Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Andrey Mardanov | -- | 2401 | 2023-11-02 07:51:24 | | | |
| 2 | Wendy Huang | Meta information modification | 2401 | 2023-11-03 02:21:29 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Shalamitskiy, M.Y.; Tanashchuk, T.N.; Cherviak, S.N.; Vasyagin, E.A.; Ravin, N.V.; Mardanov, A.V. Wine Microorganisms' Role in Formation of Ethyl Carbamate. Encyclopedia. Available online: https://encyclopedia.pub/entry/51068 (accessed on 07 February 2026).
Shalamitskiy MY, Tanashchuk TN, Cherviak SN, Vasyagin EA, Ravin NV, Mardanov AV. Wine Microorganisms' Role in Formation of Ethyl Carbamate. Encyclopedia. Available at: https://encyclopedia.pub/entry/51068. Accessed February 07, 2026.
Shalamitskiy, Maksim Yu., Tatiana N. Tanashchuk, Sofia N. Cherviak, Egor A. Vasyagin, Nikolai V. Ravin, Andrey V. Mardanov. "Wine Microorganisms' Role in Formation of Ethyl Carbamate" Encyclopedia, https://encyclopedia.pub/entry/51068 (accessed February 07, 2026).
Shalamitskiy, M.Y., Tanashchuk, T.N., Cherviak, S.N., Vasyagin, E.A., Ravin, N.V., & Mardanov, A.V. (2023, November 02). Wine Microorganisms' Role in Formation of Ethyl Carbamate. In Encyclopedia. https://encyclopedia.pub/entry/51068
Shalamitskiy, Maksim Yu., et al. "Wine Microorganisms' Role in Formation of Ethyl Carbamate." Encyclopedia. Web. 02 November, 2023.
Copy Citation
Ethyl carbamate, the ethyl ester of carbamic acid, has been identified in fermented foods and alcoholic beverages. Since ethyl carbamate is a probable human carcinogen, reduction of its content is important for food safety and human health. In alcoholic beverages, ethyl carbamate is mostly formed from the reaction of ethanol with urea, citrulline and carbamyl phosphate during fermentation and storage. These precursors are generated from arginine metabolism by wine yeasts and lactic acid bacteria.
ethyl carbamate
wine
yeast
lactic acid bacteria
fermented foods
genetic engineering
1. Introduction
Ethyl carbamate is formed as a result of the reaction of ethanol and urea, cyanate, citrulline (Cit), carbamyl phosphate or other N-carbamyl compounds (Figure 1) [1][2][3][4][5]. The main source of ethyl carbonate in fermented foods is the reaction between urea and ethanol, and the high ethyl carbonate content in stone fruit spirits is mainly due to the cyanogenic glycosides present in their pits [6]. The most important ethyl carbamate precursors in wine are urea, citrulline and carbamyl phosphate [3][5][7][8].
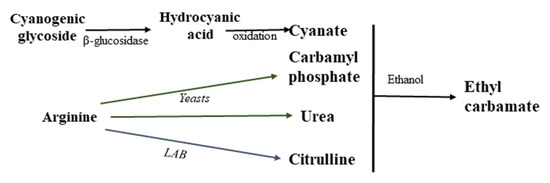
Figure 1. Mechanism of ethyl carbamate formation in alcoholic beverages.
In wine, ethyl carbamate is formed as follows: arginine, usually one of the most abundant amino acids available to yeast in grape juice, undergoes enzymatic hydrolysis in the early and middle stages of alcoholic fermentation to form urea [5][9]. Specifically, arginase (EC 3.5.3.1) converts L-arginine into L-ornithine and urea. When urea cannot be further metabolized in the cell and accumulates above a critical concentration, the yeast secretes it into the wine during or at the end of fermentation. Subsequently, urea reacts with ethanol to form ethyl carbamate (Figure 1).
Citrulline is already present in grape must, but it can also be formed as a result of arginine anabolism by the yeast Saccharomyces cerevisiae, when ornithine and carbamoyl phosphate react to form arginine, with citrulline acting as an intermediate [5]. Ethyl carbamate can also appear in wine as a result of the ethanolysis with citrulline and carbamyl phosphate, formed as a result of the degradation of arginine with the participation of certain strains of lactic acid bacteria (LAB) and yeast (Figure 1).
