojunctions for visible light photocatalytic redox degradation of environmental pollutants. Appl. Catal. B 2021, 295, 120279.
- Zeng, Y.; Yin, Q.; Liu, Z.; Dong, H. Attapulgite-interpenetrated g-C3N4/Bi2WO6 quantum-dots Z-scheme heterojunction for 2-mercaptobenzothiazole degradation with mechanism insight. Chem. Eng. J. 2022, 435, 134918.
- Ding, F.; Chen, P.; Liu, F.; Chen, L.; Guo, J.-K.; Shen, S.; Zhang, Q.; Meng, L.-H.; Au, C.-T.; Yin, S.-F. Bi2MoO6/g-C3N4 of 0D/2D heterostructure as efficient photocatalyst for selective oxidation of aromatic alkanes. Appl. Surf. Sci. 2019, 490, 102–108.
- Sun, Y.; Yuan, X.; Wang, Y.; Zhang, W.; Li, Y.; Zhang, Z.; Su, J.; Zhang, J.; Hu, S. CeO2 quantum dots anchored g-C3N4: Synthesis, characterization and photocatalytic performance. Appl. Surf. Sci. 2022, 576, 151901.
- Zhu, X.; Dong, Z.M.; Xu, J.D.; Lin, S.Y.; Liu, J.Y.; Cheng, Z.P.; Cao, X.H.; Wang, Y.Q.; Liu, Y.H.; Zhang, Z.B. Visible-light induced electron-transfer in MoO3 QDs/g-C3N4 nanosheets for efficient photocatalytic reduction of U(VI). J. Alloys Compd. 2022, 926, 10.
- Guo, R.T.; Liu, X.Y.; Qin, H.; Ang, Z.Y.; Shi, X.; Pan, W.G.; Fu, Z.G.; Tang, J.Y.; Jia, P.Y.; Miao, Y.F.; et al. Photocatalytic reduction of CO2 into CO over nanostructure Bi2S3 quantum dots/g-C3N4 composites with Z-scheme mechanism. Appl. Surf. Sci 2020, 500, 144059.
- Qin, Y.Y.; Li, H.; Lu, J.; Ma, C.C.; Liu, X.L.; Meng, M.J.; Yan, Y.S. Fabrication of magnetic quantum dots modified Z-scheme Bi2O4/g-C3N4 photocatalysts with superior hydroxyl radical productivity for the degradation of rhodamine B. Appl. Surf. Sci. 2019, 493, 458–469.
- Zhao, Z.W.; Fan, J.Y.; Deng, X.Y.; Liu, J. One-step synthesis of phosphorus-doped g-C3N4/Co3O4 quantum dots from vitamin B12 with enhanced visible-light photocatalytic activity for metronidazole degradation. Chem. Eng. J. 2019, 360, 1517–1529.
- Zou, Y.Z.; Xie, Y.; Yu, S.; Chen, L.; Cui, W.; Dong, F.; Zhou, Y. SnO2 quantum dots anchored on g-C3N4 for enhanced visible-light photocatalytic removal of NO and toxic NO2 inhibition. Appl. Surf. Sci. 2019, 496, 8.
- Sun, C.; Yang, J.; Zhu, Y.; Xu, M.; Cui, Y.; Liu, L.; Ren, W.; Zhao, H.; Liang, B. Synthesis of 0D SnO2 nanoparticles/2D g-C3N4 nanosheets heterojunction: Improved charge transfer and separation for visible-light photocatalytic performance. J. Alloys Compd. 2021, 871, 159561.
- Mohammad, A.; Khan, M.E.; Karim, M.R.; Cho, M.H. Synergistically effective and highly visible light responsive SnO2-g-C3N4 nanostructures for improved photocatalytic and photoelectrochemical performance. Appl. Surf. Sci. 2019, 495, 143432.
- Van Pham, V.; Mai, D.-Q.; Bui, D.-P.; Van Man, T.; Zhu, B.; Zhang, L.; Sangkaworn, J.; Tantirungrotechai, J.; Reutrakul, V.; Cao, T.M. Emerging 2D/0D g-C3N4/SnO2 S-scheme photocatalyst: New generation architectural structure of heterojunctions toward visible-light-driven NO degradation. Environ. Pollut. 2021, 286, 117510.
