Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Theodoros Chatzimitakos | -- | 2564 | 2023-11-07 23:38:18 | | | |
| 2 | Wendy Huang | Meta information modification | 2564 | 2023-11-08 03:05:25 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Athanasiadis, V.; Chatzimitakos, T.; Kotsou, K.; Kalompatsios, D.; Bozinou, E.; Lalas, S.I. Applications of Pulsed Electric Field in Vegetables. Encyclopedia. Available online: https://encyclopedia.pub/entry/51265 (accessed on 07 February 2026).
Athanasiadis V, Chatzimitakos T, Kotsou K, Kalompatsios D, Bozinou E, Lalas SI. Applications of Pulsed Electric Field in Vegetables. Encyclopedia. Available at: https://encyclopedia.pub/entry/51265. Accessed February 07, 2026.
Athanasiadis, Vassilis, Theodoros Chatzimitakos, Konstantina Kotsou, Dimitrios Kalompatsios, Eleni Bozinou, Stavros I. Lalas. "Applications of Pulsed Electric Field in Vegetables" Encyclopedia, https://encyclopedia.pub/entry/51265 (accessed February 07, 2026).
Athanasiadis, V., Chatzimitakos, T., Kotsou, K., Kalompatsios, D., Bozinou, E., & Lalas, S.I. (2023, November 07). Applications of Pulsed Electric Field in Vegetables. In Encyclopedia. https://encyclopedia.pub/entry/51265
Athanasiadis, Vassilis, et al. "Applications of Pulsed Electric Field in Vegetables." Encyclopedia. Web. 07 November, 2023.
Copy Citation
Polyphenols are a wide array of bioactive compounds that naturally occur in food sources derived from plants. They are recognized for their potential as preventive agents against chronic illnesses, such as cardiovascular diseases and diabetes. Pulsed electric field (PEF) is a processing method that implies a higher polyphenol extraction. The PEF treatment is a non-thermal technique employed for food preservation, which involves the application of short bursts of electrical power to inactivate microorganisms while minimizing any detrimental impact on the food’s quality. This implies that the PEF treatment aspires to enhance the accessibility of consumer-grade, polyphenol-abundant food products of superior quality.
polyphenols
flavonoids
pulsed electric field (PEF)
vegetables
potato
asparagus
mushroom
olives
1. Introduction
Polyphenols are naturally present in plant-based foods and show an extensive variety of complicated chemical structures [1][2][3]. They are composed of a phenolic ring which serves as the fundamental monomer [4]. The primary classes of polyphenols include phenolic acids, flavonoids, stilbenes, and lignans [5]. Flavonoids consist of flavones, flavonols, flavanones, isoflavones, flavanols, and anthocyanins, whereas hydroxybenzoic and hydroxycinnamic acids are types of phenolic acids [6]. These compounds are present in the human diet and are mainly derived from plant sources, including fruits, vegetables, grains, and coffee [7]. Polyphenols are a wide array of bioactive compounds that naturally occur in food sources derived from plants [8]. They are recognized for their potential as preventive agents against chronic illnesses, such as cardiovascular diseases and diabetes [9]. The four major types of polyphenols are phenolic acids, flavonoids, stilbenes, and lignans [10][11]. A visual representation of these compounds is illustrated in Figure 1. Flavonoids are highly prevalent in the context of dietary intake [12][13]. Catechin is found in a variety of fruits and beverages, most notably in tea [14]. Citrus fruits are known for their high content of hesperidin [15], whereas red fruits and berries are primarily characterized by their cyanidin content [16]. Fruits, such as apples, are known to possess proanthocyanidins and quercetin [17]. Proanthocyanidins are also present in grapes and cocoa [18], while quercetin can be found in onions and tea [19]. Finally, it should be noted that the soybean plant is primarily characterized by the presence of daidzein [20]. Polyphenolic compounds, such as lignans, are predominantly found in grains and flaxseed [21]. Due to the significant impact of polyphenols on human health, a multitude of research studies have been conducted to examine the physiological effects displayed by these compounds [22][23][24][25]. The consumption of foods rich in polyphenols has been demonstrated to contribute to a reduction in the incidence of several health conditions, such as colon cancer, liver disorders [26], cardiovascular diseases [27], and obesity [28].
