
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Elena Danilova | -- | 3828 | 2023-11-10 14:21:38 | | | |
| 2 | Lindsay Dong | + 3 word(s) | 3831 | 2023-11-13 03:49:09 | | |
Video Upload Options
One of the primary challenges regarding chronic kidney disease (CKD) diagnosis is the absence of reliable methods to detect early-stage kidney damage. A metabolomic approach is expected to broaden the current diagnostic modalities by enabling timely detection and making the prognosis more accurate. Analysis performed on urine has several advantages, such as the ease of collection using noninvasive methods and its lower protein and lipid content compared with other bodily fluids.
1. Introduction
2. Analytical Methods in CKD Metabolomics Studies
2.1. NMR Spectroscopy
2.2. Mass Spectrometry-Based Methods
3. Biomarkers and Pathways
3.1. Pathogenesis of CKD
3.2. Markers of CKD
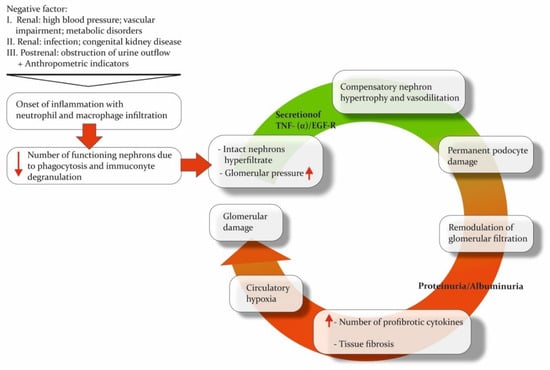
As previously mentioned, chronic kidney disease (CKD) is a polyetiological disease. The primary issue with CKD is that the inflammatory response and impaired renal function are accompanied by processes that partially sustain them. CKD is linked to various osmolytes and uremic solutes, which are associated with biochemical pathways in the body.
Uremic solutes are toxic compounds. They are associated with uremia and subsequent changes in organ function. Robert D. Mair and colleagues performed urine metabolomic analysis by using LC-MS to study the clearance of uremic solutes [55]. However, this approach required urine and plasma samples to calculate the fractional clearance formula. Specifically, in patients with advanced disease, the most significant changes in clearance compared with control samples were observed for p-cresol sulfate, indoxyl sulfate, hippurate, and phenylacetylglutamine.
This is consistent with the results of another study in which elevated indoxyl sulfate levels in urine were also correlated with elevated serotonin sulfate levels. The authors suggested that both markers could be considered significant signals for the early diagnosis of CKD [56]. However, it is uncertain whether a single molecule can definitively resolve the issue of delayed CKD diagnosis. Targeting analysis of compounds related to indoxyl sulfate metabolism would be valuable to confirm these findings.
Osmolytes are the compounds involved in cellular osmotic regulation, which alters CKD. Ryan B. Gil and colleagues confirmed the altered concentration of uremic toxins (e.g., hippuric acid, indoxyl sulfate, and p-cresol sulfate) and included osmolytes in the developed diagnostic panel [14]. Two of the most important protective compounds from the osmotic stress effect are betaine and myo-inositol. These molecules showed the best prognostic value, also agreeing with eGFR. The confirming transcriptomic analysis accompanied these results on a murine CKD model.
In addition to osmotic stress, kidney tissue is also affected by oxidative stress during the development of CKD. Markers of this process were observed in the Balkan Endemic Nephropathy (BEN) study. Additionally, the uremic toxin p-cresol was detected in the urine of a group of patients.
Amino acids (AAs) undergo filtration and reabsorption in the kidney. Altered reabsorption of AAs in the proximal tubule may explain the increased urinary levels and decreased serum levels of AAs in patients with chronic kidney disease (CKD). With progressive renal dysfunction, the urinary levels of AAs increase, indicating impairment of both glomerular and tubular structures, even in the early stages of CKD. In the study by Galavan et al., phenylalanine was also included in the metabolomic profile of CKD [56]. This amino acid has been observed by researchers investigating different subtypes of CKD. This can be explained by the data published this year on phenylalanine hydroxylase (PAH) activity. PAH is commonly found in the kidney, liver, and pancreas.
