Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Rosalie M Luiten | -- | 3091 | 2023-10-30 17:27:55 | | | |
| 2 | Wendy Huang | Meta information modification | 3091 | 2023-10-31 05:42:27 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Bakker, D.; Bakker, W.J.; Bekkenk, M.W.; Luiten, R.M. Immunity against Non-Melanoma Skin Cancer. Encyclopedia. Available online: https://encyclopedia.pub/entry/50945 (accessed on 08 February 2026).
Bakker D, Bakker WJ, Bekkenk MW, Luiten RM. Immunity against Non-Melanoma Skin Cancer. Encyclopedia. Available at: https://encyclopedia.pub/entry/50945. Accessed February 08, 2026.
Bakker, Dixie, Walbert J. Bakker, Marcel W. Bekkenk, Rosalie M. Luiten. "Immunity against Non-Melanoma Skin Cancer" Encyclopedia, https://encyclopedia.pub/entry/50945 (accessed February 08, 2026).
Bakker, D., Bakker, W.J., Bekkenk, M.W., & Luiten, R.M. (2023, October 30). Immunity against Non-Melanoma Skin Cancer. In Encyclopedia. https://encyclopedia.pub/entry/50945
Bakker, Dixie, et al. "Immunity against Non-Melanoma Skin Cancer." Encyclopedia. Web. 30 October, 2023.
Copy Citation
Non-melanoma skin cancers (NMSCs), including basal cell carcinoma (BCC) and cutaneous squamous cell carcinoma (cSCC), are the most frequent types of cancers among Caucasians. Non-melanoma skin cancers (NMSCs) occur frequently in the Caucasian population and are considered a burden for health care. Risk factors include ultraviolet (UV) radiation, ethnicity and immunosuppression. This indicates the importance of immunosurveillance in preventing NMSC. However, the immunological mechanisms mediating immunosurveillance against NMSC are not fully known.
non-melanoma skin cancer
dendritic cells
macrophages
myeloid-derived suppressor cells
neutrophils
mast cells
innate lymphoid cells
NKT cells
γδ T cells
1. Introduction
Non-melanoma skin cancers (NMSCs), including basal cell carcinoma (BCC) and cutaneous squamous cell carcinoma (cSCC), are the most frequent types of cancers among Caucasians. While overall mortality rates of these cancers are low, the steadily increasing number of cases continues to cause burden to society and health care. Risk factors for developing NMSC include chronic exposure to ultraviolet radiation (UVR), age, ethnicity and family history of skin cancer [1]. More than 90% of BCC show the upregulation of the Hedgehog pathway, involving the Patched 1 (PTCH1) gene and smoothened (SMO) and glioma-associated (GLI) oncogenes. The Hedgehog pathway is implicated in self-renewal, survival and angiogenesis and can drive a cancer stem phenotype. Although BCC rarely metastasize, local invasion and tissue destruction cause high morbidity. In cSCC, a more diverse (UV-induced) gene mutational profile has been found, including tumor suppressors, epigenetic regulators and DNA repair pathways, leading to a high neoantigen burden [1]. The mortality of cSCC is due to its metastasizing behavior to distant sites. Immunosuppression is another major risk factor, as demonstrated by the increased incidence rate among HIV patients and solid organ transplant recipients (SOTRs) undergoing a long-term immunosuppressive regimen [2][3]. This indicates the importance of immunosurveillance in preventing NMSC. However, the immunological mechanisms mediating immunosurveillance against NMSC are not fully known.
2. Dendritic Cells
Dendritic cells residing in the skin have a central role in detecting aberrant keratinocytes and, upon activation and migration to the lymph node, prime adaptive immune responses (Figure 1). Dendritic cells are considered to act as a bridge between the innate and adaptive immune system [4]. Functionally, there are two main types of dendritic cells: conventional dendritic cells (cDCs) and plasmacytoid dendritic cells (pDCs). Although pDCs are usually not found in normal skin, they produce type I interferons, such as IFNα, which play an important role in antitumor immunity. Several studies have shown that intralesional injection with IFN-⍺, a type I interferon, can be an effective treatment for both cSCC and BCC [5][6]. Furthermore, the expression of type I interferon signaling protein ISFG-3 is suppressed in early skin carcinogenesis, suggesting a role for these cytokines during cancer development [7].
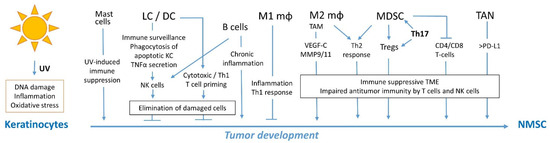
Figure 1. Overview of immune cells involved in immunosurveillance and immunity against NMSC. Abbreviations, LC, Langerhans cells; DC, dendritic cells; Mφ, macrophages; TAM, tumor-associated macrophages; Tregs, regulatory T cells; TAN, tumor-associated neutrophils; TME, tumor microenvironment.