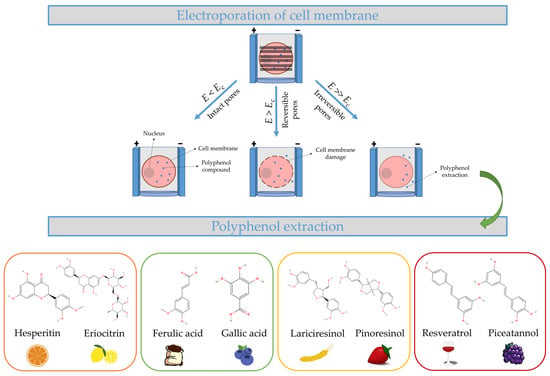
Figure 1. Electroporated cell membrane with bioactive compounds within several electric field strengths. Impact of critical electric field strength in the cell membrane. The four different types of polyphenols found in plant-based foods are transferred from the cell to the extraction solvent.
The investigation of diverse conventional methodologies has been conducted to extract bioactive compounds from specific fresh food products or food waste materials [29][30][31]. Common extraction techniques include soaking, maceration, infusion, percolation, and Soxhlet extraction [32]. The efficacy of these techniques is influenced by various factors, including the choice of solvent, the solvent’s solvation capacity, the degree of agitation, and the temperature [29]. Traditional extraction methods are associated with several challenges, including extended processing durations, reduced extraction efficiencies, excessive solvent usage, possible degradation of thermolabile bioactive compounds, and the utilization of hazardous chemicals [32][33]. A selective extraction is not adequately served by conventional extraction techniques [34][35]. Furthermore, the extracted products, such as proteins and polysaccharides, may not be of high quality if these methods are used [36]. Pulsed electric field (PEF) is a processing method that implies a higher polyphenol extraction [37]. The PEF treatment is a non-thermal technique employed for food preservation, which involves the application of short bursts of electrical power to inactivate microorganisms while minimizing any detrimental impact on the food’s quality [38]. This implies that the PEF treatment aspires to enhance the accessibility of consumer-grade, polyphenol-abundant food products of superior quality [39].
The use of PEF to diffuse, osmose, press, and dry food waste and by-products has gained popularity [40]. It reduces the negative effects of regular heating methods [41][42]. Since PEF can electroporate cell membranes, it is also used as a pretreatment to boost recoveries of bioactive compounds, such as polyphenols, carotenoids, and proteins [43][44]. When applied to water, the PEF technique exhibited lower temperatures, lower solvent consumption, and improved constituent extraction rates [45]. The extraction yield could be increased with reduced energy costs, and heat-sensitive substances could be preserved; all of these by also incorporating a “green” extraction method [46][47][48]. The PEF method exhibits greater environmental sustainability and economic efficiency due to its reduced overall energy consumption and lower energy requirements per unit of processed product [49]. For this reason, various sectors of the food industry have experimented with PEF-based maceration over the past decade [50]. Apart from fresh fruits and vegetables, biomass waste generated in the agricultural and food industries is increasingly seen as a valuable bioresource that can be converted into useful products. Large quantities of wastes, including processing residues, are generated as a result of agro-industrial activity [51].
2. Potato
The fourth most important food grown and consumed in the world, potatoes are a staple that can be found in almost every region of the country [52]. They are rich in carbohydrates and have a small amount of fat. They are also known to contain various nutrients such as vitamins, proteins, and fiber. Bioactive compounds such as anthocyanins and polyphenols are commonly found in the skin and flesh of potatoes [53].
To improve the extraction of polyphenols with significant antioxidant properties from potato peels, Frontuto et al. [54] studied the optimal PEF-assisted extraction conditions. To verify this, they applied electric field strength from 1–5 kV/cm and total specific energy inputs from 1–10 kJ/kg. Total polyphenol yield from PEF pretreated sample extracts was 1295 mg GAE/kg fw (+10% from the control) when using 5 kV/cm fields strength and 10 kJ/kg specific energy output, 52% ethanol as a solvent, 230 min of extraction time, and 50 °C for the subsequent solid-liquid extraction. The HPLC-DAD analysis revealed that the predominant polyphenolic compounds detected were chlorogenic, caffeic, syringic, protocatechuic, and p-coumaric acids. There was no indication of substantial degradation of these individual polyphenols as a result of the application of PEF.
3. Asparagus
Beneficial bioactive compounds have been found in Asparagus officinalis root. The primary goal of the research conducted by Symes et al. [55] was to improve the efficiency with which polyphenol and flavonoid extraction was accomplished from the roots of green asparagus by combining PEF and ionic liquids. Antioxidant activity (oxygen radical absorbance capacity, ORAC), FRAP and DPPH•), TPC, and TFC were all measured in this research. When compared to the standard solvent extraction method, the extraction yield was higher when PEF was used under the optimum conditions of electric field strength at 1.6 kV/cm, pulse width of 20 μs, and frequency of 200 Hz. All assays, except for ORAC, showed improvements in PEF-treated samples compared to untreated samples. Extraction yield was increased by 23%, TPC by 5%, TFC by 6%, and FRAP by 4%. Ionic liquids, on the other hand, were discovered to be more efficient than PEF treatment. For instance, ionic liquids were found to have a TFC of 122 mg RE/g. This value was ~80 times greater than the TFC achieved by PEF treatment. While ionic liquids performed better than PEF in asparagus root samples, more research is needed to determine their safety for use in the food industry.