3.3. Markers for CKD Subtypes
3.4. Markers of Acute Kidney Injury
3.5. Markers of Renal Impairment in Children
4. Conclusions
References
- Cockwell, P.; Fisher, L.A. The global burden of chronic kidney disease. Lancet 2020, 395, 662–664.
- Webster, A.C.; Nagler, E.V.; Morton, R.L.; Masson, P. Masson, Chronic Kidney Disease. Lancet 2017, 389, 1238–1252.
- Taherkhani, A.; Yekta, R.F.; Mohseni, M.; Saidijam, M.; Oskouie, A.A. Chronic kidney disease: A review of proteomic and metabolomic approaches to membranous glomerulonephritis, focal segmental glomerulosclerosis, and IgA nephropathy biomarkers. Proteome. Sci. 2019, 17, 7.
- Hill, N.R.; Fatoba, S.T.; Oke, J.L.; Hirst, J.A.; O’Callaghan, C.A.; Lasserson, D.S.; Hobbs, F.D.R. Global Prevalence of Chronic Kidney Disease—A Systematic Review and Meta-Analysis. PLoS ONE 2016, 11, e0158765.
- Lowe, K.M.; Cruz, J.B.; Jones, K.M. Complications in Patients with Chronic Kidney Disease. Crit. Care Nurs. Clin. North. Am. 2022, 34, 395–407.
- Yan, M.T.; Chao, C.T.; Lin, S.H. Chronic Kidney Disease: Strategies to Retard Progression. Int. J. Mol. Sci. 2021, 22, 10084.
- Sanchez-Niño, M.D.; Sanz, A.B.; Ramos, A.M.; Fernandez-Fernandez, B.; Ortiz, A. Clinical proteomics in kidney disease as an exponential technology: Heading towards the disruptive phase. Clin. Kidney. J. 2017, 10, 188–191.
- Karczewski, K.J.; Snyder, M.P. Integrative omics for health and disease. Nat. Rev. Genet. 2018, 19, 299–310.
- Liu, J.-J.; Liu, S.; Gurung, R.L.; Ching, J.; Kovalik, J.-P.; Tan, T.Y.; Lim, S.C. Urine Tricarboxylic Acid Cycle Metabolites Predict Progressive Chronic Kidney Disease in Type 2 Diabetes. J. Clin. Endocrinol. Metab. 2018, 103, 4357–4364.
- Macioszek, S.; Wawrzyniak, R.; Kranz, A.; Kordalewska, M.; Struck-Lewicka, W.; Dudzik, D.; Biesemans, M.; Maternik, M.; Żurowska, A.M.; Markuszewski, M.J. Comprehensive Metabolic Signature of Renal Dysplasia in Children. A Multiplatform Metabolomics Concept. Front. Mol. Biosci. 2021, 8, 665661.
- Lee, A.M.; Hu, J.; Xu, Y.; Abraham, A.G.; Xiao, R.; Coresh, J.; Rebholz, C.; Chen, J.; Rhee, E.P.; Feldman, H.I.; et al. Using Machine Learning to Identify Metabolomic Signatures of Pediatric Chronic Kidney Disease Etiology. J. Am. Soc. Nephrol. 2022, 33, 375–386.
- Jacob, M.; Lopata, A.L.; Dasouki, M.; Rahman, A.M.A. Metabolomics toward personalized medicine. Mass Spectrom. Rev. 2019, 38, 221–238.
- Bell, J.D.; Lee, J.A.; Lee, H.A.; Sadler, P.J.; Wilkie, D.R.; Woodham, R.H. Nuclear magnetic resonance studies of blood plasma and urine from subjects with chronic renal failure: Identification of trimethylamine-N-oxide. Biochim. Biophys. Acta 1991, 1096, 101–107.
- Gil, R.B.; Ortiz, A.; Sanchez-Niño, M.D.; Markoska, K.; Schepers, E.; Vanholder, R.; Glorieux, G.; Schmitt-Kopplin, P.; Heinzmann, S.S. Increased urinary osmolyte excretion indicates chronic kidney disease severity and progression rate. Nephrol. Dial. Transplant. 2018, 33, 2156–2164.
- Dalili, N.; Chashmniam, S.; Khoormizi, S.M.H.; Salehi, L.; Jamalian, S.A.; Nafar, M.; Kalantari, S. Urine and serum NMR-based metabolomics in pre-procedural prediction of contrast-induced nephropathy. Intern. Emerg. Med. 2020, 15, 95–103.