Ethyl carbamate is also formed in distilled ethyl alcohol, especially obtained from stone fruits (cherries, apricots or plums), as a result of the reaction of ethanol and isocyanate, a by-product of the enzymatic hydrolysis of cyanoglycosides present in these fruits [1][2][6][7][10][11][12]. The amount of ethyl carbamate in distilled spirits is influenced by the distillation temperature, ethanol content and the design of the distillation apparatus [6]. The content of this compound during exposure and storage can increase significantly. About 80% of the ethyl carbamate present in alcohols is formed during the distillation step and/or during the first 48 h after distillation [2].
Yeast strains can differ in their ability to catabolize arginine and urea during fermentation. Yeasts that secrete a large amount of urea into the medium usually have a high ability to break down arginine to urea, but a weak ability to hydrolyse it, which may be the result of low urea amidolyase activity, its inhibition by high ammonia content, a deficiency of cofactors necessary for enzyme action or hyperactivity of arginase. Genetic factors, as well as environmental factors such as pH and temperature, also affect the amount of urea released by cells [7][9][13]. An increase in the content of arginine in wine may be due to the effect of ethanol on the porosity of yeast membranes [13] or yeast autolysis [14].
When studying the effect of yeast strains on the formation of ethyl carbamate in alcoholic products, it was found that the S. bayanus yeast produces significantly fewer ethyl carbamate precursors than S. cerevisiae [13]. In wines obtained with the use of the yeast Wickerhamomyces anomalus, higher urea content (910 μg/L) was noted than with S. cerevisiae (300 μg/L). Moreover, the concentration of urea increased to 1261 μg/L with the joint introduction of the yeast of these two genera. At the same time, the transcriptional activity of the arginase gene (CAR1) in W. anomalus increased by 140%, while in S. cerevisiae it decreased by 40%.
There is evidence that representatives of the non-Saccharomyces yeast of the genera Pichia, Schizosaccharomyces and Zygosaccharomyces can be active regulators of the formation of urea from arginine. Some members of the genera Schizosaccharomyces and Zygosaccharomyces can produce less urea and decompose it more efficiently than S. cerevisiae [15]. The gene coding for ATP-independent urease (URE) was mainly found in the yeast of the genus Schizosaccharomyces. Another urea degradation pathway (involving urea carboxylase and allophanate hydrolase) has been identified predominantly in yeasts of the genus Pichia and Zygosaccharomyces. The authors point out that the use of such yeast allowed for the reduction of the urea content during fermentation [15].
2. Ethyl Carbamate in Wine: The Role of Lactic Acid Bacteria
Despite the fact that urea produced by yeast is the main precursor of ethyl carbamate in wine, LAB can also contribute to its formation [16]. Arginine is available for both wine yeast and LAB metabolism during malolactic fermentation (MLF), which usually proceeds after alcoholic fermentation. Depending on the LAB strain used, the amount of ethyl carbamate in wine may slightly increase [17].
The catabolism of arginine by LAB via the arginine deiminase (ADI) pathway leads to the production of ammonia, ornithine and CO2 (Figure 2). This pathway is the most common for arginine degradation under anaerobic conditions [18]. The first enzyme of this pathway, arginine deiminase (ArcA), is responsible for the degradation of arginine to form citrulline, which can react with ethanol to form ethyl carbamate (Figure 2) [19][20]. The formation of ammonia from arginine contributes to an increase in the pH of the wine, which may be physiologically significant for the adaptation of LAB to a low pH value. It has been shown that the ADI pathway is an important source of energy for bacterial growth, and LAB strains capable of obtaining energy from arginine catabolism may be more competitive in wine than strains unable to degrade arginine [21][22]. There are reports that the ADI pathway is sometimes indirectly associated with the production of biogenic amines, especially putrescine, because this amine can be formed from ornithine with the participation of LAB [23].
