
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Pedro Caetano Caetano-Pinto | -- | 2761 | 2023-11-02 11:55:52 | | | |
| 2 | Jason Zhu | Meta information modification | 2761 | 2023-11-03 02:15:51 | | |
Video Upload Options
Organic anion transporters 1 and 3 (OAT1 and OAT3) play a crucial role in kidney function by regulating the secretion of multiple renally cleared small molecules and toxic metabolic by-products. Assessing the activity of these transporters is essential for drug development purposes as they can significantly impact drug disposition and safety. OAT1 and OAT3 are amongst the most abundant drug transporters expressed in human renal proximal tubules. However, their expression is lost when cells are isolated and cultured in vitro, which is a persistent issue across all human and animal renal proximal tubule cell models, including primary cells and cell lines. Although it is well known that the overall expression of drug transporters is affected in vitro, the underlying reasons for the loss of OAT1 and OAT3 are still not fully understood.
1. Introduction
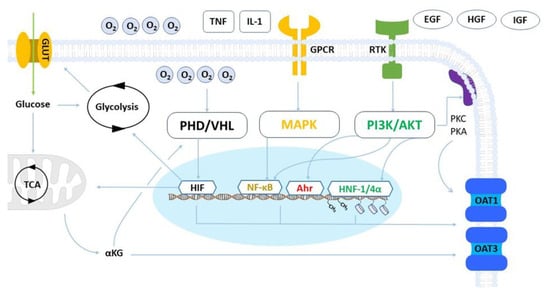
2. Metabolism and Hypoxia
3. Inflammatory and Growth Factors
4. MicroRNAs
5. Epigenetic Modifications
6. Cellular Adhesion
7. Post-Translational Regulation and Trafficking
References
- Zou, L.; Stecula, A.; Gupta, A.; Prasad, B.; Chien, H.C.; Yee, S.W.; Wang, L.; Unadkat, J.D.; Stahl, S.H.; Fenner, K.S.; et al. Molecular mechanisms for species differences in organic anion transporter 1, OAT1: Implications for renal drug toxicity. Mol. Pharmacol. 2018, 94, 689–699.
- Drozdzik, M.; Drozdzik, M.; Oswald, S. Membrane carriers and transporters in kidney physiology and disease. Biomedicines 2021, 9, 426.
- Pinto, P.C.; Rönnau, C.; Burchardt, M.; Wolff, I. Kidney Cancer and Chronic Kidney Disease: Too Close for Comfort. Biomedicines 2021, 9, 1761.
- Haase, V.H. Mechanisms of hypoxia responses in renal tissue. J. Am. Soc. Nephrol. 2013, 24, 537–541.
- Schödel, J.; Grampp, S.; Maher, E.R.; Moch, H.; Ratcliffe, P.J.; Russo, P.; Mole, D.R. Hypoxia, hypoxia-inducible transcription factors, and renal cancer. Eur. Urol. 2016, 69, 646–657.
- Jamshidi, N.; Nigam, S.K. Drug transporters OAT1 and OAT3 have specific effects on multiple organs and gut microbiome as revealed by contextualized metabolic network reconstructions. Sci. Rep. 2022, 12, 18308.
- Kierans, S.J.; Taylor, C.T. Regulation of glycolysis by the hypoxia-inducible factor (HIF): Implications for cellular physiology. J. Physiol. 2021, 599, 23–37.
- Li, S.; Zhang, Q.; You, G. Three ubiquitination sites of organic anion transporter-1 synergistically mediate protein kinase c-dependent endocytosis of the transporter. Mol. Pharmacol. 2013, 84, 139–146.
- Phatchawan, A.; Chutima, S.; Varanuj, C.; Anusorn, L. Decreased renal organic anion transporter 3 expression in type 1 diabetic rats. Am. J. Med. Sci. 2014, 347, 221–227.
- Zhang, J.; Yu, Z.; You, G. Insulin-like growth factor 1 modulates the phosphorylation, expression, and activity of organic anion transporter 3 through protein kinase A signaling pathway. Acta Pharm. Sin. B 2020, 10, 186–194.
- Clemmons, D.R. Role of insulin-like growth factor I in maintaining normal glucose homeostasis. Horm. Res. 2004, 62, 77–82.