Skin DCs comprise Langerhans cells (LCs) and cDCs and, in the skin, are referred to as dermal dendritic cells (dDCs). The role of skin DCs in NMSC has been shown in studies of UV-induced chronic inflammation and immune suppression in the skin, which both contribute to NMSC development [8]. UVB induces the apoptosis of epidermal skin cells, predominantly keratinocytes, and cutaneous inflammation. In a mouse model, UVB-induced inflammation was exacerbated and prolonged when LCs were depleted, coinciding with an increased number of apoptotic cells. LCs were shown to phagocytose apoptotic keratinocytes upon UVB exposure, and thereby play an essential role in the resolution of UVB-induced skin inflammation [9]. The role of LCs in skin cancer surveillance was also shown in a murine model of chemical skin carcinogenesis [10]. Initiation and early events during carcinogenesis induced epidermal LCs in order to secrete TNF⍺, resulting in the recruitment of NK cells to the epidermis and prevention of cSCC development. Conversely, the depletion of both LCs and NK cells resulted in increased tumor growth [10]. Regarding UV-induced immunosuppression, Furio et al. (2005) [11] reported that the UVA irradiation of ex vivo human skin resulted in a decreased number of DC emigrating from the skin and showing impaired DC maturation. These findings suggest that even though surviving UVA-exposed dDCs can reach the lymph nodes, they exhibit impaired antigen presentation and might fail to induce an effective T cell response in vivo. In support of this, DCs in human cSCC tissue were shown to have impaired ability to stimulate T cell proliferation compared to DCs from healthy skin [12].
Altogether, these findings suggest that skin DCs play an important role in skin cancer immunosurveillance and in initiating adaptive immune responses against NMSC.
3. Macrophages
Tumor-associated macrophages (TAMs) are found within and around cSCC and BCC as part of the tumor microenvironment (TME). Pro-inflammatory/M1 macrophages have antitumor properties and promote inflammation, whereas anti-inflammatory/M2 macrophages contribute to an immunosuppressive environment and decreased immunosurveillance that allows tumor growth (Figure 1) [13]. Under the influence of the TME, in most types of cancer, monocytes are stimulated to differentiate into M2 macrophages. Increased M2 macrophage infiltration is often associated with poor cancer prognosis [14][15]. TAMs with a M2 phenotype promote a Type 2 T cell response (Th2) and attract immunosuppressive T regulatory cells (Tregs), resulting in an inefficient antitumor response. This type of immune response also stimulates differentiation of TAMs into M2 macrophages as a positive feedback loop [16]. However, Pettersen et al. (2011) [17] reported that not M2, but M1 gene sets were enriched in cSCC compared to normal skin, indicating the activation of M1 macrophages and the promotion of a Type 1 T cell response (Th1). In addition, several M2-specific genes were upregulated and the TAMs also expressed Th2-associated products. This suggests that the composition of macrophages in the TME is heterogeneous and consists of both M1 and M2 macrophages and a strong Th1 response. TAMs can promote carcinogenesis in cSCC by releasing cytokines, including vascular endothelial growth factor-C (VEGF-C) and matrix metalloproteinase (MMP) 9 and 11, which stimulate lymph-angiogenesis and tumor growth [17][18]. The expression of VEGFC in cSCC TME coincided with enhanced lymphatic density and reorganized lymphatic endothelial vessels in the peritumoral dermis, which may facilitate metastases [18]. In BCC, macrophages were found more abundantly in tumors that did not respond to immunotherapy, as compared to responding tumors. However, in contrast to melanoma, macrophages in BCC displayed a low anti-inflammatory gene expression profile, suggesting a minor impact on tumor immunity [19].
4. Myeloid-Derived Suppressor Cells
Myeloid-derived suppressor cells (MDSCs) are frequently found in the TME of NMSCs [20][21]. This is a heterogeneous population of myeloid cells with immunosuppressive activity, and their presence in tumors is often associated with poor clinical outcome (Figure 1) [22]. In various types of cancer, including melanoma, MDSCs were shown to induce tumor growth and progression by promoting the immunosuppressive TME, e.g., by inhibiting NK cells, CD4+ T cells and CD8+ T cells, and stimulating the activity of regulatory T cells (Tregs) [23][24]. STAT3 is a transcription factor and known oncogene activated by pro-inflammatory cytokines. In cSCC, STAT3 was shown to stimulate the activation and proliferation of MDSCs but also promote the MDSC-induced switch from TAMs to an immunosuppressive M2 phenotype [25].