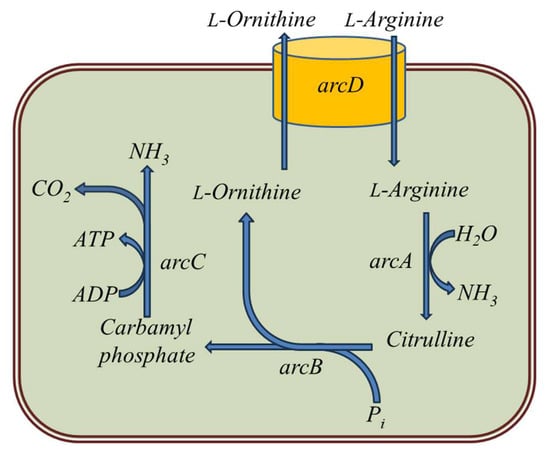
Figure 2. Arginine deiminase pathway in LAB. The enzymes of this pathway are (1) arcD—arginine: ornithine antiporter; (2) arcA—arginine deiminase (ADI); (3) arcB—ornithine transcarbamylase (OTC); and (4) arcC—carbamate kinase (CK).
In one of the studies, the presented assessment of the ability of industrial acid-reducing strains to secrete and use citrulline confirmed that LAB secretes citrulline as a result of arginine degradation. At the same time, in an arginine-rich medium, citrulline was retained in the cell during cell growth and released during cell lysis. All strains have been found to utilize citrulline, and some of them are able to recycle previously secreted citrulline [24].
LAB strains are distinguished by their ability to degrade arginine, but there is little information on the potential of LAB species to degrade arginine under winemaking conditions. Some strains are able to form small amounts of citrulline from arginine. Uncontrolled enrichment of the must with nitrogenous substances can increase the likelihood of developing LAB at the end of fermentation, which can lead to an increase in the level of ethyl carbamate even at optimal temperatures during long-term storage.
An alternative way to prevent the formation of ethyl carbamate in wine is the use of Lactobaccilus hilgardii and Oenococcus oeni to utilize arginine and citrulline [2][25]. The presence of these bacteria in the starter cultures reduced the ethyl carbamate content in the fermented food products [25]. In the second work, LAB was used as starter cultures for malolactic fermentation after the end of alcoholic fermentation [2]. Generally, all heterofermentative wine LABs, including strains of L. buchneri and L. brevis [20][26][27] and O. oeni [18] hydrolyze arginine. There are reports showing that some representatives of L. plantarum consume arginine through the ADI pathway [28]. Araque et al. [29] found a correlation between the presence of genes of the ADI pathway and the ability to degrade arginine by LAB isolated from wine. This feature was also noted for all strains of heterofermentative lactobacilli, O. oeni, Pediococcus pentosaceus and some strains of Leuconostoc mesenteroides and L. plantarum [18][29]. There is evidence that during alcoholic fermentation, some strains of Lactobacillus decomposed arginine simultaneously with yeast, which led to a decrease in the production of urea by yeast [15].
3. Genetic Modification of Wine Yeast to Reduce the Formation of Ethyl Carbamate
Selection of industrial yeast strains based on the application of a complex of modern techniques and approaches of postgenomic biology, metabolic engineering and genome editing are relevant for winemaking. Modification of yeast wine strains by genetic techniques provides significant progress in the inhibition of urea metabolism.
Import of arginine from the culture medium into the yeast cell is primarily enabled by the Can1 arginine amino acid transporter localized to the plasma membrane of yeast (Figure 3) [30]. In addition, general amino-acid permease Gap1 is responsible for the transport of arginine and related compounds such as ornithine and citrulline into the cell. Arginase, encoded by the CAR1 gene, cleaves arginine to urea, which is either excreted by DUR4 permease or metabolized by urea amidolyase (DUR1,2), responsible for the conversion of urea to ammonia and carbon dioxide. Active transport of urea from the medium into the yeast cells is performed by the urea-proton symporter (DUR3) (Figure 3). Thus, inhibition of CAR1 expression and increased expression of DUR1,2 and DUR3 leads to a decrease in the concentration of urea in the medium and, consequently, to a decrease in the formation of ethyl carbamate [3][31][32][33].
