Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Valentina Vassilenko | -- | 5799 | 2023-11-14 12:41:00 | | | |
| 2 | Mona Zou | Meta information modification | 5799 | 2023-11-15 07:53:52 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Vassilenko, V.; Moura, P.C.; Raposo, M. Diagnosis of Carcinogenic Pathologies through Breath Biomarkers. Encyclopedia. Available online: https://encyclopedia.pub/entry/51538 (accessed on 08 February 2026).
Vassilenko V, Moura PC, Raposo M. Diagnosis of Carcinogenic Pathologies through Breath Biomarkers. Encyclopedia. Available at: https://encyclopedia.pub/entry/51538. Accessed February 08, 2026.
Vassilenko, Valentina, Pedro Catalão Moura, Maria Raposo. "Diagnosis of Carcinogenic Pathologies through Breath Biomarkers" Encyclopedia, https://encyclopedia.pub/entry/51538 (accessed February 08, 2026).
Vassilenko, V., Moura, P.C., & Raposo, M. (2023, November 14). Diagnosis of Carcinogenic Pathologies through Breath Biomarkers. In Encyclopedia. https://encyclopedia.pub/entry/51538
Vassilenko, Valentina, et al. "Diagnosis of Carcinogenic Pathologies through Breath Biomarkers." Encyclopedia. Web. 14 November, 2023.
Copy Citation
The assessment of volatile breath biomarkers has been targeted with a lot of interest by the scientific and medical communities during the past decades due to their suitability for an accurate, painless, non-invasive, and rapid diagnosis of health states and pathological conditions.
carcinogenic diseases
cancer
biomarkers
volatile organic compounds (VOCs)
exhaled air
breath analysis
1. Carcinogenic Biomarkers from Breath
Endogenous Volatile organic compounds (VOCs) identified in exhaled air may arise from metabolic activity in lung tissue and airways or have a systemic origin (produced in any part of the body, including other organs and tissues). However, when produced systemically, these VOCs are captured and distributed in the bloodstream. Thus, the gas exchange of compounds between alveoli and capillaries allows the excretion of exhaled compounds in the form of air, which is exhaled together with respiratory droplets and atmospheric gases. Therefore, the importance of exhaled endogenous VOCs as the main source of biomarkers with clear associations with metabolic status is highlighted.
It is important to highlight that VOCs related to certain diseases may result from metabolic processes that occur, for example, inside a tumour cell and in the surrounding tissues that “react” to the presence of cancer. Lipid peroxidation of polyunsaturated fatty acids, for example, is a biological mechanism that leads to the production of saturated hydrocarbons. Known to be formed in different proportions through chain reactions, ethane and pentane are expelled in greater quantities in situations of mental and/or physical stress during lipid peroxidation. Some methylated hydrocarbons were also identified from breath, although their metabolic pathways are not elucidative enough to confirm their full diagnostic potential [1]. All the compounds and the respective formation processes mentioned here as examples of the origin of the analytes later exhaled in breath are well-known and often detected biomarkers, as will be addressed in due time.
However, to date, only a few VOCs have been officially approved as disease biomarkers. For breath tests, the Food and Drug Administration (FDA) has only approved the following compounds: ethanol (for assessment of blood alcohol content), hydrogen (carbohydrate metabolism), nitric oxide (a biomarker of asthma), carbon monoxide (a biomarker of neonatal jaundice), 13CO2 (a biomarker of H. pylori infection), and branched hydrocarbons (biomarkers of organ transplant rejection) [2]. For exemplification purposes, ethanol is often used by authorities and police figures to assess the alcoholic intoxication levels of drivers [3]. Nitric oxide, in turn, is commonly used in clinical scenarios for the diagnosis of asthma, as mentioned [4]. As can be seen, the world of biomarkers is gaining relevance. Despite the small number of approved biomarkers, several other respiratory metabolites have been referenced as putative breath VOC biomarkers of cancerogenic disease indicators.
Figure 1 schematically represents the pronounced variability of oncological diseases for which potential breath biomarkers have already been identified.
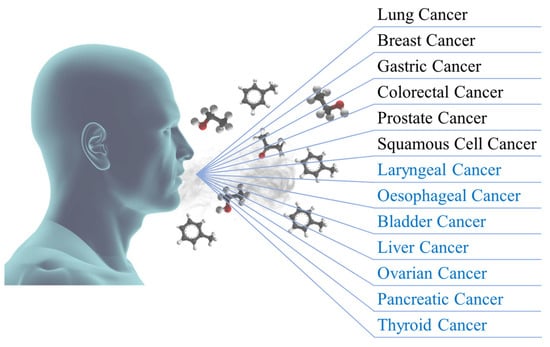
Figure 1. Representation of oncological diseases for which potential breath biomarkers have already been identified (in black) and with future potential biomarkers (in blue).
2. Lung Cancer
Lung cancer is the leading and third cause of death among men and women, respectively. Usually caused by behaviours of risk like smoking, it can also be a consequence of long-term exposure to hazardous compounds or environments. In fact, the American Cancer Society estimates that, in the United States of America, a total of 238,340 new cases and 127,070 deaths will occur during the entire year of 2023, which values are extrapolatable for the rest of the world [5]. Due to its high rate of mortality, an accurate and rapid diagnosis is mandatory. However, lung cancer is usually identified through a histological or cytological approach. Both procedures are highly invasive and have some associated risks [6][7][8]. Several biomarkers have been studied and assessed as suitable tools for more rapid and accurate identification of the pathology. Some have even already been validated, and others have presented very promising results [9][10].
In vitro cultures of lung cells were prepared and analysed by Sponring et al. (2010). For that, a thermo-desorption–gas chromatography–mass spectrometry (TD-GC-MS) device was used to separate the analytes present in 200 mL samples of headspace emitted by the cultures. Six specific VOCs were seen to be specifically produced and emitted by the lung cancer cells, in this way, these analytes represent potential biomarkers of the disease [11]. To detect the 18 analytes described as potential lung cancer biomarkers, Chatterjee et al. (2013) developed an electronic nose based on conductive polymer nanocomposite quantum resistive sensors. By applying principal component analysis (PCA) to the collected data, the authors were able to discriminate all the analytes with a total explained variance of 98.34% (PC1—65.11%, PC2—23.69%, PC3—9.54%) [12]. A colourimetric sensor array was developed and applied by Hou et al. (2013) to identify and quantify analytes known for their suitability for the role of lung cancer biomarkers. The authors focused their study on four specific analytes, hexanal, isoprene, p-xylene, and styrene. The device and protocol developed in the study enabled the authors to quantify the mentioned analytes with limits of detection ranging from 50 ppbv to 500 ppbv [13].
The suitability of nanoparticles with magnetic properties to detect several aldehydes in breath samples of lung cancer patients was assessed by Xu et al. (2014). The target VOCs were nonanal, octanal, heptanal, hexanal, pentanal, and butanal, and the authors were able of quantifying them for limits of detection ranging from 2.9 to 21.5 nmolL−1 [14]. Gregis et al. (2018) developed an analytical device around a metal oxide-based gas sensor to detect and quantify analytes in the exhaled breath that can correspond to potential biomarkers for lung cancer diagnosis. The device enabled the authors to successfully assess the parameters of four analytes, o-xylene, cyclohexane, propanol, and toluene. The limits of detection for these four compounds were established at around 5, 112, 21, and 24 ppbv, respectively [15]. Saalberg et al. (2017) were able to detect six specific VOCs that are widely assessed as possible lung cancer biomarkers. For that, the authors developed a sensor based on photoacoustic spectroscopy with an optical-parametric oscillator as the radiation source. The analytes hexanal, styrene, ethylbenzene, isoprene, propanol, and 2-butanone were identified with detection limits of 15.4, 141.6, 8.6, 36.6, 8.4, and 5.7 ppbv [16].
An ion mobility spectrometer was used to analyse exhaled breath samples of 19 lung cancer patients. The samples were collected through the working channel of a flexible bronchoscope inserted into the patient. The authors were able to detect two analytes whose concentration is significantly higher and three whose concentration is considerably lower in the breath of lung cancer patients. Unfortunately, no information on the identification of the compounds is provided [17]. Mazzone et al. (2012), aiming to describe the breath signature of lung cancer, analysed the exhaled breath of 92 lung cancer patients and 137 healthy controls with a colourimetric sensor array. Even without providing information on the identified VOCs, the authors used the breath signatures of both groups to develop prediction models that were posteriorly validated considering the colour changes of the sensor. These models enabled the authors to differentiate and classify the analysed groups with accuracy levels ranging from 82.5% to 89.0% [18]. A GC-MS device was used by Santonico et al. (2012) to analyse breath samples of healthy individuals and lung cancer patients. The breath collection was performed through an endoscopic probe. Authors could use the respective breath signatures to successfully classify the breath samples in 75% of the cases [19].