4. Mushroom
Mushrooms are frequently consumed as a staple in vegetarian diets. They are recognized for their wide variety of health advantages, including their anti-carcinogenic and anti-infectious attributes. Furthermore, the abundant presence of polyphenols in these substances renders them highly versatile for utilization as pharmaceutical agents or dietary supplements [56][57]. The only type of mushroom that was applied to the PEF system with the purpose of greater extraction of polyphenols was Agaricus bisporus.
The utilization of chemical or thermal methods for the extraction of valuable compounds is widely employed across various disciplines. The utilization of PEF during the extraction process reduces the likelihood of nutrient damage in the extracted product. To that end, the study by Xue et al. [58] aimed to investigate the impact of continuous PEF treatment on the extraction process of a white button mushroom suspension with a concentration of 9% w/w. PEF with intensities ranging from 12.4–38.4 kV/cm were applied using bipolar square pulses lasting 2 μs. The mushroom suspension was exposed to electric pulses with a field intensity of 38.4 kV/cm for a duration of 272 μs at 85 °C. Based on estimations, it was determined that the optimal extraction yields would be 98% (7.9 mg/g mushroom) of polysaccharide, 51% (1.6 mg GAE/g mushroom) of total polyphenols, and 49% (2.7 mg/g mushroom) of proteins. However, traditional mushroom extraction methods yielded only 56% (6.4 mg/g of mushroom) polysaccharide, 25% of total polyphenols (1.3 mg GAE/g of mushroom), and 45% (2.6 mg/g of mushroom) proteins after processing the 9% w/w mushroom suspension at 95 °C for 1 h. For all of these substances, the yield from conventional extraction carried out at the same temperature and for a similar amount of time was negligible. This indicates that a synergistic effect of electric pulses and temperature on the extraction performance is responsible for the improvement in extraction performance observed with PEF and that this improvement cannot be attributed solely to ohmic heat generated during PEF treatment.
5. Olives
Olives of several cultivars are key in Mediterranean dishes and an essential agricultural crop for European countries such as Greece, Spain, and Italy. Olives are the fruit of the olive tree with the scientific name Olea europaea, which means “European olive”. They are cultivated throughout the Mediterranean basin as well as in South America, South Africa, India, China, Australia, New Zealand, Mexico, and the United States. Antioxidant compounds, such as polyphenols and flavonoids, are characteristic in olives, especially oleuropein. The content of extra virgin olive oil in polyphenols is 500 mg/L [59]. It has been claimed that the health benefits of olives and olive oil can protect the human organism from a variety of illnesses [60]. For this reason, the effect of PEF pretreatment on the increase of their polyphenol content was evaluated for all parts of the olive plant as well as for extra virgin olive oil. For instance, in the previously mentioned study by Ziagova et al. [61], PEF-treated leaves and unripe fruit yielded TPC of 105 and 12 mg GAE/g dw, respectively.
Andreou et al. [62] improved the recovery of high value-added compounds from olive pomace as a result of the combined use of PEF. Pretreatments with PEF (1.0–6.5 kV/cm, 0.9–51.1 kJ/kg, and 15 μs pulse width) were applied to olive pomace (cv Tsounati). Solid-liquid extraction of intracellular compounds with 50% v/v aqueous ethanol solution for 1 h at 25 °C was then used for analysis. At electric field strengths over 3 kV/cm, the polyphenol concentration increased significantly, reaching as high as 91.6% from the untreated sample (~1500 mg GAE/L). This method may be useful in conjunction with traditional solvent extraction. With PEF pretreatment, olive pomace can be valorized by increasing the yields of intracellular compounds with high antioxidant properties.
The same research team [63] investigated the application of non-thermal processing techniques to maximize the production and quality of virgin olive oil. Different PEF conditions (electric field strength of 0.5–2.0 kV/cm, 0–2500 pulses, energy input of 0.5–57.5 kJ/kg) were applied to olive paste. Quality, bioactive compounds, oxidative stability, and sensory evaluation of olive oils generated under optimum conditions (electric field strength of 1.5 kV/cm, 100 pulses) were assessed using response surface methodology. The olive oil yield from the PEF-pretreated sample was ~3% higher (25.4%), had ~57% more α-tocopherol (66.9 mg/kg oil), and had ~7% more polyphenols (822.3 mg GAE/kg oil) than the yielded oil from the untreated control sample. Consequently, PEF seeks to increase the production of a sustainable and cost-effective product in the olive oil industry.