- Chasapi, S.A.; Karagkouni, E.; Kalavrizioti, D.; Vamvakas, S.; Zompra, A.; Takis, P.G.; Goumenos, D.S.; Spyroulias, G.A. NMR-Based Metabolomics in Differential Diagnosis of Chronic Kidney Disease (CKD) Subtypes. Metabolites 2022, 12, 490.
- Mutter, S.; Valo, E.; Aittomäki, V.; Nybo, K.; Raivonen, L.; Thorn, L.M.; Forsblom, C.; Sandholm, N.; Würtz, P.; Groop, P.H. Urinary metabolite profiling and risk of progression of diabetic nephropathy in 2670 individuals with type 1 diabetes. Diabetologia 2022, 65, 140–149.
- Lucio-Gutiérrez, J.R.; Cordero-Pérez, P.; Farías-Navarro, I.C.; Tijerina-Marquez, R.; Sánchez-Martínez, C.; Ávila-Velázquez, J.L.; García-Hernández, P.A.; Náñez-Terreros, H.; Coello-Bonilla, J.; Pérez-Trujillo, M.; et al. Using nuclear magnetic resonance urine metabolomics to develop a prediction model of early stages of renal disease in subjects with type 2 diabetes. J. Pharm. Biomed. Anal. 2022, 219, 114885.
- Muhle-goll, C.; Eisenmann, P.; Luy, B.; Kölker, S.; Tönshoff, B.; Fichtner, A.; Westhoff, J.H. Urinary NMR Profiling in Pediatric Acute Kidney Injury—A Pilot Study. Int. J. Mol. Sci. 2020, 21, 1187.
- Cheng, L.; Wang, L.; Chen, B.; Wang, C.; Wang, M.; Li, J.; Gao, X.; Zhang, Z.; Han, L. A multiple-metabolites model to predict preliminary renal injury induced by iodixanol based on UHPLC/Q-Orbitrap-MS and 1H-NMR. Metabolomics 2022, 18, 85.
- Zhang, W.R.; Parikh, C.R. Biomarkers of Acute and Chronic Kidney Disease. Annu. Rev. Physiol. 2019, 81, 309–333.
- Eckardt, K.U.; Coresh, J.; Devuyst, O.; Johnson, R.J.; Köttgen, A.; Levey, A.S.; Levin, A. Evolving importance of kidney disease: From subspecialty to global health burden. Lancet 2013, 382, 158–169.
- Ordodi, V.L.; Hădărugă, N.G.; Hădărugă, D.I.; Lukinich-Gruia, A.T.; Mărgineanu, M.; Tatu, C.A.; Păunescu, V. Urine Metabolomic Signature of People Diagnosed with Balkan Endemic Nephropathy and Other Types of Chronic Kidney Disease Compared with Healthy Subjects in Romania. Metabolites 2023, 13, 609.
- Ligor, T.; Zawadzka, J.; Strączyński, G.; Paredes, R.M.G.; Wenda-Piesik, A.; Ratiu, I.A.; Muszytowski, M. Searching for Potential Markers of Glomerulopathy in Urine by HS-SPME-GC × GC TOFMS. Molecules 2021, 26, 1817.
- Shao, M.; Lu, H.; Yang, M.; Liu, Y.; Yin, P.; Li, G.; Wang, Y.; Chen, L.; Chen, Q.; Zhao, C.; et al. Serum, and urine metabolomics reveal potential biomarkers of T2DM patients with nephropathy. Ann. Transl. Med. 2020, 8, 199.
- Taherkhani, A.; Nafar, M.; Arefi-Oskouei, A.; Broumandnia, N.; Parvin, M.; Mahmoudieh, L.; Kalantari, S. Metabolomic Analysis of Membranous Glomerulonephritis: Identification of a Diagnostic Panel and Pathogenic Pathways. Arch. Med. Res. 2019, 50, 159–169.
- Hirakawa, Y.; Yoshioka, K.; Kojima, K.; Yamashita, Y.; Shibahara, T.; Wada, T.; Nangaku, M.; Inagi, R. Potential progression biomarkers of diabetic kidney disease determined using comprehensive machine learning analysis of non-targeted metabolomics. Sci. Rep. 2022, 12, 16287.