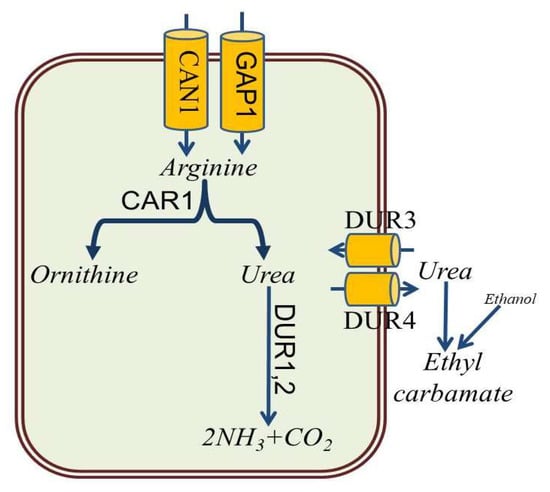
Figure 3. Main metabolic pathways of arginine degradation in S. cerevisiae.
Despite numerous and successful attempts to improve the characteristics of wine yeast strains by genetic engineering, only two of them, strain ML01 carrying genes of the MLF pathway [34] and strain ECMo01 [25], are officially registered for use in the USA, Canada, and Moldova. Wider use of GM strains is constrained by both the conservatism of winemakers and well-known public prejudice and legislative restrictions in the field of genetic engineering in the food industry [35]. Many studies were aimed at obtaining yeast strains with low ethyl carbamate production in wine [25]. The ECMo01 strain contains an additional copy of the DUR1,2 amidolyase gene under the control of regulatory sequences of the phosphoglycerate kinase PGK1. In this strain, the expression of the DUR1,2 gene was increased by 17 times, which led to a decrease in the concentration of urea, and the produced ammonia was used as a source of nitrogen. The concentration of ethyl carbamate in wine obtained using the ECMo01 strain was reduced by 90%, while the strain was equivalent in its phenotypic characteristics to the parental strain 522. Subsequently, the obtained wine yeast strain 522DUR3 with elevated expression of the DUR3 transporter gene enabled an 81% reduction in ethyl carbamate content in wine [36].
Genetically engineered yeast strains with a null mutation in CAR1 were specially adapted to reduce the concentration of ethyl carbamate in sake, cherry spirits and Chinese rice wine. There were no significant differences in yeast fermentation activity compared to the corresponding parental strains [37].
To reduce the production of urea, a recombinant strain YZ22 (Δcarl/Δcarl) with arginase deficiency was obtained from diploid wine yeast strain WY1 by successive deletion of two CAR1 alleles. The results of RT-qPCR showed that strain YZ22 almost did not express the CAR1 gene, and the arginase activity was 12.6 times lower than that of the parental strain WY1. According to the results of alcoholic fermentation, it was found that the content of urea and ethyl carbamate in wine decreased by 77.9 and 73.8%, respectively. In addition, the lower urea content of the wine resulted in slower and quantitatively less formation of ethyl carbamate during storage. The resulting strain in fermentation activity was equivalent to the parental strain [37].
The use of CRISPR/Cas-mediated genome editing is a promising technique for creating improved yeast strains since it does not bear the risks associated with the introduction of foreign genes and genetic elements, markers of antibiotic resistance, into the genome of yeast food strains, i.e., the obtained strains are safe, according to the regulatory restrictions adopted in some countries. The CRISPR/Cas9 system was used to make wine strains with reduced urea production. Derivatives of wine strains EC1118 and AWRI1796, defective in both alleles of the CAN1 gene, were obtained [38].
Another way to reduce urea production is to inactivate transporters responsible for the uptake of arginine into yeast cells from the culture medium. Modified wine yeast strains with alterations in arginine catabolism pathways have already been developed for the production of sake and sherry, but there is no information on the role of CAN1 in urea production during yeast fermentation. The resulting recombinant strains ScEC1118can1 and ScAWRI796can1 with eliminating the CAN1 arginine permease pathway were characterized by reduced urea production (by 18–36% compared to the original ones) under the conditions of experimental winemaking with the preserved ability to ferment the synthetic substrate, although with a slightly reduced growth rate [38]. The advantage of introducing a mutation in the CAN1 gene compared to other methods of modifying arginine utilization pathways is that this technique is less sensitive to fluctuations in the content of nitrogen sources in the composition of the wort and has less effect on the growth parameters of yeast strains.