- Schneider, R.; Sauvant, C.; Betz, B.; Otremba, M.; Fischer, D.; Holzinger, H.; Wanner, C.; Galle, J.; Gekle, M. Downregulation of organic anion transporters OAT1 and OAT3 correlates with impaired secretion of para-aminohippurate after ischemic acute renal failure in rats. Am. J. Physiol.-Ren. Physiol. 2007, 292, F1599–F1605.
- Dodd, K.M.; Yang, J.; Shen, M.H.; Sampson, J.R.; Tee, A.R. mTORC1 drives HIF-1α and VEGF-A signalling via multiple mechanisms involving 4E-BP1, S6K1 and STAT3. Oncogene 2015, 34, 2239–2250.
- Hagos, Y.; Schley, G.; Scḧdel, J.; Krick, W.; Burckhardt, G.; Willam, C.; Burckhardt, B.C. α-Ketoglutarate-related inhibitors of HIF prolyl hydroxylases are substrates of renal organic anion transporters 1 (OAT1) and 4 (OAT4). Pflug. Arch. Eur. J. Physiol. 2012, 464, 367–374.
- Taylor, C.T.; Colgan, S.P. Regulation of immunity and inflammation by hypoxia in immunological niches. Nat. Rev. Immunol. 2017, 17, 774–785.
- Zhang, J.; Wang, H.; Fan, Y.; Yu, Z.; You, G. Regulation of organic anion transporters: Role in physiology, pathophysiology, and drug elimination. Pharmacol. Ther. 2021, 217, 107647.
- Sirijariyawat, K.; Ontawong, A.; Palee, S.; Thummasorn, S.; Maneechote, C.; Boonphang, O.; Chatsudthipong, V.; Chattipakorn, N.; Srimaroeng, C. Impaired renal organic anion transport 1 (SLC22A6) and its regulation following acute myocardial infarction and reperfusion injury in rats. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2019, 1865, 2342–2355.
- Pou Casellas, C.; Jansen, K.; Rookmaaker, M.B.; Clevers, H.; Verhaar, M.C.; Masereeuw, R. Regulation of Solute Carriers Oct2 and Oat1/3 in the Kidney: A Phylogenetic, Ontogenetic, and Cell Dynamic Perspective. Physiol. Rev. 2022, 102, 993–1024.
- Soodvilai, S.; Wright, S.H.; Dantzler, W.H.; Chatsudthipong, V. Involvement of tyrosine kinase and PI3K in the regulation of OAT3-mediated estrone sulfate transport in isolated rabbit renal proximal tubules. Am. J. Physiol.-Ren. Physiol. 2005, 289, F1057–F1064.
- Liu, N.; Xu, L.; Shi, Y.; Fang, L.; Gu, H.; Wang, H.; Ding, X.; Zhuang, S. Pharmacologic targeting ERK1/2 attenuates the development and progression of hyperuricemic nephropathy in rats. Oncotarget 2017, 8, 33807–33826.
- Li, T.T.; An, J.X.; Xu, J.Y.; Tuo, B.G. Overview of organic anion transporters and organic anion transporter polypeptides and their roles in the liver. World J. Clin. Cases 2019, 7, 3915–3933.
- Caetano-Pinto, P.; Jamalpoor, A.; Ham, J.; Goumenou, A.; Mommersteeg, M.; Pijnenburg, D.; Ruijtenbeek, R.; Sanchez-Romero, N.; Van Zelst, B.; Heil, S.G.; et al. Cetuximab Prevents Methotrexate-Induced Cytotoxicity in Vitro through Epidermal Growth Factor Dependent Regulation of Renal Drug Transporters. Mol. Pharm. 2017, 14, 2147–2157.
- Serocki, M.; Bartoszewska, S.; Janaszak-Jasiecka, A.; Ochocka, R.J.; Collawn, J.F.; Bartoszewski, R. miRNAs regulate the HIF switch during hypoxia: A novel therapeutic target. Angiogenesis 2018, 21, 183–202.
- Liu, Y.; Nie, H.; Zhang, K.; Ma, D.; Yang, G.; Zheng, Z.; Liu, K.; Yu, B.; Zhai, C.; Yang, S. A feedback regulatory loop between HIF-1α and miR-21 in response to hypoxia in cardiomyocytes. FEBS Lett. 2014, 588, 3137–3146.