- Chen, C.; Zhang, P.; Bao, G.; Fang, Y.; Chen, W. Discovery of potential biomarkers in acute kidney injury by ultra-high-performance liquid chromatography-tandem quadrupole time-of-flight mass spectrometry (UPLC-Q/TOF–MS). Int. Urol. Nephrol. 2021, 53, 2635–2643.
- Piedrafita, A.; Balayssac, S.; Mayeur, N.; Gazut, S.; Grossac, J.; Buleon, M.; Alves, M.; Klein, J.; Minville, V.; Marcheix, B.; et al. The tryptophan pathway and nicotinamide supplementation in ischaemic acute kidney injury. Clin. Kidney J. 2021, 14, 2490–2496.
- Saito, R.; Hirayama, A.; Akiba, A.; Kamei, Y.; Kato, Y.; Ikeda, S.; Kwan, B.; Pu, M.; Natarajan, L.; Shinjo, H.; et al. Urinary Metabolome Analyses of Patients with Acute Kidney Injury Using Capillary Electrophoresis-Mass Spectrometry. Metabolites 2021, 11, 671.
- Wang, S.; Xiao, C.; Liu, C.; Li, J.; Fang, F.; Lu, X.; Zhang, C.; Xu, F. Identification of Biomarkers of Sepsis-Associated Acute Kidney Injury in Pediatric Patients Based on UPLC-QTOF/MS. Inflammation 2020, 43, 629–640.
- Kwan, B.; Fuhrer, T.; Zhang, J.; Darshi, M.; Van Espen, B.; Montemayor, D.; de Boer, I.H.; Dobre, M.; Hsu, C.Y.; Kelly, T.N.; et al. Metabolomic Markers of Kidney Function Decline in Patients with Diabetes: Evidence from the Chronic Renal Insufficiency Cohort (CRIC) Study. Am. J. Kidney Dis. 2020, 76, 511–520.
- Kim, Y.; Lee, J.; Kang, M.S.; Song, J.; Kim, S.G.; Cho, S.; Huh, H.; Lee, S.; Park, S.; Jo, H.A.; et al. Urinary metabolite profile predicting the progression of chronic kidney disease. Kidney 2023, 4, 1048–1057.
- Yoshioka, K.; Hirakawa, Y.; Kurano, M.; Ube, Y.; Ono, Y.; Kojima, K.; Iwama, T.; Kano, K.; Hasegawa, S.; Inoue, T.; et al. Lysophosphatidylcholine mediates fast decline in kidney function in diabetic kidney disease. Kidney Int. 2022, 101, 510–526.
- Feng, Q.; Li, Y.; Yang, Y.; Feng, J. Urine Metabolomics Analysis in Patients with Normoalbuminuric Diabetic Kidney Disease. Front. Physiol. 2020, 11, 578799.
- Bai, Y.; Zhang, H.; Wu, Z.; Huang, S.; Luo, Z.; Wu, K.; Hu, L.; Chen, C. Use of ultra high-performance liquid chromatography with high resolution mass spectrometry to analyze urinary metabolome alterations following acute kidney injury in post-cardiac surgery patients. J. Mass Spectrom. Adv. Clin. Lab. 2022, 24, 31–40.
- Lv, W.; Booz, G.W.; Wang, Y.; Fan, F.; Roman, R.J. Inflammation and renal fibrosis: Recent developments on key signaling molecules as potential therapeutic targets. Eur. J. Pharmacol. 2018, 820, 65–76.
- Chagnac, A.; Zingerman, B.; Rozen-Zvi, B.; Herman-Edelstein, M. Consequences of Glomerular Hyperfiltration: The Role of Physical Forces in the Pathogenesis of Chronic Kidney Disease in Diabetes and Obesity. Nephron 2019, 143, 38–42.
- Lindhardt, M.; Persson, F.; Currie, G.; Pontillo, C.; Beige, J.; Delles, C.; Von Der Leyen, H.; Mischak, H.; Navis, G.; Noutsou, M.; et al. Proteomic prediction and Renin angiotensin aldosterone system Inhibition prevention of early diabetic nephropathy in TYpe 2 diabetic patients with normoalbuminuria (PRIORITY): Essential study design and rationale of a randomised clinical multicentre trial. BMJ Open 2016, 6, e010310.