The relationship between breath composition and the disease stage was studied by Schmekel et al. (2014) For that, 10 healthy individuals and 22 lung cancer patients were selected as volunteers for breath sample collection and analysis. The analyte separation was performed with an electronic nose (e-nose) and the authors were able to successfully distinguish between the two considered groups. The working principle of the e-nose used by these authors was based on an array of independent sensors, specifically: 10 metal-oxide-semiconductor field effect transistors and 12 metal-oxide semiconductor sensors, capable of detecting alkanes and nucleophilic compounds, respectively [20]. Nine volatile organic compounds were assessed by Li et al. (2015) regarding their suitability for the role of lung cancer biomarkers. For that, 85 healthy individuals, 34 benign pulmonary nodules patients, and 85 lung cancer patients were considered for breath analysis by Fourier transform–ion cyclotron–mass spectrometry (FT-ICR-MS). By considering the respective breath signatures, authors could successfully distinguish lung cancer patients from non-smokers (97% accuracy), smokers (95% accuracy), and benign pulmonary nodule patients (89% accuracy) [21].
To validate the suitability of breath biomarkers as a diagnostic tool for lung cancer in two independent cohorts, Phillips et al. (2015) developed a dual study. The first considered cohort was composed of 35 healthy individuals, 82 healthy smokers, 84 high-risk symptomatic patients, and 100 lung cancer patients. The second cohort, in its turn, included 19 healthy individuals, 70 healthy smokers, 51 high-risk symptomatic patients, and 75 lung cancer patients. By considering the respective breath prints, the authors could discriminate the lung cancer patient group in the first cohort with sensitivity and specificity levels of 68.0% and 68.4%. The sensitivity and specificity levels of lung cancer patient group discrimination in the second cohort were 70.1% and 68.0%, respectively [22]. The control group (89 individuals) and lung cancer patient group (81 individuals) were considered in a study developed by Callol-Sanchez et al. (2017). Here, the collected samples of breath were analysed with a GC-MS device, and special emphasis was given to nonanoic acid, propanoic acid, nonanal, octanal, heptanal, and hexanal. All of these VOCs have been studied as high-potential analytes for lung cancer diagnosis. In fact, the authors were able to prove that nonanoic acid, for example, is 2.5 times more likely to be present in the breath of lung cancer patients than in healthy breath samples [23].
A proton transfer reaction–mass spectrometry (PTR-MS) device was used to separate the analytes of exhaled breath samples collected from lung cancer patients (30 subjects) and healthy individuals (30 subjects). By considering the respective breath signatures, Sun et al. (2019) were able to discriminate the groups with promising interval levels of accuracy (74–99%), sensitivity (60–100%), and specificity (73–97%) [24]. Exhaled breath samples of healthy volunteers (12 subjects) and lung cancer patients (32 subjects) were collected into inert Tedlar bags and analysed with a GC-ToF-MS device by Saidi et al. (2020). The authors were able to detect 30 VOCs with potential for the role of lung cancer biomarkers and, additionally, four of those stand out from the others due to their even higher discriminant capacity. Unfortunately, the authors did not provide the identification of these four VOCs [25].
Nonanal, octanal, hexanal, and pentanal were identified as discriminant analytes for lung cancer by Fuchs et al. (2010). The concentration levels of these analytes were considerably higher in the breath of lung cancer patients than in healthy individuals’ breath; hence, the four VOCs were considered to be potential biomarkers for lung cancer. To achieve such results, the authors performed analyses of the breath of 12 lung cancer patients and 12 healthy subjects. The identification and quantification of the analytes was made possible by a GC-MS device [26]. Several aldehydes were also assessed as potential lung cancer biomarkers by Poli et al. (2010). These seven analytes (propanal, butanal, pentanal, hexanal, heptanal, and octanal) were identified and quantified in breath samples collected from lung cancer patients (40 subjects) and healthy individuals (38 subjects), with a solid-phase microextraction–gas chromatography–mass spectrometry (SPME-GC-MS) device [27]. Exhaled breath samples of 23 lung cancer patients and 30 healthy volunteers were collected into 1 L Tedlar bags and posteriorly analysed with a GC-ToF-MS device. Among all the detected analytes, Rudnicka et al. (2011) were able to identify five specific VOCs (isopropyl alcohol, ethylbenzene, 2-propenal, carbon disulphide, and propane) that enable the best discrimination between both groups. Consequently, the mentioned analytes have a high potential for the role of lung cancer biomarkers [28].
A total of 12 VOCs were identified by Buszewski et al. (2012), with a GC-ToF-MS device and with limits of detection ranging from 0.31 ppbv to 0.75 ppbv. A cohort of 73 individuals (29 lung cancer patients and 44 healthy subjects) was gathered for the breath analyses. The authors conclude that all 12 analytes show an increase in their concentration levels in the exhaled breath of lung cancer patients [29]. In a parallel study, Buszewski et al. (2012) used an SPME-GC-MS device to analyse the headspace emitted by tissue samples collected from oncologic patients. The authors conclude that eight VOCs (2-pentanone, 2-butanone, 2-propanol, 1-propanol, dimethyl sulphide, carbon disulphide, acetone, and ethanol) presented higher levels of concentration when emitted from carcinogenic tissues when compared with normal tissues [30].
A Fourier transform–ion cyclotron resonance–mass spectrometry (FT-ICR-MS) device was used by Fu et al. (2013) to assess the breath analytes of healthy volunteers (88 subjects) and lung cancer patients (97 subjects). From the dozens of detected analytes, four VOCs exhibited a similar change of behaviour between healthy volunteers and lung cancer patients. The concentration levels of 4-hydroxy-hexenal, 3-hydroxy-2-butanone, 2-butanone, and 2-hydroxy-acetaldehyde were consistently higher in the breath of lung cancer scenarios [31]. Ma et al. (2014) used a solid-phase microextraction–gas chromatography–gas chromatography (SPME-GC-GC) device to analyse the exhaled breath samples of 38 individuals (25 healthy volunteers and 13 lung cancer patients). Among all the detected analytes, five specific VOCs (propanol, pentane, methanol, isoprene, and acetone) were identified as potential biomarkers. In fact, these analytes present higher concentration levels in the breath of lung cancer patients than in healthy volunteers’ breath [32].
An ion mobility spectrometry (IMS) device was the analytical technique selected by Handa et al. (2014) to analyse exhaled breath samples collected from 39 healthy volunteers and 50 lung cancer patients. Among the 115 detected VOCs, a single analyte (dodecane) enabled the authors to differentiate both groups with sensitivity and specificity levels up to 76% and 100%, respectively [33]. Eight volatile organic compounds were identified by Ligor et al. (2015) as potential lung cancer biomarkers due to their discriminating capacity between oncologic and healthy cases. A cohort of 484 volunteers (361 healthy subjects and 123 lung cancer patients) was used for breath analyses with an SPME-GC-MS device. The mentioned VOCs enabled the authors to differentiate both groups with accuracy, sensitivity, and specificity levels of 65.0%, 63.5%, and 72.4%, respectively [34]. Octanal was identified as a potential biomarker for lung cancer detection by Jouyban et al. (2017). For that, the authors collected 1000 mL of exhaled breath into a homemade extraction device. The separated VOCs were condensed in 0.5 mL of acetone and posteriorly analysed with a GC-MS device. The limits of detection and quantification for the octanal were 0.008 ng/mL and 0.026 ng/mL, respectively [35].
As reviewed, the study of exhaled breath biomarkers for the diagnosis of lung cancer, in the form of volatile organic compounds is very complete and deeply assessed. There are several VOCs already validated and certified as biomarkers; nevertheless, other analytes require further investigation to assess their suitability.
3. Breast Cancer
Breast cancer is among the most common types of carcinomas in women and, additionally, leads to thousands of deaths every year. In fact, the American Cancer Society and the Cancer Research Institute of the United Kingdom estimate that a total of 297,790 and 55,920 new cases will be diagnosed during the entire year of 2023, respectively, for the United States of America and the United Kingdom. Regarding the deaths, a total of 43,700 and 11,499 are, respectively, expected during 2023—impressive numbers [36][37].
Contemporary unhealthy lifestyles like poor nutrition or lack of exercise, and changes in reproductive behaviour are some of the main risk factors for the rise of breast cancer incidence [38][39]. In addition to their origins, the current methods for breast cancer screening and diagnosis are often aggressive and painful due to the necessity of invasive procedures. This fact leads to a later diagnosis of the pathology and, consequently, to more dangerous comorbidities [40][41]. Several studies have been developed aiming at the identification of possible biomarkers emitted mainly in the breath, but also in the urine, of the patients, that allow fast, non-invasive, and painless screening for breast cancer [42][43][44][45].