Extracting polyphenols from olive leaves using the PEF method was evaluated by Pappas et al. [64]. Water, ethanol, and various mixtures thereof were used across a gradient of 25% in this research. The optimal conditions for PEF extraction took 30 min and required an electric field strength of 1 kV/cm. In addition, they explored a range of pulse durations, from 10–100 ns. Results obtained from PEF-treated extracts were compared to those obtained from untreated extracts. With a pulse duration of 10 μs and a 25% v/v ethanol aqueous solution, PEF was found to have the greatest effect. Significant increases of 31.85% in total polyphenols and 265.67% in specific metabolites were observed when comparing pre- and post-harvest samples. Differential scanning calorimetry revealed that 569 °C was the highest temperature at which oxidation occurred. The higher the oxidation temperature, the more resistant to oxidation the sample. This remarkable temperature was achieved by subjecting the samples to pulses with a duration of 100 μs and a period of 1000 μs. When the above PEF parameters were applied, the main metabolite luteolin-7-O-glucoside showed a significant increase of 71.87%, amounting to a total of 0.82 mg/g dw. Under a 100 μs pulse, however, oleuropein alone showed the highest extraction yield.
The same research team conducted an investigation [65] into the efficacy of PEF in concentrating polyphenol extracts, thereby evaluating the potential worth of olive leaves. The researchers thoroughly investigated the optimal methods for enhancing the PEF process in order to improve the extraction of olive leaf compounds. A comprehensive investigation was conducted to examine various parameters of the extraction chamber, including its geometric configuration, electric field intensity, pulse duration, pulse period (and frequency), and extraction duration. The researchers employed experimental methods to ensure the optimal duration of PEF-assisted solid-liquid extraction of olive leaves. The implementation of PEF resulted in a significant increase of 38% in the extractability of total polyphenols compared to the untreated control sample. The TPC reached a value of 25.35 mg GAE/g dw. Furthermore, it is noteworthy to mention the remarkable 117% increase observed in the concentration levels of certain specific metabolites. The optimum conditions required a 15-min extraction with 25% v/v ethanol conducted in a rectangular extraction chamber, an electric field strength of 0.85 kV/cm, a pulse period of 100 μs, and a pulse duration of 2 μs. Regarding oxidative stability, the samples subjected to a pulse duration of 10 μs, pulse period of 1000 μs, electric field of 0.85 kV/cm, and extraction time of 30 min exhibited the most pronounced oxidation peak at 488 °C. This value was found to be 16% higher than the control sample when assessed using differential scanning calorimetry. The findings of this research indicate that the use of PEF treatment has a notable impact on enhancing extraction efficiency and improving the physicochemical properties. Table 1 provides a concise summary of the above research conducted on fresh vegetables and their by-products.
Table 1. Application of PEF on fresh vegetables and by-products with the treatment effects.
| Sample | PEF Conditions | Treatment Effect | Ref. |
|---|---|---|---|
| Potato peel | 5 kV/cm, 10 kJ/kg | Increased TPC by ~10% (from ~1160 to 1295 mg GAE/kg fw) | [54] |
| Asparagus root | 1.6 kV/cm, 200 Hz, 20 μs pulse width | Increased values of extraction yield from 47.7 to 58.8% (23%), TPC from 32.6 to 34.4 mg GAE/g extract (5%), TFC from 0.16 to 0.17 mg RE/g extract (6%), and FRAP from 1363 to 1418 mM FeSO4 E/g extract (4%) | [55] |
| Mushrooms | 38.4 kV/cm, 272 μs duration | Estimated ~26% or 1.6 mg GAE/g higher polyphenol extraction yield | [58] |
| Olive | 0.5–2 kV/cm | High TPC value (12 mg GAE/g dw) | [61] |
| Olive pomace | 3 kV/cm, 15 μs pulse width | Notable increase in TPC (91.6%) from ~1500 to ~2900 mg/L | [62] |
| Olive paste | 1.5 kV/cm, 100 pulses | Increased recovery yield to 25.4% (by ~3%), TPC (by ~7%) from ~760 mg GAE/Kg oil | [63] |
| Olive leaf | 1 kV/cm, 10 ns pulse duration | Increased TPC (by 31.85%) from 15.74 to 20.75 mg GAE/g dw | [64] |
| 0.85 kV/cm, 100 μs pulse period, 2 μs pulse duration | TPC increase by 38.5% (from 18.30 to 25.35 mg GAE/g dw) | [65] | |
| 0.5–2 kV/cm | High TPC value (105 mg GAE/g dw) | [61] |
References
- Quideau, S.; Deffieux, D.; Douat-Casassus, C.; Pouységu, L. Plant Polyphenols: Chemical Properties, Biological Activities, and Synthesis. Angew. Chem. Int. Ed. 2011, 50, 586–621.