- Gomez, I.G.; MacKenna, D.A.; Johnson, B.G.; Kaimal, V.; Roach, A.M.; Ren, S.; Nakagawa, N.; Xin, C.; Newitt, R.; Pandya, S.; et al. Anti-microRNA-21 oligonucleotides prevent Alport nephropathy progression by stimulating metabolic pathways. J. Clin. Investig. 2015, 125, 141–156.
- Jansen, J.; Jansen, K.; Neven, E.; Poesen, R.; Othman, A.; van Mil, A.; Sluijter, J.; Torano, J.S.; Zaal, E.A.; Berkers, C.R.; et al. Remote sensing and signaling in kidney proximal tubules stimulates gut microbiome-derived organic anion secretion. Proc. Natl. Acad. Sci. USA 2019, 116, 16105–16110.
- Bannister, A.J.; Kouzarides, T. Regulation of chromatin by histone modifications. Cell Res. 2011, 21, 381–395.
- Fisel, P.; Schaeffeler, E.; Schwab, M. DNA methylation of ADME genes. Clin. Pharmacol. Ther. 2016, 99, 512–527.
- Hirota, T.; Tanaka, T.; Takesue, H.; Ieiri, I. Epigenetic regulation of drug transporter expression in human tissues. Expert Opin. Drug Metab. Toxicol. 2017, 13, 19–30.
- Jin, L.; Kikuchi, R.; Saji, T.; Kusuhara, H.; Sugiyama, Y. Regulation of tissue-specific expression of renal organic anion transporters by hepatocyte nuclear factor 1 α/β and DNA methylation. J. Pharmacol. Exp. Ther. 2012, 340, 648–655.
- Zhou, S.; Shu, Y. Special Section on New Era of Transporter Science: Unraveling the Functional Role of Orphan Transporters-Minireview Transcriptional Regulation of Solute Carrier Drug Transporters. Drug Metab. Dispos. 2022, 50, 1238–1250.
- Litke, C.; Hagenston, A.M.; Kenkel, A.K.; Paldy, E.; Lu, J.; Kuner, R.; Mauceri, D. Organic anion transporter 1 is an HDAC4-regulated mediator of nociceptive hypersensitivity in mice. Nat. Commun. 2022, 13, 875.
- Martovetsky, G.; Tee, J.B.; Nigam, S.K. Hepatocyte nuclear factors 4α and 1α regulate kidney developmental expression of drug-metabolizing enzymes and drug transporters. Mol. Pharmacol. 2013, 84, 808–823.
- Ogasawara, K.; Terada, T.; Asaka, J.I.; Katsura, T.; Inui, K.I. Hepatocyte nuclear factor-4α regulates the human organic anion transporter 1 gene in the kidney. Am. J. Physiol.-Ren. Physiol. 2007, 292, F1819–F1826.
- Parsons, J.T.; Horwitz, A.R.; Schwartz, M.A. Cell adhesion: Integrating cytoskeletal dynamics and cellular tension. Nat. Rev. Mol. Cell Biol. 2010, 11, 633–643.
- Duan, P.; You, G. Short-term regulation of organic anion transporters. Pharmacol. Ther. 2010, 125, 55–61.
- Epstein, F.H.; Fish, E.M.; Molitoris, B.A. Alterations in Epithelial Polarity and the Pathogenesis of Disease States. N. Engl. J. Med. 1994, 330, 1580–1588.
- Pou Casellas, C.; Rookmaaker, M.B.; Verhaar, M.C. Controlling cellular plasticity to improve in vitro models for kidney regeneration. Curr. Opin. Biomed. Eng. 2021, 20, 100345.
- Jansen, J.; Fedecostante, M.; Wilmer, M.J.; Peters, J.G.; Kreuser, U.M.; Van Den Broek, P.H.; Mensink, R.A.; Boltje, T.J.; Stamatialis, D.; Wetzels, J.F.; et al. Bioengineered kidney tubules efficiently excrete uremic toxins. Sci. Rep. 2016, 6, 26715.
- Rougerie, P.; Pieuchot, L.; dos Santos, R.S.; Marteau, J.; Bigerelle, M.; Chauvy, P.F.; Farina, M.; Anselme, K. Topographical curvature is sufficient to control epithelium elongation. Sci. Rep. 2020, 10, 14784.