Aiming to differentiate between sub-types of breast cancer tumours, Barash et al. (2015) analysed the breath of 276 women with different types of lesions. As in some of the previously addressed papers, a GC-MS device was the analytical technique selected to perform the analyses. A total of 23 analytes of interest were detected and 13 were accurately identified [46]. To study the fingerprint patterns of VOCs emitted from four different types of breast cancer cells, Lavra et al. (2015) also used a GC-MS device. From the in vitro cultures of cells, the authors were able to successfully identify 13 specific VOCs with elevated potential for being breast cancer biomarkers [47]. In vitro cultures of breast cancer cells were also prepared and studied by Arshad et al. (2014). By using a Fourier-transform infrared (FTIR) spectroscopy device, the authors were able to remark methanol as a clear biomarker for breast cancer [48]. Ethyl acetate, 2-methyl butanoate, ethyl propanoate, 3-methyl-3-buten-1-ol, 2-heptanone, and 2-pentanone were identified in the headspace of in vitro cell cultures, by Silva et al. (2017) as VOCs with high potentiality for being biomarkers of breast cancer [49].
To assess the suitability of VOCs as biomarkers for breast cancer diagnosis, Phillips et al. (2014) analysed the exhaled breath of 244 women divided into two groups, healthy individuals and women with abnormal screening mammograms (breast cancer). Through the detection of breath signatures, the authors were able to differentiate both groups with accuracy, specificity, and sensitivity levels of 79, 70 and 81.8%, respectively. Screening mammograms were used to check the results [50]. Ji et al. (2014), with a similar goal, analysed the exhaled breath of 63 volunteers (24 healthy individuals, 22 breast cancer patients, and 17 benign breast tumour patients). The breath signatures collected from the three groups enabled a successful differentiation among them. The distinction between healthy women and breast cancer patients was achieved with sensitivity and specificity levels of 68.2 and 91.7%, respectively. The sensitivity and specificity values for the differentiation between benign and malignant breast cancer were 91.7 and 95.8%, respectively [51]. GC-MS was used by Phillips et al. (2017) to analyse the exhaled breath of 258 women (54 breast cancer patients and 204 healthy volunteers). Even without information on the identified VOCs, 21 analytes were detected as potential biomarkers. The discrimination of breast cancer patients from healthy women considering these VOCs was achieved with an accuracy level of 77% [52]. In a more recent study, Phillips et al. (2018) analysed the exhaled breath of 178 women (54 breast cancer patients and 124 healthy volunteers) with two analytical techniques, a GC-MS device and a gas chromatography–surface acoustic wave detection (GC-SAW) device, for comparison purposes. Again, no information on the identification of VOCs is provided; however, the breath signatures detected by GC-MS enabled the differentiation of both groups with 90% accuracy. The results of the GC-SAW measurements enabled the authors to distinguish between both groups with 86% accuracy [53]. Finally, Yang et al. (2021) were able to differentiate both groups of healthy individuals (n = 88) and breast cancer patients (n = 351) through their respective VOCs signatures in breath, with accuracy, sensitivity, and specificity levels of 91, 86, and 97%, respectively [54].
To identify breath signatures for breast cancer with an electronic nose, León-Martínez et al. (2020) analysed the breath samples of 443 women (181 healthy volunteers and 262 breast cancer patients). The commercial e-nose used by the authors bases its working principle on the variation of electrical resistance of 32 polymer-based sensors once exposed to the target metabolites. Unfortunately, the authors do not provide information on the identified analytes, but they were able to use the VOCs’ patterns in the breath to differentiate between healthy women and breast cancer patients in 98% of the cases [55]. In a study developed by the same research group, Rodríguez-Aguilar et al. (2021) were able to distinguish breast cancer patients from lung cancer patients and chronic obstructive pulmonary disease (COPD) patients through the breath signatures collected from each group. The measurement data were processed by principal component analysis (PCA). The differentiation between lung and breast cancer patients was achieved with a total explained variance of 98.6% (PC1—80.1%, PC2—17.2%, PC3—1.3%). Regarding the differentiation between COPD and breast cancer patients, the resulting total explained variance was 99.2% (PC1—96.3%, PC2—2.0%, PC3—0.9%) [56].
Phillips et al. (2010) analysed the exhaled breath of 258 women (54 breast cancer patients and 204 healthy volunteers) with a GC-MS device. The authors employed a portable breath collection apparatus to sample 1 L of alveolar breath, during 2 min of normal breathing through a disposable inert mouthpiece. From the detected analytes, authors isolated 10 with a higher potential for being biomarkers and, for their identification, 26 possibilities were gathered [57]. A cohort of 20 individuals (10 healthy individuals and 10 breast cancer patients) was also analysed with GC-MS by Mandy et al. (2012). Samples of 1 L of alveolar breath were collected and four VOCs, decene, naphthalene, caryophyllene, trichloroethylene, and 3-methyl hexane, were identified due to their high potential for being breast cancer biomarkers [58]. Xu et al. (2013) developed a chip with a highly sensitive single nanowire array to detect and identify VOCs in breath potentially related to breast cancer. Four specific VOCs, heptanal, 2-propanol, isopropyl myristate, and acetophenone, were successfully identified by the authors as potential biomarkers. The detection limits ranged from 798 ppbv to 129.5 ppmv [59]. P-xylene, cyclohexanol, 2,4-dimethyl-benzaldehyde and 2-ethyl-1-hexanol were identified by Huang et al. (2016) as potential biomarkers for discriminating breast cancer cells from healthy mammary cells. In vitro cultures of both healthy and cancerous cells were prepared, and the emitted headspace was analysed with a solid-phase microextraction–gas chromatography–mass spectrometry (SPME-GC-MS) device [60]. Fifteen VOCs were identified by Zhang et al. (2020) as potential biomarkers for breast cancer diagnosis. For that, the authors analysed the exhaled breath of 203 volunteers with an SPME-GC-MS device [61]. As the reviewed studies prove, the field of breast cancer biomarkers, and specifically, VOCs as biomarkers, is a developing world that already has very promising and paradigm-changing results.
4. Gastric Cancer
Most of the techniques currently used for diagnosing gastric cancer involve invasive painful procedures. For example, a digestive endoscopy with biopsy for posterior diagnosis through histopathological analysis requires the introduction of medical devices for the collection of tissue samples. The development of non-invasive but accurate techniques for gastric cancer diagnosis would enable a faster reaction and treatment of the disease [62][63][64]. The rapidness and effectiveness of the diagnostic are particularly important since the number of new cases is impressive. In fact, just for the United States of America, the American Cancer Society estimates that, during 2023, 26,500 new cases and 11,130 deaths will occur of gastric cancer [65].
Some studies have been developed to identify possible biomarkers for the diagnosis of gastric cancer. Salivary VOCs, for example, were studied for this purpose by Bel’skaya et al. (2020). The identified VOCs (ethanol, 2-propanol, acetone, and acetaldehyde) enabled the authors to differentiate between healthy individuals and gastric cancer patients with specificity and sensitivity levels of 90.9% and 95.7%, respectively [66]. Regarding the exhaled breath, an electronic nose (e-nose) was used by Schuermans et al. (2018) to analyse a cohort of 44 individuals (28 healthy volunteers and 16 gastric cancer patients). This device bases its working principle on the heating and cooling of three micro-hotplate metal-oxide sensors that, once exposed to the metabolites exhaled in breath, alter their conductivity, leading to the formation of VOC-specific patterns. The identification of the VOC breath signature for each group enabled the authors to differentiate them with accuracy, sensitivity, and selectivity levels of 75%, 81%, and 71%, respectively. Unfortunately, information about the detected VOCs is not provided [67]. Zhang et al. (2014), in their turn, studied the analytes emitted by in vitro cultures of gastric cancer cells. The headspace emitted by the cultures of cells was analysed by gas chromatography–mass spectrometry (GC-MS). Eight volatile organic compounds were successfully identified as being produced solely by the cancer cells and, hence, are potential biomarkers for gastric cancer diagnostics [68].
Xu et al. (2013) developed a breath test whose aim is to differentiate benign gastric cancer from gastric cancer by examining the breath signatures of both groups. For that, the exhaled breath of a total of 130 patients (37 gastric cancer patients and 93 patients with non-oncologic gastric pathologies) was analysed. The results obtained enabled the authors to identify five VOCs with high suitability for being biomarkers. These five VOCs allowed differentiation between gastric cancer and less severe gastric pathologies with a sensitivity and specificity of 84% and 87%, respectively [69]. Furfural, 2-propene-nitrile, and 2-butoxy-ethanol were also identified as gastric cancer biomarkers, among other VOCs, by Amal et al. (2016). In addition, four other VOCs were also remarked on as being potential biomarkers (4-methyl octane, 2-butanone, 1,2,3-trimethylbenzene, and α-methyl-styrene). The identification of these analytes from the exhaled breath analysis of 484 patients (99 gastric cancer patients and 385 healthy volunteers) enabled the authors to differentiate between both groups with levels of accuracy, sensitivity, and specificity of 92%, 73%, and 98% [70].