- Kim, K.; Aronov, P.; Zakharkin, S.O.; Anderson, D.; Perroud, B.; Thompson, I.M.; Weiss, R.H. Urine metabolomics analysis for kidney cancer detection and biomarker discovery. Mol. Cell Proteom. 2009, 8, 558–570.
- Romagnani, P.; Remuzzi, G.; Glassock, R.; Levin, A.; Jager, K.J.; Tonelli, M.; Massy, Z.; Wanner, C.; Anders, H.J. Chronic kidney disease. Nat. Rev. Dis. Primers 2017, 3, 17088.
- Humphreys, B.D. Mechanisms of Renal Fibrosis. Annu. Rev. Physiol. 2018, 80, 309–326.
- Wang, Y.N.; Ma, S.X.; Chen, Y.Y.; Chen, L.; Liu, B.L.; Liu, Q.Q.; Zhao, Y.Y. Chronic kidney disease: Biomarker diagnosis to therapeutic targets. Clin. Chim. Acta 2019, 499, 54–63.
- Schnaper, H.W. The Tubulointerstitial Pathophysiology of Progressive Kidney Disease. Adv. Chronic. Kidney Dis. 2017, 24, 107–116.
- Duffield, J.S. Cellular and molecular mechanisms in kidney fibrosis. J. Clin. Investig. 2014, 124, 2299–2306.
- Fu, Q.; Colgan, S.P.; Shelley, C.S. Hypoxia: The Force that Drives Chronic Kidney Disease. Clin. Med. Res. 2016, 14, 15–39.
- Tanaka, S.; Tanaka, T.; Nangaku, M. Hypoxia and Dysregulated Angiogenesis in Kidney Disease. Kidney Dis. 2015, 1, 80–89.
- Carbonare, L.D.; Valenti, M.T.; Giannini, S.; Gallieni, M.; Stefani, F.; Ciresa, R.; Politi, C.; Fusaro, M. Bone Biopsy for Histomorphometry in Chronic Kidney Disease (CKD): State-of-the-Art and New Perspectives. J. Clin. Med. 2021, 10, 4617.
- Misra, B.B.; Upadhayay, R.P.; Cox, L.A.; Olivier, M. Optimized GC–MS metabolomics for the analysis of kidney tissue metabolites. Metabolomics 2018, 14, 75.
- Hanifa, M.A.; Skott, M.; Maltesen, R.G.; Rasmussen, B.S.; Nielsen, S.; Frøkiær, J.; Ring, T.; Wimmer, R. Tissue, urine and blood metabolite signatures of chronic kidney disease in the 5/6 nephrectomy rat model. Metabolomics 2019, 15, 112.
- Mussap, M.; Noto, A.; Fanos, V.; Van Den Anker, J.N. Emerging Biomarkers and Metabolomics for Assessing Toxic Nephropathy and Acute Kidney Injury (AKI) in Neonatology. Biomed. Res. Int. 2014, 2014, 602526.
- Gupta, J.; Mitra, N.; Kanetsky, P.A.; Devaney, J.; Wing, M.R.; Reilly, M.; Shah, V.O.; Balakrishnan, V.S.; Guzman, N.J.; Girndt, M.; et al. Association between albuminuria; kidney function, and inflammatory biomarker profile in CKD in CRIC. Clin. J. Am. Soc. Nephrol. 2012, 7, 1938–1946.
- Nkuipou-Kenfack, E.; Duranton, F.; Gayrard, N.; Argilés, À.; Lundin, U.; Weinberger, K.M.; Dakna, M.; Delles, C.; Mullen, W.; Husi, H.; et al. Assessment of metabolomic and proteomic biomarkers in detection and prognosis of progression of renal function in chronic kidney disease. PLoS ONE 2014, 9, e96955.
- Uwaezuoke, S.N.; Ayuk, A.C.; Muoneke, V.U.; Mbanefo, N.R. Chronic kidney disease in children: Using novel biomarkers as predictors of disease. Saudi. J. Kidney Dis. Transpl. 2018, 29, 775–784.
- Mair, R.D.; Lee, S.; Plummer, N.S.; Sirich, T.L.; Meyer, T.W. Impaired tubular secretion of organic solutes in advanced chronic kidney disease. J. Am. Soc. Nephrology 2021, 32, 2877.