- van Genderen, A.M.; Jansen, K.; Kristen, M.; van Duijn, J.; Li, Y.; Schuurmans, C.C.L.; Malda, J.; Vermonden, T.; Jansen, J.; Masereeuw, R.; et al. Topographic Guidance in Melt-Electrowritten Tubular Scaffolds Enhances Engineered Kidney Tubule Performance. Front. Bioeng. Biotechnol. 2021, 8, 617364.
- Garreta, E.; Prado, P.; Tarantino, C.; Oria, R.; Fanlo, L.; Martí, E.; Zalvidea, D.; Trepat, X.; Roca-Cusachs, P.; Gavaldà-Navarro, A.; et al. Fine tuning the extracellular environment accelerates the derivation of kidney organoids from human pluripotent stem cells. Nat. Mater. 2019, 18, 397–405.
- Batchelder, C.A.; Martinez, M.L.; Tarantal, A.F. Natural scaffolds for renal differentiation of human embryonic stem cells for kidney tissue engineering. PLoS ONE 2015, 10, e0143849.
- Bonventre, J.V.; Verhaar, M.C. Editorial overview: Kidney regeneration; cells, organoids and whole organ engineering? Curr. Opin. Biomed. Eng. 2022, 23, 100403.
- Ohi, M.D.; Kenworthy, A.K. Emerging Insights into the Molecular Architecture of Caveolin-1. J. Membr. Biol. 2022, 255, 375–383.
- Fridolfsson, H.N.; Roth, D.M.; Insel, P.A.; Patel, H.H. Regulation of intracellular signaling and function by caveolin. FASEB J. 2014, 28, 3823–3831.
- Barros, S.A.; Srimaroeng, C.; Perry, J.L.; Walden, R.; Dembla-Rajpal, N.; Sweet, D.H.; Pritchard, J.B. Activation of protein kinase Cζ increases OAT1 (SLC22A6)-and OAT3 (SLC22A8)-mediated transport. J. Biol. Chem. 2009, 284, 2672–2679.
- Xu, D.; Wang, H.; You, G. Posttranslational Regulation of Organic Anion Transporters by Ubiquitination: Known and Novel. Med. Res. Rev. 2016, 36, 964–979.
- Preising, C.; Schneider, R.; Bucher, M.; Gekle, M.; Sauvant, C. Regulation of expression of renal organic anion transporters OAT1 and OAT3 in a model of ischemia/reperfusion injury. Cell. Physiol. Biochem. 2015, 37, 1–13.
- Tanaka, K.; Xu, W.; Zhou, F.; You, G. Role of Glycosylation in the Organic Anion Transporter OAT1. J. Biol. Chem. 2004, 279, 14961–14966.
- Celen, A.B.; Sahin, U. Sumoylation on its 25th anniversary: Mechanisms, pathology, and emerging concepts. FEBS J. 2020, 287, 3110–3140.
- Wang, H.; Zhang, J.; You, G. Activation of Protein Kinase A Stimulates SUMOylation, Expression, and Transport Activity of Organic Anion Transporter 3. AAPS J. 2019, 21, 30.
- Yu, Z.; Liu, C.; Zhang, J.; Liang, Z.; You, G. Protein kinase C regulates organic anion transporter 1 through phosphorylating ubiquitin ligase Nedd4–2. BMC Mol. Cell Biol. 2021, 22, 53.
- Xu, D.; Wang, H.; You, G. An Essential Role of Nedd4-2 in the Ubiquitination, Expression, and Function of Organic Anion Transporter-3. Mol. Pharm. 2016, 13, 621–630.
- Fan, Y.; Wang, H.; Yu, Z.; Liang, Z.; Li, Y.; You, G. Inhibition of proteasome, but not lysosome, upregulates organic anion transporter 3 in vitro and in vivo. Biochem. Pharmacol. 2023, 208, 115387.
- Fan, Y.; Liang, Z.; Zhang, J.; You, G. Oral proteasomal inhibitors ixazomib, oprozomib, and delanzomib upregulate the function of organic anion transporter 3 (OAT3): Implications in OAT3-mediated drug-drug interactions. Pharmaceutics 2021, 13, 314.