Nine analytes were identified in the exhaled breath of 43 volunteers (17 healthy subjects and 26 gastric cancer patients), by Jung et al. (2021). A proton-transfer-reaction time-of-flight mass spectrometry (PTR-ToF-MS) device was the analytical technique selected for the analysis. The identification of the mentioned VOCs as potential gastric cancer biomarkers was achieved with an accuracy level of 82% [71]. A thermal desorption–single-photon ionization–mass spectrometry (TD-SPI-MS) device was used by Hong et al. (2021) to analyse the exhaled breath of a large cohort (174 volunteers: 78 healthy subjects and 96 gastric cancer patients). Among all the detected analytes, the authors were able to identify seven specific VOCs with the potentiality of being gastric cancer biomarkers. By using these seven VOCs in a single pattern, the differentiation of both groups was achieved with an accuracy level of 96.2% [72]. Chen et al. (2016), in their turn, were able to identify 14 volatile organic compounds as potential biomarkers for gastric cancer diagnosis. A surface-enhanced Raman scattering (SERS) sensor was the selected procedure to analyse and differentiate between healthy volunteers and gastric cancer patients. The differentiation was achieved by the authors with sensitivity and specificity values of 83% and 92% [73]. As reviewed, there are several promising results in the field of gastric cancer biomarkers. Further scientific studies must be developed to make this possibility a reality.
5. Colorectal Cancer
Colorectal cancer (CRC) is one of the cancers with a higher incidence and mortality. In fact, just in the United States of America, 106,970 new cases and 46,050 deaths are expected during 2023 [74]. Since it can be a very silent pathology, CRC is usually detected only in the late stages of development, leading to very low rates of cure [75][76]. New, precise and, more importantly, rapid diagnostic tools are mandatory. The application of VOCs as an option for an accurate diagnosis of CRC has been deeply studied and this has already provided substantial results [77][78].
Altomare et al. (2013) collected breath samples into 1 L inert Tedlar bags, from 41 healthy individuals and 37 colorectal cancer patients. The samples were analysed with a GC-MS device. Among all the identified compounds, the authors were able to build a pattern composed of 15 VOCs that distinguishes both groups with sensitivity and specificity levels of 86% and 83%, respectively [79]. In follow-up work, the same research group analysed the breath of 103 volunteers (55 healthy individuals and 48 CRC patients) with a thermal-desorption–gas chromatography–mass spectrometry (TD-GC-MS) device. The authors were able to identify 12 of the 15 VOCs detected in the previous work. In addition, the differentiation between both considered groups, based on their breath signatures, was achieved with accuracy, sensitivity, and specificity levels of 98.6%, 100.0%, and 97.9%, respectively [80]. Finally, in a third study, Altomare et al. (2020) analysed the exhaled breath of 83 CRC patients and 90 healthy subjects. Among all the detected analytes, 14 VOCs were identified as high-potential biomarkers for the diagnosis of CRC [81].
Nine VOCs were identified by Wang et al. (2014) as potential biomarkers for the diagnosis of colorectal cancer. To achieve this, the exhaled breath of 40 individuals (20 healthy subjects and 20 CRC patients) was collected and analysed with an SPME-GC-MS apparatus [82]. A total of 122 healthy individuals and 87 CRC patients were considered by Amal et al. (2016) for breath analysis with a GC-MS. The authors concluded that two specific VOCs, ethyl acetate and acetone, increase their concentration levels (in the ppbv range) in the breath of CRC patients. On the other hand, 4-methyl octane and ethanol decrease their concentration (in the ppbv range) in these cases. In conclusion, the referred to VOCs represent potential biomarkers for the diagnosis of CRC [83]. Even without identifying the VOCs, Keulen et al. (2020), could differentiate healthy individuals (68 subjects) from CRC patients (42 subjects). This discrimination was based on the breath signatures of both groups, and it was achieved with sensitivity and specificity levels of 83% and 60%, respectively [84]. Four acids (butanoic, pentanoic, dodecanoic and octanoic acids) and seven other VOCs were recently identified by Vietro et al. (2020) as potential CRC biomarkers. For that, exhaled breath samples were analysed with a GC-MS apparatus [85]. Considering the reviewed works, it is possible to state that volatile organic compounds play a crucial role in contemporary procedures for CRC diagnosis.
6. Prostate Cancer
As with other urogenital system cancers, prostate cancer has a very challenging diagnosis, and, in most cases, identification of the pathology is only determined in the late stages when the cure probabilities are diminished. An accurate methodology for diagnosing prostate cancer commonly involves blood analysis and, in most cases, biopsy-based procedures that are deeply invasive and present some risks to the patient. In this way, this pathology, which leads to thousands of deaths among men every year (12,000 and 34,700 deaths, respectively, are expected in the United Kingdom and the United States of America during 2023, [86][87]), requires more rapid and more accurate diagnostic methodologies to tackle the current challenges [88][89]. A lot of work has been developed around urinary VOCs, however, breath biomarkers for prostate cancer remain a scientific area filled with opportunities [90][91].
Waltman et al. (2018) have recently addressed the suitability of an electronic nose device to identify patterns of VOCs in the exhaled breath of both prostate cancer patients (32 individuals) and healthy volunteers (53 individuals). The working principle of this commercially available e-nose consists of the creation of compound-specific patterns by hotplate metal-oxide sensors, whose conductivity varies once exposed to the target analytes. This variant, as mentioned, is directly related to the detected analyte, leading to a unique fingerprint of the studied matrix. Unfortunately, information about the detected VOCs is not provided but the authors were able to use breath signatures to distinguish both groups with accuracy, sensitivity, and specificity levels of 75%, 84%, and 70%, respectively [92]. In even more recent work, Maiti et al. (2021) analysed breath samples collected from healthy subjects (19 individuals) and prostate cancer patients (28 individuals) in the search for potential biomarkers. Six VOCs, methyl butyrate, ethyl butyrate, acetaldehyde, ethyl vinyl ketone, propyl propionate and acetic anhydride, were identified by the authors as being of extreme relevance for the diagnosis of prostate cancer through breath biomarkers [93].
7. Squamous Cell Cancer
Commonly known as head and neck cancer (HNC), squamous cell carcinoma (SCC) encompases several types of cancer. Tumours whose development occurs in any head structure, like craniofacial bones, mucosa of the oral cavity, soft tissues, or even skin, are usually defined as HNC. The treatment of HNCs is especially difficult due to their location. Any kind of treatment deeply interferes with very important structures involved with necessary-to-life activities, like eating or even breathing. In this way, a rapid and accurate diagnosis of the tumour type and location is crucial to avoid more severe and invasive treatments [94][95][96]. The physicians and researchers working with HNC patients are in constant search of new methodologies that enable a faster and more accurate diagnosis of the pathology. The identification of specific VOCs as disease biomarkers has been interpreted as one of the solutions with higher potential [97][98][99].
To assess the suitability of patterns of VOCs as potential biomarkers for HNC diagnosis, Leunis et al. (2013) analysed exhaled breath samples with an electronic nose device. This device employs a total of 12 metal-oxide sensors specifically developed for the detection of four types of metabolites (CH4, CO, NOx, and Pt). Samples of the exhaled breath from 59 individuals (23 healthy subjects and 36 HNC patients) were collected into 5 L Tedlar bags and posteriorly analysed with the e-nose. The authors claimed that the differentiation between both groups was achieved with a specificity and sensitivity of 80% and 90% [100]. In a three-dimensional study, Goor et al. (2016) evaluated the breath of patients with three distinct pathologies: colon (28 patients), bladder (40 patients), and head and neck (100 patients) cancers. Even without information on the detected VOCs, the authors were able to use the detected patterns to differentiate HNC patients from colon and bladder cancer patients with accuracy levels of 81% and 84%, respectively [101]. In a more recent study, the same research group analysed the exhaled breath of HNC patients after treatment procedures. A cohort of 40 volunteers (20 patients with tumour recurrence and 20 patients without evidence of a tumour) was analysed with an e-nose device. The e-nose specifically used in this study consists of three hotplate metal-oxide sensors that are continuously heated and cooled down and that, once exposed to the target analytes, vary their conductivity. This process leads to the formation of VOC-specific patterns that act as a fingerprint of the samples under analysis. In this situation, the pattern of VOCs was not identified; however, the authors were able to use the patients’ breath signatures to discriminate between the two groups with an accuracy level of 83% [102]. Hartwig et al. (2017), in their turn, used a GC-MS device to analyse the exhaled breath of 14 volunteers (4 healthy controls and 10 HNC patients). Among the 125 analytes detected in the exhaled breath, the authors found eight VOCs with high potential as HNC biomarkers. Three of these eight compounds disappeared after surgery to remove the tumour [103]. The addressed results prove the potentiality of breath signatures to rapidly, accurately, and noninvasively diagnose HNC.