- Wang, X.; He, Y.; Xu, L.; Xia, Y.; Gang, R. SnO2 particles as efficient photocatalysts for organic dye degradation grown in-situ on g-C3N4 nanosheets by microwave-assisted hydrothermal method. Mater. Sci. Semicond. Process. 2021, 121, 105298.
- Zhang, B.; He, X.; Yu, C.; Liu, G.; Ma, D.; Cui, C.; Yan, Q.; Zhang, Y.; Zhang, G.; Ma, J.; et al. Degradation of tetracycline hydrochloride by ultrafine TiO2 nanoparticles modified g-C3N4 heterojunction photocatalyst: Influencing factors, products and mechanism insight. Chin. Chem. Lett. 2022, 33, 1337–1342.
- Ni, S.; Fu, Z.; Li, L.; Ma, M.; Liu, Y. Step-scheme heterojunction g-C3N4/TiO2 for efficient photocatalytic degradation of tetracycline hydrochloride under UV light. Colloids Surf. A Physicochem. Eng. Asp. 2022, 649, 129475.
- Guo, F.; Sun, H.; Huang, X.; Shi, W.; Yan, C. Fabrication of TiO2/high-crystalline g-C3N4 composite with enhanced visible-light photocatalytic performance for tetracycline degradation. J. Chem. Technol. Biotechnol. 2020, 95, 2684–2693.
- Gündoğmuş, P.; Park, J.; Öztürk, A. Preparation and photocatalytic activity of g-C3N4/TiO2 heterojunctions under solar light illumination. Ceram. Int. 2020, 46, 21431–21438.
- Li, Y.; Zhang, Q.; Lu, Y.; Song, Z.; Wang, C.; Li, D.; Tang, X.; Zhou, X. Surface hydroxylation of TiO2/g-C3N4 photocatalyst for photo-Fenton degradation of tetracycline. Ceram. Int. 2022, 48, 1306–1313.
- Jung, H.; Pham, T.-T.; Shin, E.W. Effect of g-C3N4 precursors on the morphological structures of g-C3N4/ZnO composite photocatalysts. J. Alloys Compd. 2019, 788, 1084–1092.
- Ngullie, R.C.; Alaswad, S.O.; Bhuvaneswari, K.; Shanmugam, P.; Pazhanivel, T.; Arunachalam, P. Synthesis and Characterization of Efficient ZnO/g-C3N4 Nanocomposites Photocatalyst for Photocatalytic Degradation of Methylene Blue. Coatings 2020, 10, 500.
- Tan, X.; Wang, X.; Hang, H.; Zhang, D.; Zhang, N.; Xiao, Z.; Tao, H. Self-assembly method assisted synthesis of g-C3N4/ZnO heterostructure nanocomposites with enhanced photocatalytic performance. Opt. Mater. 2019, 96, 109266.
- Naseri, A.; Samadi, M.; Pourjavadi, A.; Ramakrishna, S.; Moshfegh, A.Z. Enhanced photocatalytic activity of ZnO/g-C3N4 nanofibers constituting carbonaceous species under simulated sunlight for organic dye removal. Ceram. Int. 2021, 47, 26185–26196.
- Mathialagan, A.; Manavalan, M.; Venkatachalam, K.; Mohammad, F.; Oh, W.C.; Sagadevan, S. Fabrication and physicochemical characterization of g-C3N4/ZnO composite with enhanced photocatalytic activity under visible light. Opt. Mater. 2020, 100, 109643.
- Xu, X.; Huang, T.; Xu, Y.; Hu, H.; Liao, S.; Hu, X.; Chen, D.; Zhang, M. Highly dispersed CeO2–x nanoparticles with rich oxygen vacancies enhance photocatalytic performance of g-C3N4 toward methyl orange degradation under visible light irradiation. J. Rare Earths 2022, 40, 1255–1263.
- Subashini, A.; Varun Prasath, P.; Sagadevan, S.; Anita Lett, J.; Fatimah, I.; Mohammad, F.; Al-Lohedan, H.A.; Alshahateet, S.F.; Chun Oh, W. Enhanced photocatalytic degradation efficiency of graphitic carbon nitride-loaded CeO2 nanoparticles. Chem. Phys. Lett. 2021, 769, 138441.
- Barathi, D.; Rajalakshmi, N.; Ranjith, R.; Sangeetha, R.; Meyvel, S. Controllable synthesis of CeO2/g-C3N4 hybrid catalysts and its structural, optical and visible light photocatalytic activity. Diam. Relat. Mat. 2021, 111, 108161.