- Glavan, M.R.; Socaciu, C.; Socaciu, A.I.; Gadalean, F.; Cretu, O.M.; Vlad, A.; Muntean, D.M.; Bob, F.; Milas, O.; Suteanu, A.; et al. Untargeted Metabolomics by Ultra-High-Performance Liquid Chromatography Coupled with Electrospray Ionization-Quadrupole-Time of Flight-Mass Spectrometry Analysis Identifies a Specific Metabolomic Profile in Patients with Early Chronic Kidney Disease. Biomedicines 2023, 11, 1057.
- Zhang, J.; Fuhrer, T.; Ye, H.; Kwan, B.; Montemayor, D.; Tumova, J.; Darshi, M.; Afshinnia, F.; Scialla, J.J.; Anderson, A.; et al. High-Throughput Metabolomics and Diabetic Kidney Disease Progression: Evidence from the Chronic Renal Insufficiency (CRIC) Study. Am. J. Nephrol. 2022, 53, 215–225.
- Vanweert, F.; Schrauwen, P.; Phielix, E. Role of branched-chain amino acid metabolism in the pathogenesis of obesity and type 2 diabetes-related metabolic disturbances BCAA metabolism in type 2 diabetes. Nutr. Diabetes 2022, 12, 35.
- Masania, J.; Faustmann, G.; Anwar, A.; Hafner-Giessauf, H.; Rajpoot, N.; Grabher, J.; Rajpoot, K.; Tiran, B.; Obermayer-Pietsch, B.; Winklhofer-Roob, B.M.; et al. Urinary Metabolomic Markers of Protein Glycation, Oxidation, and Nitration in Early-Stage Decline in Metabolic, Vascular, and Renal Health. Oxid. Med. Cell Longev. 2019, 2019, 4851323.
- Mills, E.L.; Kelly, B.; Logan, A.; Costa, A.S.H.; Varma, M.; Bryant, C.E.; Tourlomousis, P.; Däbritz, J.H.M.; Gottlieb, E.; Latorre, I.; et al. Succinate Dehydrogenase Supports Metabolic Repurposing of Mitochondria to Drive Inflammatory Macrophages. Cell 2016, 167, 457–470.e13.
- Chouchani, E.T.; Pell, V.R.; Gaude, E.; Aksentijević, D.; Sundier, S.Y.; Robb, E.L.; Logan, A.; Nadtochiy, S.M.; Ord, E.N.J.; Smith, A.C.; et al. Ischaemic accumulation of succinate controls reperfusion injury through mitochondrial ROS. Nature 2014, 515, 431–435.
- Ye, Z.; Wang, S.; Zhang, C.; Zhao, Y. Coordinated Modulation of Energy Metabolism and Inflammation by Branched-Chain Amino Acids and Fatty Acids. Front. Endocrinol. 2020, 11, 559087.
- Reiss, A.B.; De Leon, J.; Pan, X. The Roles of Fatty Acids and Apolipoproteins in the Kidneys. Metabolites 2022, 12, 462.
- Sun, Y.; Zhou, C.; Chen, Y.; He, X.; Gao, F.; Xue, D. Quantitative increase in short-chain fatty acids, especially butyrate protects kidney from ischemia/reperfusion injury. J. Investig. Med. 2022, 70, 29–35.
- Cui, F.Q.; Wang, Y.F.; Gao, Y.B.; Meng, Y.; Cai, Z.; Shen, C.; Liu, Z.Q.; Jiang, X.C.; Zhao, W.J.; Zhao, R. Effects of BSF on Podocyte Apoptosis via Regulating the ROS-Mediated PI3K/AKT Pathway in DN. J. Diabetes Res. 2019, 2019, 9512406.
- Franiek, A.; Sharma, A.; Cockovski, V.; Wishart, D.S.; Zappitelli, M.; Blydt-Hansen, T.D. Urinary metabolomics to develop predictors for pediatric acute kidney injury. Pediatr. Nephrol. 2022, 37, 2079–2090.
- Scalabre, A.; Clément, Y.; Guillière, F.; Ayciriex, S.; Gaillard, S.; Demède, D.; Bouty, A.; Lanteri, P.; Mure, P.Y. Early detection of ureteropelvic junction obstruction in neonates with prenatal diagnosis of renal pelvis dilatation using 1H NMR urinary metabolomics. Sci. Rep. 2022, 12, 13406.