Some scientific research has been carried out regarding urine-emitted VOCs as biomarkers for several types of cancer, as that completed by Opitz et al. (2018) [104]. Regarding the VOCs emitted in the exhaled breath, interesting studies have been conducted. A cohort of 47 subjects (34 healthy individuals and 13 neck cancer patients) was considered for a study developed by Zhou et al. (2017). The use of a proton transfer reaction–mass spectrometry (PTR-MS) enabled the authors to detect four characteristic analytes with high potential for HNC diagnostic. The complete identification of these four analytes was not achieved but the authors were able to reduce the list of possibilities to just 22 VOCs [105]. Hakim et al. (2011), in a previous study, addressed the HNC issue. From the analyses of the breath samples of 87 individuals with a nanoscale artificial nose (NA-NOSE), the authors built the patterns of VOCs for both healthy people and HNC patients; with a GC-MS device, it was possible to identify the detected VOCs. Among all the detected volatile organic compounds, 12 were identified as high-potential biomarkers for diagnosing and distinguishing HNC from other pathologies [106]. A GC-IMS apparatus was used by Taware et al. (2018) to identify four volatile organic compounds whose behaviour is directly related to the diagnosis of HNC. From the breath analyses of 59 individuals (27 healthy individuals and 32 HNC patients), 48 analytes were detected [107]. Hydrogen cyanide was the VOC identified by Chandran et al. (2019) as a clear biomarker for HNC diagnosis. The differentiation of HNC patients and healthy groups through this analyte was achieved with an accuracy level of 95% [108]. Gruber et al. (2014), in their turn, identified three volatile organic compounds, undecane, ethanol, and 2-propenonitrile, as HNC-differentiating markers [109].
All the reviewed works prove that the role of VOCs as HNC biomarkers is of extreme relevance; however, further work must be carried out. One can state that the final aim of this field is to achieve the certification of breath biomarkers for HNC diagnosis and, consequently, their implementation in real clinical scenarios. To do so, additional techniques with better separation and detection capacities must be tested. Techniques like ion mobility spectrometry (IMS), whose limits of detection can achieve levels as low as ppbv and even pptv, can be a suitable option to detect eventual metabolites not identified yet as relevant for this field [110]. In addition, newer collection and storage procedures based on new devices must be developed and tested in order to ensure the repeatability of the data and the relevancy of the results [111][112]. Beyond all the current challenges, the future of breath biomarkers for HNC diagnosis is auspicious and should not be overlooked.
References
- Miekisch, W.; Schubert, J.K.; Noeldge-Schomburg, F.E. Diagnostic potential of breath analysis--focus on volatile organic compounds. Clin. Chim. Acta 2004, 347, 25–39.
- Focus Area: Biomarkers. Available online: https://www.fda.gov/science-research/focus-areas-regulatory-science-report/focus-area-biomarkers (accessed on 3 October 2023).
- Jones, A.W.; Andersson, L. Determination of ethanol in breath for legal purposes using a five-filter infrared analyzer: Studies on response to volatile interfering substances. J. Breath Res. 2008, 2, 026006.
- Taylor, D.R. Advances in the clinical applications of exhaled nitric oxide measurements. J. Breath Res. 2012, 6, 047102.
- Key Statistics for Lung Cancer. Available online: https://www.cancer.org/cancer/types/lung-cancer/about/key-statistics.html (accessed on 3 October 2023).
- Travis, W.D. Pathology of Lung Cancer. Clin. Chest Med. 2011, 32, 669–692.
- Tanoue, L.T.; Tanner, N.T.; Gould, M.K.; Silvestri, G.A. Lung cancer screening. Am. J. Respir. Crit. Care Med. 2015, 191, 19–33.
- Wakelee, H.; Chang, E.T.; Gomez, S.L.; Keegan, T.H.M.; Feskanich, D.; Clarke, C.A.; Holmberg, L.; Yong, L.C.; Kolonel, L.N.; Gould, M.K.; et al. Lung cancer incidence in never-smoking. J. Clin. Oncol. 2007, 25, 472–478.
- Thriumani, R.; Zakaria, A.; Hashim, Y.Z.; Jeffree, A.I.; Helmy, K.M.; Kamarudin, L.M.; Omar, I.; Shakaff, A.Y.; Adom, A.H.; Persaud, K.C. A study on volatile organic compounds emitted by in-vitro lung cancer cultured cells using gas sensor array and SPME-GCMS. BMC Cancer 2018, 18, 362.
- Kischkel, S.; Miekisch, W.; Sawacki, A.; Straker, E.M.; Trefz, P.; Amann, A.; Schubert, J.K. Breath biomarkers for lung cancer detection and assessment of smoking related effects—Confounding variables, influence of normalization and statistical algorithms. Clin. Chim. Acta 2010, 411, 1637–1644.
- Sponring, A.; Filipiak, W.; Ager, C.; Schubert, J.; Miekisch, W.; Amann, A.; Troppmair, J. Analysis of volatile organic compounds (VOCs) in the headspace of NCI-H1666 lung cancer cells. Cancer Biomark. 2010, 7, 153–161.
- Chatterjee, S.; Castro, M.; Feller, J.F. An e-nose made of carbon nanotube based quantum resistive sensors for the detection of eighteen polar/nonpolar VOC biomarkers of lung cancer. J. Mater. Chem. B 2013, 1, 4563.
- Hou, C.; Lei, J.; Huo, D.; Song, K.; Li, J.; Luo, X.; Yang, M.; Fa, H. Discrimination of lung cancer related volatile organic compounds with a colorimetric sensor array. Anal. Lett. 2013, 46, 2048–2059.
- Xu, H.; Wei, Y.; Zhu, L.; Huang, J.; Li, Y.; Liu, F.; Wang, S.; Liu, S. Bifunctional magnetic nanoparticles for analysis of aldehyde metabolites in exhaled breath of lung cancer patients. J. Chromatogr. A 2014, 1324, 29–35.
- Gregis, G.; Sanchez, J.B.; Bezverkhyy, I.; Weber, G.; Berger, F.; Fierro, V.; Bellat, J.P.; Celzard, A. Detection and quantification of lung cancer biomarkers by a micro-analytical device using a single metal oxide-based gas sensor. Sens. Actuators B Chem. 2018, 255, 391–400.
- Saalberg, Y.; Bruhns, H.; Wolff, M. Photoacoustic Spectroscopy for the Determination of Lung Cancer Biomarkers—A Preliminary Investigation. Sensors 2017, 17, 210.
- Darwiche, K.; Baumbach, J.I.; Sommerwerck, U.; Teschler, H.; Freitag, L. Bronchoscopically Obtained Volatile Biomarkers in Lung Cancer. Lung 2011, 189, 445–452.
- Mazzone, P.J.; Wang, X.F.; Xu, Y.; Mekhail, T.; Beukemann, M.C.; Na, J.; Kemling, W.J.; Suslick, K.S.; Sasidhar, M. Exhaled Breath Analysis with a Colorimetric Sensor Array for the Identification and Characterization of Lung Cancer. J. Thorac. Oncol. 2012, 7, 137–142.
- Santonico, M.; Lucantoni, G.; Pennazza, G.; Capuano, R.; Galluccio, G.; Roscioni, C.; Delfa, G.L.; Consoli, D.; Martinelli, E.; Paolesse, R.; et al. In situ detection of lung cancer volatile fingerprints using bronchoscopic air-sampling. Lung Cancer 2012, 77, 46–50.
- Schmekel, B.; Winquist, F.; Vikström, A. Analysis of breath samples for lung cancer survival. Anal. Chim. Acta 2014, 840, 82–86.
- Li, M.; Yang, D.; Brock, G.; Knipp, R.J.; Bousamra, M.; Nantz, M.H.; Fu, X.A. Breath carbonyl compounds as biomarkers of lung cancer. Lung Cancer 2015, 90, 92–97.
- Phillips, M.; Bauer, T.L.; Cataneo, R.N.; Lebauer, C.; Mundada, M.; Pass, H.I.; Ramakrishna, N.; Rom, W.N.; Vallières, E. Blinded Validation of Breath Biomarkers of Lung Cancer, a Potential Ancillary to Chest CT Screening. PLoS ONE 2015, 10, e0142484.
- Callol-Sanchez, L.; Munoz-Lucas, M.A.; Gomez-Martin, O.; Maldonado-Sanz, J.A.; Civera-Tejuca, A.; Gutierrez-Ortega, C.; Rodriguez-Trigo, G.; Jareno-Esteban, J. Observation of nonanoic acid and aldehydes in exhaled breath of patients with lung cancer. J. Breath Res. 2017, 11, 026004.