- Kaur, M.; Singh, S.; Mehta, S.K.; Kansal, S.K.; Umar, A.; Ibrahim, A.A.; Baskoutas, S. CeO2 quantum dots decorated g-C3N4 nanosheets: A potential scaffold for fluorescence sensing of heavy metals and visible-light driven photocatalyst. J. Alloys Compd. 2023, 960, 170637.
- Wei, X.; Wang, X.; Pu, Y.; Liu, A.; Chen, C.; Zou, W.; Zheng, Y.; Huang, J.; Zhang, Y.; Yang, Y.; et al. Facile ball-milling synthesis of CeO2/g-C3N4 Z-scheme heterojunction for synergistic adsorption and photodegradation of methylene blue: Characteristics, kinetics, models, and mechanisms. Chem. Eng. J. 2021, 420, 127719.
- Rathi, V.; Panneerselvam, A.; Sathiyapriya, R. A novel hydrothermal induced BiVO4/g-C3N4 heterojunctions visible-light photocatalyst for effective elimination of aqueous organic pollutants. Vacuum 2020, 180, 109458.
- Xu, X.; Zhang, J.; Tao, F.; Dong, Y.; Wang, L.; Hong, T. Facile construction of Z-scheme g-C3N4/BiVO4 heterojunctions for boosting visible-light photocatalytic activity. Mater. Sci. Eng. B 2022, 279, 115676.
- Reddy, C.V.; Nagar, A.; Shetti, N.P.; Reddy, I.N.; Basu, S.; Shim, J.; Kakarla, R.R. Novel g-C3N4/BiVO4 heterostructured nanohybrids for high efficiency photocatalytic degradation of toxic chemical pollutants. Chemosphere 2023, 322, 138146.
- Mao, J.; Hong, B.; Wei, J.; Xu, J.; Han, Y.; Jin, H.; Jin, D.; Peng, X.; Li, J.; Yang, Y.; et al. Enhanced Ciprofloxacin Photodegradation of Visible-Light-Driven Z-Scheme g-C3N4/Bi2WO6 Nanocomposites and Interface Effect. ChemistrySelect 2019, 4, 13716–13723.
- Yasmeen, H.; Zada, A.; Li, W.; Xu, M.; Liu, S. Suitable energy platform of Bi2WO6 significantly improves visible-light degradation activity of g-C3N4 for highly toxic diuron pollutant. Mater. Sci. Semicond. Process. 2019, 102, 104598.
- Lian, X.; Xue, W.; Dong, S.; Liu, E.; Li, H.; Xu, K. Construction of S-scheme Bi2WO6/g-C3N4 heterostructure nanosheets with enhanced visible-light photocatalytic degradation for ammonium dinitramide. J. Hazard. Mater. 2021, 412, 125217.
- Yang, Q.; An, J.; Xu, Z.; Liang, S.; Wang, H. Performance and mechanism of atrazine degradation using Co3O4/g-C3N4 hybrid photocatalyst with peroxymonosulfate under visible light irradiation. Colloids Surf. A Physicochem. Eng. Asp. 2021, 614, 126161.
- Liu, L.; Huang, J.; Yu, H.; Wan, J.; Liu, L.; Yi, K.; Zhang, W.; Zhang, C. Construction of MoO3 nanopaticles/g-C3N4 nanosheets 0D/2D heterojuntion photocatalysts for enhanced photocatalytic degradation of antibiotic pollutant. Chemosphere 2021, 282, 131049.
- Xue, S.; Wu, C.; Pu, S.; Hou, Y.; Tong, T.; Yang, G.; Qin, Z.; Wang, Z.; Bao, J. Direct Z-Scheme charge transfer in heterostructured MoO3/g-C3N4 photocatalysts and the generation of active radicals in photocatalytic dye degradations. Environ. Pollut. 2019, 250, 338–345.
- Liu, Y.; Huang, H.; Cao, W.; Mao, B.; Liu, Y.; Kang, Z. Advances in carbon dots: From the perspective of traditional quantum dots. Mat. Chem. Front. 2020, 4, 1586–1613.
More
Information
Subjects:
Electrochemistry; Chemistry, Physical
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.0K
Revisions:
2 times
(View History)
Update Date:
15 Nov 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