- Lund, M.N. Reactions of Plant Polyphenols in Foods: Impact of Molecular Structure. Trends Food Sci. Technol. 2021, 112, 241–251.
- Gomez-Pinilla, F.; Nguyen, T.T.J. Natural Mood Foods: The Actions of Polyphenols against Psychiatric and Cognitive Disorders. Nutr. Neurosci. 2012, 15, 127–133.
- El Gharras, H. Polyphenols: Food Sources, Properties and Applications—A Review. Int. J. Food Sci. Technol. 2009, 44, 2512–2518.
- Bešlo, D.; Golubić, N.; Rastija, V.; Agić, D.; Karnaš, M.; Šubarić, D.; Lučić, B. Antioxidant Activity, Metabolism, and Bioavailability of Polyphenols in the Diet of Animals. Antioxidants 2023, 12, 1141.
- Mutha, R.E.; Tatiya, A.U.; Surana, S.J. Flavonoids as Natural Phenolic Compounds and Their Role in Therapeutics: An Overview. Future J. Pharm. Sci. 2021, 7, 25.
- Román, G.C.; Jackson, R.E.; Gadhia, R.; Román, A.N.; Reis, J. Mediterranean Diet: The Role of Long-Chain ω-3 Fatty Acids in Fish; Polyphenols in Fruits, Vegetables, Cereals, Coffee, Tea, Cacao and Wine; Probiotics and Vitamins in Prevention of Stroke, Age-Related Cognitive Decline, and Alzheimer Disease. Rev. Neurol. 2019, 175, 724–741.
- Pandey, K.B.; Rizvi, S.I. Plant Polyphenols as Dietary Antioxidants in Human Health and Disease. Oxid. Med. Cell. Longev. 2009, 2, 270–278.
- Abbas, M.; Saeed, F.; Anjum, F.M.; Afzaal, M.; Tufail, T.; Bashir, M.S.; Ishtiaq, A.; Hussain, S.; Suleria, H.A.R. Natural Polyphenols: An Overview. Int. J. Food Prop. 2017, 20, 1689–1699.
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food Sources and Bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747.
- Hazafa, A.; Iqbal, M.O.; Javaid, U.; Tareen, M.B.K.; Amna, D.; Ramzan, A.; Piracha, S.; Naeem, M. Inhibitory Effect of Polyphenols (Phenolic Acids, Lignans, and Stilbenes) on Cancer by Regulating Signal Transduction Pathways: A Review. Clin. Transl. Oncol. 2022, 24, 432–445.
- Maleki, S.J.; Crespo, J.F.; Cabanillas, B. Anti-Inflammatory Effects of Flavonoids. Food Chem. 2019, 299, 125124.
- Rufino, A.T.; Costa, V.M.; Carvalho, F.; Fernandes, E. Flavonoids as Antiobesity Agents: A Review. Med. Res. Rev. 2021, 41, 556–585.
- Rothwell, J.A.; Madrid-Gambin, F.; Garcia-Aloy, M.; Andres-Lacueva, C.; Logue, C.; Gallagher, A.M.; Mack, C.; Kulling, S.E.; Gao, Q.; Praticò, G. Biomarkers of Intake for Coffee, Tea, and Sweetened Beverages. Genes Nutr. 2018, 13, 15.
- Miles, E.A.; Calder, P.C. Effects of Citrus Fruit Juices and Their Bioactive Components on Inflammation and Immunity: A Narrative Review. Front. Immunol. 2021, 12, 712608.
- Bortolini, D.G.; Maciel, G.M.; Fernandes, I.d.A.A.; Rossetto, R.; Brugnari, T.; Ribeiro, V.R.; Haminiuk, C.W.I. Biological Potential and Technological Applications of Red Fruits: An Overview. Food Chem. Adv. 2022, 1, 100014.
- Thilakarathna, S.H.; Rupasinghe, H.P.V. Flavonoid Bioavailability and Attempts for Bioavailability Enhancement. Nutrients 2013, 5, 3367–3387.
- Kruger, M.J.; Davies, N.; Myburgh, K.H.; Lecour, S. Proanthocyanidins, Anthocyanins and Cardiovascular Diseases. Food Res. Int. 2014, 59, 41–52.
- Dabeek, W.M.; Marra, M.V. Dietary Quercetin and Kaempferol: Bioavailability and Potential Cardiovascular-Related Bioactivity in Humans. Nutrients 2019, 11, 2288.