- Sun, Y.; Chen, Y.; Sun, C.; Liu, H.; Wang, Y.; Jiang, X. Analysis of volatile organic compounds from patients and cell lines for the validation of lung cancer biomarkers by proton-transfer-reaction mass spectrometry. Anal. Methods 2019, 11, 3188–3197.
- Saidi, T.; Moufid, M.; Beleño-Saenz, K.J.; Welearegay, T.G.; Bari, N.E.; Jaimes-Mogollon, A.L.; Ionescu, R.; Bourkadi, J.E.; Benamor, J.; Ftouh, M.E.; et al. Non-invasive prediction of lung cancer histological types through exhaled breath analysis by UV-irradiated electronic nose and GC/QTOF/MS. Sens. Actuators B Chem. 2020, 311, 127932.
- Fuchs, P.; Loeseken, C.; Schubert, J.K.; Mieksich, W. Breath gas aldehydes as biomarkers of lung cancer. Int. J. Cancer 2010, 126, 2663–2670.
- Poli, D.; Goldoni, M.; Corradi, M.; Acampa, O.; Carbognani, P.; Internullo, E.; Casalini, A.; Mutti, A. Determination of aldehydes in exhaled breath of patients with lung cancer by means of on-fiber-derivatisation SPME-GC/MS. J. Chromatogr. B 2010, 878, 2643–2651.
- Rudnicka, J.; Kowalkowski, T.; Ligor, T.; Buszewski, B. Determination of volatile organic compounds as biomarkers of lung cancer by SPME-GC-TOF/MS and chemometrics. J. Chromatogr. B 2011, 879, 3360–3366.
- Buszewski, B.; Ligor, T.; Jezierski, T.; Wenda-Piesik, A.; Walczak, M.; Rudnicka, J. Identification of volatile lung cancer markers by gas chromatography-mass spectrometry: Comparison with discrimination by canines. Anal. Bioanal. Chem. 2012, 404, 141–146.
- Buszewski, B.; Ulanowska, A.; Kowalkowski, T.; Cieslinski, K. Investigation of lung cancer biomarkers by hyphenated separation techniques and chemometrics. Clin. Chem. Lab. Med. 2011, 50, 573–581.
- Fu, X.A.; Li, M.; Knipp, R.J.; Nantz, M.H.; Bousamra, M. Noninvasive detection of lung cancer using exhaled breath. Cancer Med. 2014, 3, 174–181.
- Ma, H.; Li, X.; Chen, J.; Wang, H.; Cheng, T.; Chen, K.; Xu, S. Analysis of human breath samples of lung cancer patients and healthy controls with solid-phase microextraction (SPME) and flow-modulated comprehensive two-dimensional gas chromatography (GC-GC). Anal. Methods 2014, 6, 6841–6849.
- Handa, H.; Usuba, A.; Maddula, S.; Baumbach, J.I.; Mineshita, M.; Miyazawa, T. Exhaled Breath Analysis for Lung Cancer Detection Using Ion Mobility Spectrometry. PLoS ONE 2014, 9, e114555.
- Ligor, T.; Pater, L.; Buszewski, B. Application of an artificial neural network model for selection of potential lung cancer biomarkers. J. Breath Res. 2015, 9, 027106.
- Jouyban, A.; Djozan, D.; Mohammadandashti, P.; Alizadeh-Nabil, A.; Ghorbanpour, H.; Khoubnasabjafari, M.; Mohammadzadeh, M. Co-liquefaction with acetone and GC analysis of volatile compounds in exhaled breath as lung cancer biomarkers. Bioimpacts 2014, 7, 99–108.
- Key Statistics for Breast Cancer. Available online: https://www.cancer.org/cancer/types/breast-cancer/about/how-common-is-breast-cancer.html (accessed on 3 October 2023).
- Breast Cancer Statistics. Available online: https://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/breast-cancer (accessed on 3 October 2023).
- Benson, J.R.; Jatoi, I.; Keisch, M.; Esteva, F.J.; Makris, A.; Jordan, V.C. Early breast cancer. Lancet 2009, 373, 1463–1479.
- Waks, A.G.; Winer, E.P. Breast Cancer Treatment: A Review. J. Am. Med. Assoc. 2019, 321, 288–300.
- Warner, E. Breast-Cancer Screening. N. Engl. J. Med. 2011, 365, 1025–1032.
- Grimm, L.J.; Avery, C.S.; Hendrick, E.; Baker, J.A. Benefits and Risks of Mammography Screening in Women Ages 40 to 49 Years. J. Prim. Care Community Health 2022, 13, 21501327211058322.
- Silva, C.L.; Passos, M.; Câmara, J.S. Solid phase microextraction, mass spectrometry and metabolomic approaches for detection of potential urinary cancer biomarkers—A powerful strategy for breast cancer diagnosis. Talanta 2012, 89, 360–368.
- Byrnes, R.; Phillips, M.; Cataneo, R.N.; Chaturvedi, A.; Kaplan, P.D.; Libardoni, M.; Mehta, V.; Mundada, M.; Patel, U.; Ramakrishna, N.; et al. Detection of volatile biomarkers of therapeutic radiation in breath. J. Breath Res. 2013, 7, 036002.
- Taunk, K.; Taware, R.; More, T.H.; Porto-Figueira, P.; Pereira, J.A.M.; Mohapatra, R.; Soneji, D.; Câmara, J.S.; Nagarajaram, H.A.; Rapole, S. A non-invasive approach to explore the discriminatory potential of the urinary volatilome of invasive ductal carcinoma of the breast. RSC Adv. 2018, 8, 25040.
- Woollam, M.; Teli, M.; Angarita-Rivera, P.; Liu, S.; Siegel, A.P.; Yokota, H.; Agarwal, M. Detection of Volatile Organic Compounds (VOCs) in Urine via Gas Chromatography—Mass Spectrometry QTOF to Differentiate Between Localized and Metastatic Models of Breast Cancer. Sci. Rep. 2019, 9, 2526.
- Barash, O.; Zhang, W.; Halpern, J.M.; Hua, Q.L.; Pan, Y.Y.; Kayal, H.; Khoury, K.; Liu, H.; Davies, M.P.A.; Haick, H. Differentiation between genetic mutations of breast cancer by breath volatolomics. Oncotarget 2015, 6, 44864–88476.
- Lavra, L.; Catini, A.; Ulivieri, A.; Capuano, R.; Salehi, L.B.; Sciacchitano, S.; Bartolazzi, A.; Nardis, S.; Paolesse, R.; Martinelli, E.; et al. Investigation of VOCs associated with different characteristics of breast cancer cells. Sci. Rep. 2015, 5, 13246.
- Arshad, A.Z.; Munajat, Y.; Ibrahim, R.K.R.; Hamdan, S.; Mahmood, N.H. Volatolomics analysis using FTIR spectroscopy for breast cancer identification in vitro. In Proceedings of the 2014 IEEE Conference on Biomedical Engineering and Sciences (IECBES), Kuala Lumpur, Malaysia, 8–10 December 2014.
- Silva, C.L.; Perestrelo, R.; Silva, P.; Tomás, H.; Câmara, J.S. Volatile metabolomic signature of human breast cancer cell lines. Sci. Rep. 2017, 7, 43969.
- Phillips, M.; Beatty, J.D.; Cataneo, R.N.; Huston, J.; Kaplan, P.D.; Lalisang, R.I.; Lambin, P.; Lobbes, M.B.I.; Mundada, M.; Pappas, N.; et al. Rapid Point-Of-Care Breath Test for Biomarkers of Breast Cancer and Abnormal Mammograms. PLoS ONE 2014, 9, e90226.
- Li, J.; Peng, Y.; Liu, Y.; Li, W.; Jin, Y.; Tang, Z.; Duan, Y. Investigation of potential breath biomarkers for the early diagnosis of breast cancer using gas chromatography-mass spectrometry. Clin. Chim. Acta 2014, 436, 59–67.
- Phillips, M.; Cataneo, R.; Lebauer, C.; Mundada, M.; Saunders, C. Breath mass ion biomarkers of breast cancer. J. Breath Res. 2017, 11, 016004.
- Phillips, M.; Cataneo, R.N.; Cruz-Ramos, J.A.; Huston, J.; Ornelas, O.; Pappas, N.; Pathak, S. Prediction of breast cancer risk with volatile biomarkers in breath. Breast Cancer Res. Treat. 2018, 170, 343–350.
- Yang, H.Y.; Wang, Y.C.; Peng, H.Y.; Huang, C.H. Breath biopsy of breast cancer using sensor array signals and machine learning analysis. Sci. Rep. 2021, 11, 103.