One more way to minimize ethyl carbamate formation is to reduce the concentration of urea by converting it into ammonia and CO2 by the bifunctional enzyme urea amidolyase encoded by DUR1,2 [25][39][40]. Gene DUR1,2 is repressed when the preferred nitrogen sources of yeast are available. Improving urea degradation through the constitutive expression of DUR1,2 in yeast has been achieved and the reduction of ethyl carbamate in wine and sake was 89.1% and 68.0%, respectively [25][36]. Wu D. et al. developed a DUR1,2 expression cassette to insert the strong PGK1 promoter upstream of the DUR1,2 gene loci in the wild-type industrial yeast N85DUR1,2 and N85DUR1,2-c with deleted CAR1 genes [41]. During small-scale fermentation, the concentrations of urea and ethyl carbamate were reduced by engineered strain N85DUR1,2 by 75.6% and 40.0% compared to the parental yeast strain, respectively. The N85DUR1,2-c strain reduced urea and ethyl carbamate by 89.1% and 55.3%, respectively [41].
Recently, a CRISPR/Cas9-based genome editing was used to modify a polyploid wild yeast S. cerevisiae strain used for the production of Korean traditional rice wine [42]. In addition to the CAR1 gene, the GZF3 gene encoding the transcription factor controlling expression levels of genes DUR1,2 and DUR3 was deleted to further reduce the formation of ethyl carbamate. The use of a double deletion strain for wine brewing resulted in a significant reduction of the ethyl carbamate content in this wine when compared to the wild-type strain without affecting fermentation capability.
References
- Larcher, R.; Moser, S.; Menolli, A.U.; Tonidandel, L.; Nicolini, G. Ethyl carbamate formation in sub-optimal wine storage conditions and influence of the yeast starter. OENO One 2013, 47, 65–68.
- Ryu, D.; Choi, B.; Kim, E.; Park, S.; Paeng, H.; Kim, C.; Lee, J.; Yoon, H.J.; Koh, E. Determination of ethyl carbamate in alco-holic beverages and fermented foods sold in Korea. Toxicol. Res. 2015, 31, 289–297.
- Monteiro, F.F.; Trousdale, E.K.; Bisson, L.F. Ethyl carbamate formation in wine: Use of radioactively labeled precursors to demonstrate the involvement of urea. Am. J. Enol. Vitic. 1989, 40, 1–8.
- Leça, J.M.; Pereira, V.; Miranda, A.; Vilchez, J.L.; Marques, J.C. New insights into ethyl carbamate occurrence in fortified wines. LWT 2021, 150, 111566.
- Jiao, Z.; Dong, Y.; Chen, Q. Ethyl carbamate in fermented beverages: Presence, analytical chemistry, formation mechanism, and mitigation proposals. Compr. Rev. Food Sci. Food Saf. 2014, 13, 611–626.
- Abt, E.; Incorvati, V.; Robin, L.P.; Redan, B.W. Occurrence of ethyl carbamate in foods and beverages: Review of the formation mechanisms, advances in analytical methods, and mitigation strategies. J. Food Prot. 2021, 84, 2195–2212.
- Stevens, D.F.; Ough, C.S. Ethyl carbamate formation: Reaction of urea and citrulline with ethanol in wine under low to normal temperature conditions. Am. J. Enol. Vitic. 1993, 44, 309–312.
- Azevedo, Z.; Couto, J.A.; Hogg, T. Citrulline as the main precursor of ethyl carbamate in model fortified wines inoculated with Lactobacillus hilgardii: A marker of the levels in a spoiled fortified wine. Lett. Appl. Microbiol. 2002, 34, 32–36.
- Butzke, C.E.; Bisson, L.F. Ethyl Carbamate Preventative Action Manual, US Food and Drug Administration; Center for Food Safety and Applied Nutrition: Washington, DC, USA, 1997.
- Weber, J.V.; Sharypov, V.I. Ethyl carbamate in foods and beverages—A Review. In Climate Change, Intercropping, Pest Control and Beneficial Microorganisms. Sustainable Agriculture Reviews; Lichtfouse, E., Ed.; Springer: Paris, France, 2009; Volume 2, pp. 429–452.