- Mayo, B.; Vázquez, L.; Flórez, A.B. Equol: A Bacterial Metabolite from The Daidzein Isoflavone and Its Presumed Beneficial Health Effects. Nutrients 2019, 11, 2231.
- De Silva, S.F.; Alcorn, J. Flaxseed Lignans as Important Dietary Polyphenols for Cancer Prevention and Treatment: Chemistry, Pharmacokinetics, and Molecular Targets. Pharmaceuticals 2019, 12, 68.
- Khurana, S.; Venkataraman, K.; Hollingsworth, A.; Piche, M.; Tai, T.C. Polyphenols: Benefits to the Cardiovascular System in Health and in Aging. Nutrients 2013, 5, 3779–3827.
- Cory, H.; Passarelli, S.; Szeto, J.; Tamez, M.; Mattei, J. The Role of Polyphenols in Human Health and Food Systems: A Mini-Review. Front. Nutr. 2018, 5, 87.
- Viuda-Martos, M.; Fernández-López, J.; Pérez-Álvarez, J.A. Pomegranate and Its Many Functional Components as Related to Human Health: A Review. Compr. Rev. Food Sci. Food Saf. 2010, 9, 635–654.
- Pap, N.; Fidelis, M.; Azevedo, L.; do Carmo, M.A.V.; Wang, D.; Mocan, A.; Pereira, E.P.R.; Xavier-Santos, D.; Sant’Ana, A.S.; Yang, B. Berry Polyphenols and Human Health: Evidence of Antioxidant, Anti-Inflammatory, Microbiota Modulation, and Cell-Protecting Effects. Curr. Opin. Food Sci. 2021, 42, 167–186.
- Bose, M.; Lambert, J.D.; Ju, J.; Reuhl, K.R.; Shapses, S.A.; Yang, C.S. The Major Green Tea Polyphenol, (-)-Epigallocatechin-3-Gallate, Inhibits Obesity, Metabolic Syndrome, and Fatty Liver Disease in High-Fat-Fed Mice. J. Nutr. 2008, 138, 1677–1683.
- McSweeney, M.; Seetharaman, K. State of Polyphenols in the Drying Process of Fruits and Vegetables. Crit. Rev. Food Sci. Nutr. 2015, 55, 660–669.
- Lu, C.; Zhu, W.; Shen, C.L.; Gao, W. Green Tea Polyphenols Reduce Body Weight in Rats by Modulating Obesity-Related Genes. PLoS ONE 2012, 7, e38332.
- Rifna, E.J.; Misra, N.N.; Dwivedi, M. Recent Advances in Extraction Technologies for Recovery of Bioactive Compounds Derived from Fruit and Vegetable Waste Peels: A Review. Crit. Rev. Food Sci. Nutr. 2023, 63, 719–752.
- Nayak, A.; Bhushan, B. An Overview of the Recent Trends on the Waste Valorization Techniques for Food Wastes. J. Environ. Manag. 2019, 233, 352–370.
- Kumar, K.; Yadav, A.N.; Kumar, V.; Vyas, P.; Dhaliwal, H.S. Food Waste: A Potential Bioresource for Extraction of Nutraceuticals and Bioactive Compounds. Bioresour. Bioprocess. 2017, 4, 18.
- Yan, L.-G.; He, L.; Xi, J. High Intensity Pulsed Electric Field as an Innovative Technique for Extraction of Bioactive Compounds—A Review. Crit. Rev. Food Sci. Nutr. 2017, 57, 2877–2888.
- Chemat, F.; Vian, M.A.; Fabiano-Tixier, A.-S.; Nutrizio, M.; Jambrak, A.R.; Munekata, P.E.S.; Lorenzo, J.M.; Barba, F.J.; Binello, A.; Cravotto, G. A Review of Sustainable and Intensified Techniques for Extraction of Food and Natural Products. Green Chem. 2020, 22, 2325–2353.
- Barba, F.J.; Zhu, Z.; Koubaa, M.; Sant’Ana, A.S.; Orlien, V. Green Alternative Methods for the Extraction of Antioxidant Bioactive Compounds from Winery Wastes and By-Products: A Review. Trends Food Sci. Technol. 2016, 49, 96–109.
- Pojić, M.; Mišan, A.; Tiwari, B. Eco-Innovative Technologies for Extraction of Proteins for Human Consumption from Renewable Protein Sources of Plant Origin. Trends Food Sci. Technol. 2018, 75, 93–104.
- Li, K.-Y.; Ye, J.-T.; Yang, J.; Shao, J.-Q.; Jin, W.-P.; Zheng, C.; Wan, C.-Y.; Peng, D.-F.; Deng, Q.-C. Co-Extraction of Flaxseed Protein and Polysaccharide with a High Emulsifying and Foaming Property: Enrichment through the Sequence Extraction Approach. Foods 2023, 12, 1256.