- León-Martínez, L.D.; Rodríguez-Aguilar, M.; Gorocica-Rosete, P.; Domínguez-Reyes, C.A.; Martínez-Bustos, V.; Tenorio-Torres, J.A.; Ornelas-Rebolledo, O.; Cruz-Ramos, J.A.; Balderas-Segura, B.; Flores-Ramírez, R. Identification of profiles of volatile organic compounds in exhaled breath by means of an electronic nose as a proposal for a screening method for breast cancer: A case-control study. J. Breath Res. 2020, 14, 046009.
- Rodríguez-Aguilar, M.; León-Martínez, L.D.; Gorocica-Rosete, P.; Pérez-Padilla, R.; Domínguez-Reyes, C.A.; Tenorio-Torres, J.A.; Ornelas-Rebolledo, O.; Mehta, G.; Zamora-Mendoza, B.N.; Flores-Ramírez, R. Application of chemoresistive gas sensors and chemometric analysis to differentiate the fingerprints of global volatile organic compounds from diseases. Preliminary results of COPD, lung cancer and breast cancer. Clin. Chim. Acta 2021, 518, 83–92.
- Phillips, M.; Cataneo, R.N.; Saunders, C.; Hope, P.; Schmitt, P.; Wai, J. Volatile biomarkers in the breath of women with breast cancer. J. Breath Res. 2010, 4, 026003.
- Mandy, M.; Cornelia, F.; Malgorzata, L.; Oliver, S.; Achim, S.; Dorothee, S. Volatile organic compounds (VOCs) in exhaled breath of patients with breast cancer in a clinical setting. Ginekol. Pol. 2012, 83, 730–736.
- Xu, Y.; Lee, H.; Hu, Y.; Huang, J.; Kim, S.; Yun, M. Detection and Identification of Breast Cancer Volatile Organic Compounds Biomarkers Using Highly-Sensitive Single Nanowire Array on a Chip. J. Biomed. Nanotechnol. 2013, 9, 1164–1172.
- Huang, Y.; Li, Y.; Luo, Z.; Duan, Y. Investigation of biomarkers for discriminating breast cancer cell lines from normal mammary cell lines based on VOCs analysis and metabolomics. RSC Adv. 2016, 6, 41816–41824.
- Zhang, Y.; Guo, L.; Qiu, Z.; Lv, Y.; Chen, G.; Li, E. Early diagnosis of breast cancer from exhaled breath by gas chromatography-mass spectrometry (GC/MS) analysis: A prospective cohort study. J. Clin. Lab. Anal. 2020, 34, e23526.
- Xiang, L.; Wu, S.; Hua, Q.; Bao, C.; Liu, H. Volatile Organic Compounds in Human Exhaled Breath to Diagnose Gastrointestinal Cancer: A Metal-Analysis. Front. Oncol. 2021, 11, 606915.
- Kumar, S.; Huang, J.; Cushnir, J.R.; Spanel, P.; Smith, D.; Hanna, G.B. Selected Ion Flow Tube-MS Analysis of Headspace Vapor from Gastric Content for the Diagnosis of Gastro-Esophageal Cancer. Anal. Chem. 2012, 84, 9550–9557.
- Catalano, V.; Labianca, R.; Beretta, G.D.; Gatta, G.; Braud, F.; Cutsem, E. Gastric Cancer. Crit. Rev. Oncol./Hematol. 2009, 71, 127–164.
- Key Statistics About Stomach Cancer. Available online: https://www.cancer.org/cancer/types/stomach-cancer/about/key-statistics.html (accessed on 3 October 2023).
- Bel’skaya, L.V.; Sarf, E.A.; Shalygin, S.P.; Postnova, T.V.; Kosenok, V.K. Identification of salivary volatile organic compounds as potential markers of stomach and colorectal cancer: A pilot study. J. Oral Biosci. 2020, 62, 212–221.
- Schuermans, V.N.E.; Li, Z.; Jongen, A.; Wu, Z.; Shi, J.; Ji, J.; Bouvy, N.D. Pilot Study: Detection of Gastric Cancer From Exhaled Air Analyzed with an Electronic Nose in Chinese Patients. Surg. Innov. 2018, 25, 429–434.
- Zhang, Y.; Gao, G.; Liu, H.; Fu, H.; Fan, J.; Wang, K.; Chen, Y.; Li, B.; Zhang, C.; Zhi, X.; et al. Identification of Volatile Biomarkers of Gastric Cancer Cells and Ultrasensitive Electrochemical Detection based on Sensing Interface of Au-Ag Alloy coated MWCNTs. Theranostics 2014, 4, 154–162.
- Xu, Z.; Broza, Y.Y.; Ionsecu, R.; Tisch, U.; Ding, L.; Liu, H.; Song, Q.; Pan, Y.; Xiong, F.; Gu, K.; et al. A nanomaterial-based breath test for distinguishing gastric cancer from benign gastric conditions. Br. J. Cancer 2013, 108, 941–950.
- Amal, H.; Leja, M.; Funka, K.; Skarpars, R.; Sivins, A.; Ancans, G.; Liepniece-Karele, I.; Kikuste, I.; Lasina, I.; Haick, H. Detection of precancerous gastric lesions and gastric cancer through exhaled breath. Gut 2016, 65, 400–407.
- Jung, Y.J.; Seo, H.S.; Kim, J.H.; Song, K.Y.; Park, C.H.; Lee, H.H. Advanced Diagnostic Technology of Volatile Organic Compounds Real Tiem Analysis From Exhaled Breath of Gastric Cancer Patients Using Proton-Transfer-Reaction Time-of-Flight Mass Spectrometry. Front. Oncol. 2021, 11, 560591.
- Hong, Y.; Che, X.; Su, H.; Mai, Z.; Huang, Z.; Huang, W.; Chen, W.; Liu, S.; Gao, W.; Zhou, Z.; et al. Exhaled breath analysis using on-line preconcentration mass spectrometry for gastric cancer diagnosis. J. Mass Spectrom. 2021, 56, e4588.
- Chen, Y.; Zhang, Y.; Pan, F.; Liu, J.; Wang, K.; Zhang, C.; Cheng, S.; Lu, L.; Zhang, W.; Zhang, Z.; et al. Breath Analysis Based on Surface-Enhanced Raman Scattering Sensors Distinguishes Early and Advanced Gastric Cancer Patients from Healthy Persons. ACS Nano 2016, 10, 8169–8179.
- Key Statistics for Colorectal Cancer. Available online: https://www.cancer.org/cancer/types/colon-rectal-cancer/about/key-statistics.html (accessed on 3 October 2023).
- Mármol, I.; Sánchez-de-Diego, C.; Dieste, A.P.; Cerrada, E.; Yoldi, M.J.R. Colorectal Carcinoma: A General Overview and Future Perspectives in Colorectal Cancer. Int. J. Mol. Sci. 2017, 18, 197.
- Markowitz, S.D.; Bertagnolli, M.M. Molecular Basis of Colorectal Cancer. N. Engl. J. Med. 2009, 361, 2449–2460.
- Oakley-Girvan, I.; Davis, S.W. Breath based volatile organic compounds in the detection of breast, lung, and colorectal cancers: A systematic review. Cancer Biomark. 2017, 21, 29–39.
- Lena, M.; Porcelli, F.; Altomare, D.F. Volatile organic compounds as new biomarkers for colorectal cancer: A review. Colorectal Dis. 2016, 18, 654–663.
- Altomare, D.F.; Lena, M.D.; Porcelli, F.; Trizio, L.; Travaglio, E.; Tutino, M.; Dragonieri, S.; Memeo, V.; Gennaro, G. Exhaled volatile organic compounds identify patients with colorectal cancer. Br. J. Surg. 2013, 100, 144–150.
- Altomare, D.F.; Lena, M.D.; Porcelli, F.; Travaglio, E.; Longobardi, F.; Tutino, M.; Depalma, N.; Tedesco, G.; Sardaro, A.; Memeo, R.; et al. Effects of Curative Colorectal Cancer Surgery on Exhaled Volatile Organic Compounds and Potential Implications in Clinical Follow-up. Ann. Surg. 2015, 262, 862–867.
- Altomare, D.F.; Picciariello, A.; Rotelli, M.T.; Fazio, M.D.; Aresta, A.; Zambonin, C.G.; Vincenti, L.; Trerotoli, P.; Vietro, N.D. Chemical signature of colorectal cancer: Case-control study for profiling the breath print. BJS Open 2020, 4, 1189–1199.
- Wang, C.; Ke, C.; Wang, X.; Chi, C.; Guo, L.; Luo, S.; Guo, Z.; Xu, G.; Zhang, F.; Li, E. Noninvasive detection of colorectal cancer by analysis of exhaled breath. Anal. Bioanal. Chem. 2014, 406, 4757–4763.