- Kodama, S.; Suzuki, T.; Fujinawa, S.; de la Teja, P.; Yotsuzuka, F. Urea contribution to ethyl carbamate formation in commercial wines during storage. Am. J. Enol. Vitic. 1994, 45, 17–24.
- Zhao, X.; Du, G.; Zou, H.; Fu, J.; Zhou, J.; Chen, J. Progress in preventing the accumulation of ethyl carbamate in alcoholic beverages. Trends Food Sci. Technol. 2013, 32, 97–107.
- Ough, C.S.; Crowell, E.A.; Gutlove, B.R. Carbamyl compound reactions with ethanol. Am. J. Enol. Vitic. 1988, 39, 239–242.
- Hernawan, T.; Fleet, G. Chemical and cytological changes during the autolysis of yeasts. J. Ind. Microbiol. 1995, 14, 440–450.
- Du, H.; Song, Z.; Xu, Y. Ethyl carbamate formation regulated by lactic acid bacteria and nonconventional yeasts in solid-state fermentation of Chinese Moutai-flavor liquor. J. Agric. Food Chem. 2018, 66, 387–392.
- Arena, M.E.; Saguir, F.M.; De Nadra, M.M. Arginine, citrulline and ornithine metabolism by lactic acid bacteria from wine. Int. J. Food Microbiol. 1999, 52, 155–161.
- Uthurry, C.A.; Lepe, J.S.; Lombardero, J.; Del Hierro, J.G. Ethyl carbamate production by selected yeasts and lactic acid bacteria in red wine. Food Chem. 2006, 94, 262–270.
- Liu, S.Q.; Pilone, G.J. A review: Arginine metabolism in wine lactic acid bacteria and its practical significance. J. Appl. Microbiol. 1998, 84, 315–327.
- Liu, S.; Pritchard, G.G.; Hardman, M.J.; Pilone, G.J. Occurrence of arginine deiminase pathway enzymes in arginine catabolism by wine lactic acid bacteria. Appl. Environ. Microbiol. 1995, 61, 310–316.
- Liu, S.Q.; Pritchard, G.G.; Hardman, M.J.; Pilone, G.J. Arginine catabolism in wine lactic acid bacteria: Is it via the arginine deiminase pathway or the arginase-urease pathway? J. Appl. Bacteriol. 1996, 81, 486–492.
- Tonon, T.; Lonvaud-Funel, A. Metabolism of arginine and its positive effect on growth and revival of Oenococcus oeni. J. Appl. Microbiol. 2000, 89, 526–531.
- Mira de Orduña, R.; Patchett, M.L.; Liu, S.Q.; Pilone, G.J. Growth and arginine metabolism of the wine lactic acid bacteria Lactobacillus buchneri and Oenococcus oeni at different pH values and arginine concentrations. Appl. Environ. Microbiol. 2001, 67, 1657–1662.
- Mangani, S.; Guerrini, S.; Granchi, L.; Vincenzini, M. Putrescine accumulation in wine: Role of Oenococcus oeni. Curr. Microbiol. 2005, 51, 6–10.
- Mira de Orduña, R.; Liu, S.Q.; Patchett, M.L.; Pilone, G.J. Ethyl carbamate precursor citrulline formation from arginine degradation by malolactic wine lactic acid bacteria. FEMS Microbiol. Lett. 2000, 183, 31–35.
- Coulon, J.; Husnik, J.I.; Inglis, D.L.; van der Merwe, G.K.; Lonvaud, A.; Erasmus, D.J.; van Vuuren, H.J. Metabolic engineering of Saccharomyces cerevisiae to minimize the production of ethyl carbamate in wine. Am. J. Enol. Vitic. 2006, 57, 113–124.
- Edwards, C.G.; Powers, J.R.; Jensen, K.A.; Weller, K.M.; Peterson, J.C. Lactobacillus spp. from Washington State wines: Isolation and characterization. J. Food Sci. 1993, 58, 453–458.