- Liu, Z.; Esveld, E.; Vincken, J.-P.; Bruins, M.E. Pulsed Electric Field as an Alternative Pre-Treatment for Drying to Enhance Polyphenol Extraction from Fresh Tea Leaves. Food Bioprocess Technol. 2019, 12, 183–192.
- Mohamed, M.E.A.; Amer Eiss, A.H. Pulsed Electric Fields for Food Processing Technology. In Structure and Function of Food Engineering; Amer Eissa, A., Ed.; InTech: London, UK, 2012; ISBN 978-953-51-0695-1.
- Quass, D.W. Pulsed Electric Field Processing in the Food Industry; A Status Report on Pulsed Electric Field; Electric Power Research Institute: Palo Alto, CA, USA, 1997; pp. 23–35.
- Bansal, V.; Sharma, A.; Ghanshyam, C.; Singla, M.L.; Kim, K.-H. Influence of Pulsed Electric Field and Heat Treatment on Emblica Officinalis Juice Inoculated with Zygosaccharomyces bailii. Food Bioprod. Process. 2015, 95, 146–154.
- Gabrić, D.; Barba, F.; Roohinejad, S.; Gharibzahedi, S.M.T.; Radojčin, M.; Putnik, P.; Bursać Kovačević, D. Pulsed Electric Fields as an Alternative to Thermal Processing for Preservation of Nutritive and Physicochemical Properties of Beverages: A Review. J. Food Process Eng. 2018, 41, e12638.
- Mohamad, A.; Shah, N.N.A.K.; Sulaiman, A.; Mohd Adzahan, N.; Aadil, R.M. Impact of the Pulsed Electric Field on Physicochemical Properties, Fatty Acid Profiling, and Metal Migration of Goat Milk. J. Food Process. Preserv. 2020, 44, e14940.
- Tzima, K.; Brunton, N.P.; Lyng, J.G.; Frontuto, D.; Rai, D.K. The Effect of Pulsed Electric Field as a Pre-Treatment Step in Ultrasound Assisted Extraction of Phenolic Compounds from Fresh Rosemary and Thyme by-Products. Innov. Food Sci. Emerg. Technol. 2021, 69, 102644.
- Martí-Quijal, F.J.; Ramon-Mascarell, F.; Pallarés, N.; Ferrer, E.; Berrada, H.; Phimolsiripol, Y.; Barba, F.J. Extraction of Antioxidant Compounds and Pigments from Spirulina (Arthrospira Platensis) Assisted by Pulsed Electric Fields and the Binary Mixture of Organic Solvents and Water. Appl. Sci. 2021, 11, 7629.
- Soquetta, M.B.; Terra, L.d.M.; Bastos, C.P. Green Technologies for the Extraction of Bioactive Compounds in Fruits and Vegetables. CyTA J. Food 2018, 16, 400–412.
- Bozinou, E.; Karageorgou, I.; Batra, G.; Dourtoglou, V.G.; Lalas, S.I. Pulsed Electric Field Extraction and Antioxidant Activity Determination of Moringa Oleifera Dry Leaves: A Comparative Study with Other Extraction Techniques. Beverages 2019, 5, 8.
- Chemat, F.; Rombaut, N.; Meullemiestre, A.; Turk, M.; Perino, S.; Fabiano-Tixier, A.-S.; Abert-Vian, M. Review of Green Food Processing Techniques. Preservation, Transformation, and Extraction. Innov. Food Sci. Emerg. Technol. 2017, 41, 357–377.
- Jha, A.K.; Sit, N. Extraction of Bioactive Compounds from Plant Materials Using Combination of Various Novel Methods: A Review. Trends Food Sci. Technol. 2022, 119, 579–591.
- Arshad, R.N.; Abdul-Malek, Z.; Roobab, U.; Qureshi, M.I.; Khan, N.; Ahmad, M.H.; Liu, Z.W.; Aadil, R.M. Effective Valorization of Food Wastes and By-Products through Pulsed Electric Field: A Systematic Review. J. Food Process. Eng. 2021, 44, e13629.
- Baiano, A. Recovery of Biomolecules from Food Wastes—A Review. Molecules 2014, 19, 14821–14842.
- Athanasiadis, V.; Grigorakis, S.; Lalas, S.; Makris, D.P. Methyl β-Cyclodextrin as a Booster for the Extraction for Olea Europaea Leaf Polyphenols with a Bio-Based Deep Eutectic Solvent. Biomass Convers. Biorefin. 2018, 8, 345–355.