- Amal, H.; Leja, M.; Funka, K.; Lasina, I.; Skapars, R.; Sivins, A.; Ancans, G.; Kikuste, I.; Vanags, A.; Tolmanis, I.; et al. Breath testing as potential colorectal cancer screening tool. Int. J. Cancer 2016, 138, 229–236.
- Keulen, K.E.; Jansen, M.E.; Schrauwen, R.W.M.; Kolkman, J.M.; Siersema, P.D. Volatile organic compounds in breath can serve as a non-invasive diagnostic biomarker for the detection of advanced adenomas and colorectal cancer. Aliment. Pharmacol. Ther. 2020, 51, 334–346.
- Vietro, N.D.; Aresta, A.; Rotelli, M.T.; Zambonin, C.; Lippolis, C.; Picciariello, A.; Altomare, D.F. Relationship between cancer tissue derived and exhaled volatile organic compound from colorectal cancer patients. Preliminary results. J. Pharm. Biomed. Anal. 2020, 180, 113055.
- About Prostate Cancer. Available online: https://prostatecanceruk.org/prostate-information-and-support/risk-and-symptoms/about-prostate-cancer (accessed on 3 October 2023).
- Key Statistics for Prostate Cancer. Available online: https://www.cancer.org/cancer/types/prostate-cancer/about/key-statistics.html (accessed on 3 October 2023).
- Asimakopoulos, A.D.; Fabbro, D.D.; Miano, R.; Santonico, M.; Capuano, R.; Pennazza, G.; D’Amico, A.; Finazzi-Agrò, E. Prostate cancer diagnosis through electronic nose in the urine headspace setting: A pilot study. Prostate Cancer Prostatic Dis. 2014, 17, 206–211.
- Grönberg, H. Prostate cancer epidemiology. Lancet 2003, 361, 859–864.
- Deev, V.; Solovieva, S.; Andreev, E.; Protoshchak, V.; Karpushchenko, E.; Sleptsov, A.; Kartsova, L.; Bessonova, E.; Legin, A.; Kirsanov, D. Prostate cancer screening using chemometric processing of GC-MS profiles obtained in the headspace above urine samples. J. Chromatogr. B 2020, 1155, 122298.
- Jiménez-Pacheco, A.; Salinero-Bachiller, M.; Iribar, M.C.; López-Luque, A.; Miján-Ortiz, J.L.; Peinado, J.M. Furan and p-xylene as candidate biomarkers for prostate cancer. Urol. Oncol. 2018, 36, 243.e21–243.e27.
- Waltman, C.G.; Marcelissen, T.A.T.; Roermund, J.G.H. Exhaled-breath Testing for Prostate Cancer Based on Volatile Organic Compound Profiling Using an Electronic Nose Device (Aeonose): A Preliminary Report. Eur. Urol. Focus 2020, 6, 1220–1225.
- Maiti, K.S.; Fill, E.; Strittmatter, F.; Volz, Y.; Sroka, R.; Apolonski, A. Towards reliable diagnostics of prostate cancer via breath. Sci. Rep. 2021, 11, 18381.
- Chow, L.Q.M. Head and Neck Cancer. N. Engl. J. Med. 2020, 382, 60–72.
- Grénman, R.; Chevalier, D.; Gregoire, V.; Myers, E.; Rogers, S. Treatment of head and neck cancer in the elderly. Eur. Arch. Oto-Rhino-L. 2010, 267, 1619–1621.
- Bouza, M.; Gonzalez-Soto, J.; Pereiro, R.; Vicente, J.C.; Sanz-Medel, A. Exhaled breath and oral cavity VOCs as potential biomarkers in oral cancer patients. J. Breath Res. 2017, 11, 016015.
- Lang, H.P.; Loizeau, F.; Hiou-Feige, A.; Rivals, J.P.; Romero, P.; Akiyama, T.; Gerber, C.; Meyer, E. Piezoresistive Membrane Surface Stress Sensors for Characterization of Breath Samples of Head and Neck Cancer Patients. Sensors 2016, 16, 1149.
- Konings, E.; Stappers, S.; Geens, M.; Winter, B.Y.; Lamote, K.; Meerbeeck, J.P.; Specenier, P.; Vanderveken, O.M.; Ledeganck, K.J. A Literature Review of the Potential Diagnostic Biomarkers of Head and Neck Neoplasms. Front. Oncol. 2020, 10, 1020.
- Shigeyama, H.; Wang, T.; Ichinose, M.; Ansai, T.; Lee, S.W. Identification of volatile metabolites in human saliva from patients with oral squamous cell carcinoma via zeolite-based thin-film microextraction coupled with GC-MS. J. Chromatogr. B 2019, 1104, 49–58.
- Leunis, N.; Boumans, M.L.; Kremer, M.B.; Din, S.; Stobberingh, E.; Kessels, A.G.H.; Kross, K.W. Application of an Electronic Nose in the Diagnosis of Head and Neck Cancer. Laryngoscope 2014, 124, 1377–1381.
- Goor, R.; Leunis, N.; Hooren, M.; Francisca, E.; Masclee, A.; Kremer, B.; Kross, K.W. Feasibility of electronic nose technology for discriminating between head and neck, bladder, and colon carcinomas. Eur. Arch. Oto-Rhino-L. 2017, 274, 1053–1060.
- Goor, R.; Hardy, J.; Hooren, M.; Kremer, B.; Kross, K. Detecting recurrent head and neck cancer using electronic nose technology: A feasibility study. Head Neck-J. Sci. Spec. 2019, 41, 2983–2990.
- Hartwig, S.; Raguse, J.D.; Pfitzner, D.; Preissner, R.; Paris, S.; Preissner, S. Volatile Organic Compounds in the Breath of Oral Squamous Cell Carcinoma Patients: A Pilot Study. Otolaryngol. Head Neck Surg. 2017, 157, 981–987.
- Optiz, P.; Herbarth, O. The volatilome—Investigation of volatile organic metabolites (VOM) as potential tumor markers in patients with head and neck squamous cell carcinoma (HNSCC). Otolaryngol. Head Neck Surg. 2018, 47, 42.
- Zhou, W.; Huang, C.; Zou, X.; Lu, Y.; Shen, C.; Ding, X.; Wang, H.; Jiang, H.; Chu, Y. Exhaled breath online measurement for cervical cancer patients and healthy subjects by proton transfer reaction mass spectrometry. Anal. Bioanal. Chem. 2017, 409, 5603–5612.
- Hakim, M.; Billan, S.; Tisch, U.; Peng, G.; Dvrokind, I.; Marom, O.; Abdah-Bortnyak, R.; Kuten, A.; Haick, H. Diagnosis of head-and-neck cancer from exhaled breath. Br. J. Cancer 2011, 104, 1649–1655.
- Taware, R.; Taunk, K.; Pereira, J.A.M.; Shirolkar, M.; Soneji, D.; Câmara, J.S.; Nagarajaram, H.A.; Rapole, S. Volatilomic insight of head and neck cancer via the effects observed on saliva metabolites. Sci. Rep. 2018, 8, 17725.
- Chandran, D.; Ooi, E.H.; Watson, D.I.; Kholmurodova, F.; Jaenisch, S.; Yazbeck, R. The Use of Selected Ion Flow Tube-Mass Spectrometry Technology to Identify Breath Volatile Organic Compounds for the Detection of Head and Neck Squamous Cell Carcinoma: A Pilot Study. Medicina 2019, 55, 306.
- Gruber, M.; Tisch, U.; Jeries, R.; Amal, H.; Hakim, M.; Ronen, O.; Marshak, T.; Zimmerman, D.; Israel, O.; Amiga, E.; et al. Analysis of exhaled breath for diagnosing head and neck squamous cell carcinoma: A feasibility study. Br. J. Cancer 2014, 111, 790–798.
- Moura, P.C.; Vassilenko, V. Contemporary ion mobility spectrometry applications and future trends towards environmental, health and food research: A review. Int. J. Mass Spectrom. 2023, 486, 117012.
- Santos, P.; Moura, P.C.; Vassilenko, V. Suitability of Short- and Long-Term Storage of Volatile Organic Compounds Samples in Syringe-Based Containers: A Comparison Study. Metabolites 2023, 13, 903.
- Santos, P.H.C.; Vassilenko, V.B.; Moura, P.C.; Conduto, C.; Fernandes, J.M.; Bonifácio, P. Instrumentation for differentiation of exhaled breath. In Proceedings of the SPIE 12126, Fifteenth International Conference on Correlation Optics, Chernivtsi, Ukraine, 13–16 September 2021; p. 121262L.
More
Information
Subjects:
Medicine, Research & Experimental
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
599
Revisions:
2 times
(View History)
Update Date:
15 Nov 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