- Liu, S.Q.; Pritchard, G.G.; Hardman, M.J.; Pilone, G.J. Citrulline production and ethyl carbamate (urethane) precursor formation from arginine degradation by wine lactic acid bacteria Leuconostoc oenos and Lactobacillus buchneri. Am. J. Enol. Vitic. 1994, 45, 235–242.
- Spano, G.; Chieppa, G.; Beneduce, L.; Massa, S. Expression analysis of putative arcA, arcB and arcC genes partially cloned from Lactobacillus plantarum isolated from wine. J. Appl. Microbiol. 2004, 96, 185–193.
- Araque, I.; Gil, J.; Carreté, R.; Bordons, A.; Reguant, C. Detection of arc genes related with the ethyl carbamate precursors in wine lactic acid bacteria. J. Agric. Food Chem. 2009, 57, 1841–1847.
- Ahmad, M.; Bussey, H. Yeast arginine permease: Nucleotide sequence of the CAN1 gene. Curr. Genet. 1986, 10, 587–592.
- Pieper, H.J.; Seibold, R.; Luz, E.; Jung, O. Reduction of the ethyl carbamate concentration in manufacture of Kirsch (cherry spirit). Kleinbrennerei 1992, 44, 125–130.
- Schehl, B.; Senn, T.; Lachenmeier, D.W.; Rodicio, R.; Heinisch, J.J. Contribution of the fermenting yeast strain to ethyl carbamate generation in stone fruit spirits. Appl. Microbiol. Biotechnol. 2007, 74, 843–850.
- Adams, C.; van Vuuren, H.J. Effect of timing of diammonium phosphate addition to fermenting grape must on the production of ethyl carbamate in wine. Am. J. Enol. Vitic. 2010, 61, 125–129.
- Husnik, J.I.; Delaquis, P.J.; Cliff, M.A.; van Vuuren, H.J. Functional analyses of the malolactic wine yeast ML01. Am. J. Enol. Vitic. 2007, 58, 42–52.
- Grossmann, M.; Kießling, F.; Singer, J.; Schoeman, H.; Schröder, M.B.; von Wallbrunn, C. Genetically modified wine yeasts and risk assessment studies covering different steps within the wine making process. Ann. Microbiol. 2011, 61, 103–115.
- Dahabieh, M.S.; Husnik, J.I.; Van Vuuren, H.J.J. Functional enhancement of Sake yeast strains to minimize the production of ethyl carbamate in Sake wine. J. Appl. Microbiol. 2010, 109, 963–973.
- Guo, X.W.; Li, Y.Z.; Guo, J.; Wang, Q.; Huang, S.Y.; Chen, Y.F.; Du, L.P.; Xiao, D.G. Reduced production of ethyl carbamate for wine fermentation by deleting CAR1 in Saccharomyces cerevisiae. J. Ind. Microbiol. Biotechnol. 2016, 43, 671–679.
- Vigentini, I.; Gebbia, M.; Belotti, A.; Foschino, R.; Roth, F.P. CRISPR/Cas9 system as a valuable genome editing tool for wine yeasts with application to decrease urea production. Front. Microbiol. 2017, 8, 2194.
- Genbauffe, F.S.; Cooper, T.G. Induction and repression of the urea amidolyase gene in Saccharomyces cerevisiae. Mol. Cell. Biol. 1986, 6, 3954–3964.
- Hofman-Bang, J. Nitrogen catabolite repression in Saccharomyces cerevisiae. Mol. Biotechnol. 1999, 12, 35–71.
- Wu, D.; Li, X.; Lu, J.; Chen, J.; Zhang, L.; Xie, G. Constitutive expression of the DUR1,2 gene in an industrial yeast strain to minimize ethyl carbamate production during Chinese rice wine fermentation. FEMS Microbiol. Lett. 2016, 363, fnv214.
- Jung, J.Y.; Kang, M.J.; Hwang, H.S.; Baek, K.R.; Seo, S.O. Reduction of ethyl carbamate in an alcoholic beverage by CRISPR/Cas9-based genome editing of the wild yeast. Foods 2022, 12, 102.
More
Information
Subjects:
Food Science & Technology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
989
Revisions:
2 times
(View History)
Update Date:
03 Nov 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