- Aleti, G.; Nikolić, B.; Brader, G.; Pandey, R.V.; Antonielli, L.; Pfeiffer, S.; Oswald, A.; Sessitsch, A. Secondary Metabolite Genes Encoded by Potato Rhizosphere Microbiomes in the Andean Highlands Are Diverse and Vary with Sampling Site and Vegetation Stage. Sci. Rep. 2017, 7, 2330.
- Rasheed, H.; Ahmad, D.; Bao, J. Genetic Diversity and Health Properties of Polyphenols in Potato. Antioxidants 2022, 11, 603.
- Frontuto, D.; Carullo, D.; Harrison, S.M.; Brunton, N.P.; Ferrari, G.; Lyng, J.G.; Pataro, G. Optimization of Pulsed Electric Fields-Assisted Extraction of Polyphenols from Potato Peels Using Response Surface Methodology. Food Bioprocess Technol. 2019, 12, 1708–1720.
- Symes, A.; Shavandi, A.; Bekhit, A.E.A. Effects of Ionic Liquids and Pulsed Electric Fields on the Extraction of Antioxidants from Green Asparagus Roots. Int. J. Food Sci. Technol. 2023, 58, 3935–3945.
- Lee, I.H.; Huang, R.L.; Chen, C.T.; Chen, H.C.; Hsu, W.C.; Lu, M.K. Antrodia camphorata Polysaccharides Exhibit Anti-Hepatitis B Virus Effects. FEMS Microbiol. Lett. 2002, 209, 63–67.
- Chaturvedi, V.K.; Agarwal, S.; Gupta, K.K.; Ramteke, P.W.; Singh, M.P. Medicinal Mushroom: Boon for Therapeutic Applications. 3 Biotech 2018, 8, 334.
- Xue, D.; Farid, M.M. Pulsed Electric Field Extraction of Valuable Compounds from White Button Mushroom (Agaricus Bisporus). Innov. Food Sci. Emerg. Technol. 2015, 29, 178–186.
- Gorzynik-Debicka, M.; Przychodzen, P.; Cappello, F.; Kuban-Jankowska, A.; Gammazza, A.M.; Knap, N.; Wozniak, M.; Gorska-Ponikowska, M. Potential Health Benefits of Olive Oil and Plant Polyphenols. Int. J. Mol. Sci. 2018, 19, 686.
- Tsantili, E.; Evangelou, E.; Kiritsakis, A. Botanical Characteristics of Olive Trees: Cultivation and Growth Conditions—Defense Mechanisms to Various Stressors and Effects on Olive Growth and Functional Compounds. In Olives and Olive Oil as Functional Foods; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2017; pp. 13–33. ISBN 978-1-119-13534-0.
- Ziagova, M.; Mavromatidou, C.; Samiotis, G.; Amanatidou, E. Total Phenolic Content and Antioxidant Capacity of Greek Medicinal and Aromatic Plant Extracts Using Pulsed Electric Field Followed by Ultrasounds Extraction Process. J. Food Process. Preserv. 2022, 46, e16639.
- Andreou, V.; Psarianos, M.; Dimopoulos, G.; Tsimogiannis, D.; Taoukis, P. Effect of Pulsed Electric Fields and High Pressure on Improved Recovery of High-Added-Value Compounds from Olive Pomace. J. Food Sci. 2020, 85, 1500–1512.
- Andreou, V.; Kourmbeti, E.; Dimopoulos, G.; Psarianos, M.; Katsaros, G.; Taoukis, P. Optimization of Virgin Olive Oil Yield and Quality Applying Nonthermal Processing. Food Bioprocess Technol. 2022, 15, 891–903.
- Pappas, V.M.; Lakka, A.; Palaiogiannis, D.; Bozinou, E.; Ntourtoglou, G.; Batra, G.; Athanasiadis, V.; Makris, D.P.; Dourtoglou, V.G.; Lalas, S.I. Use of Pulsed Electric Field as a Low-Temperature and High-Performance “Green” Extraction Technique for the Recovery of High Added Value Compounds from Olive Leaves. Beverages 2021, 7, 45.
- Pappas, V.M.; Lakka, A.; Palaiogiannis, D.; Athanasiadis, V.; Bozinou, E.; Ntourtoglou, G.; Makris, D.P.; Dourtoglou, V.G.; Lalas, S.I. Optimization of Pulsed Electric Field as Standalone “Green” Extraction Procedure for the Recovery of High Value-Added Compounds from Fresh Olive Leaves. Antioxidants 2021, 10, 1554.
More
Information
Subjects:
Others
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.1K
Revisions:
2 times
(View History)
Update Date:
08 Nov 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