5. Neutrophils
High-risk cSCC displaying markers of increased metastatic risk are associated with high numbers of both tumor-associated neutrophils (TANs) and circulating neutrophils [26]. Neutrophils are an essential part of the innate immune system. Although neutrophils generally have antitumor properties, high numbers of TANs are associated with poor prognosis in many types of cancer [27] (Figure 1). The gene expression analysis of TANs in the DMBA/PMA-induced murine cSCC model revealed a predominant protumor gene expression signature during tumorigenesis, as compared to adjacent skin [28]. This indicates that under the influence of the TME neutrophils shift to a tumor-promoting phenotype. Moreover, in this murine cSCC model, TANs displayed an increased expression of immunosuppressive markers PD-L1, Siglec F, reactive oxygen and nitrite production and PD-L1 expression on TANs correlated to tumor growth. The depletion of TANs delayed tumor growth and restored the antitumor CD8+ T cell responses, indicating that PD-L1+ TANs suppress tumor-specific CD8+ effector T cells expressing PD-1, through PD-L1/PD-1 signaling [28].
6. Mast Cells
Mast cells are prevalent in the skin and other peripheral tissues, but their role in cancer in has not been studied in detail. In mice, the levels of dermal mast cells was shown to correlate with their susceptibility to the UV-induced suppression of systemic contact hypersensitivity responses. Studies in mast cell-depleted mice showed that mast cells are required for UV-induced immunosuppression [27][29]. In BCC patients, sun-protected skin showed a more abundant presence of dermal mast cells than in healthy individuals [30], suggesting elevated dermal mast cell level as a predisposing factor for BCC development in humans. A functional connection is suggested by the murine data that dermal mast cells promote UVB-induced immune suppression and thereby decrease cancer immunosurveillance (Figure 1) [27][29].
7. Innate lymphoid Cells and Natural Killer Cells
Group 1 innate lymphoid cells (ILC1) that lack the expression of antigen-specific receptors and produce inflammatory cytokines IFNγ, TNFα and GM-CSF. Natural killer (NK) cells are a subset of ILC that are able to kill tumor cells by secreting perforins and granzymes [31], while other ILC1 lack cytotoxic properties. The role of NK cells in NMSC surveillance has mostly been studied in murine cSCC models. In the DMBA/PMA-induced murine cSCC model, NK cells were found to accumulate in DMBA-treated skin. NK cell infiltration depended on TNFα produced by Langerhans cells, which in turn activates the epidermal production of chemokines CCL2 and CXCL10. These NK cells expressed NKG2D, by which they can kill transformed cells expressing ligands of NKG2D. This was demonstrated via NK cell depletion experiments that resulted in an accumulation of DNA-damaged cells, and an increased number of papillomas, indicating that NK cells are involved the early elimination of DNA-damaged, transformed keratinocytes [10]. These data show the cooperation between NK cells and Langerhans cells in suppressing cSCC development.
Although less characterized, the role of noncytotoxic ILC1 in antitumor immunity is thought to be in cancer immunoediting [32]. ILC1 thus mediate cancer immunity by producing inflammatory cytokines in the TME that promote local T cell activation. However, GM-CSF production by ILC1 may activate macrophages and facilitate tumor progression. In both precancerous murine cSCC lesions and cSCC tumors and human cSCC, infiltrating NK cells and ILC1 have been shown to display an atypical cytokine secretion profile, impaired cytotoxicity and are possibly incapable of eradicating tumor cells [33]. Since several studies have reported the downregulation of NK cell-activating receptors and the presence of exhaustion markers on tumor-infiltrating NK cells, possibly through IL-33 cytokine signaling, it is likely that the TME can promote the dysfunction of NK cells, thus allowing tumor development [34][35]. In addition, impaired NK function has been associated with an increased risk in cSCC [36]. The role of ILC3 and ILC2 in antitumor immunity may involve macrophage activation and eosinophil recruitment, respectively [32], but this has not yet been studied in cSCC or BCC.
This suggests that besides their antitumor response, NK cells and noncytotoxic ILC1 also play a dual role in cancer immunosurveillance and tumor establishment (Figure 1).
8. NKT Cells and γδ T Cells
NKT cells express an αβ T cell receptor that recognizes glycolipids bound to CD1d. NKT cells can have both immunosuppressive and stimulatory roles in the skin. In murine models, NKT cells were involved in the UV-induced immunosuppression and permitted outgrowth of UV-induced tumors and HPVE7 oncogene-driven skin tumors [37]. The immune suppressive effect was dependent on NKT cell-derived IFNy that likely stimulates IDO production by IFNy-responsive myeloid cells.
Intraepithelial lymphocytes comprise γδ T cells, a special subset of T cells lacking αβ T cell receptor and CD4 or CD8 coreceptor expression. Their role in skin cancer has not widely been studied. Studies in mice have shown that mice lacking γδ cells are more susceptible to skin carcinogenesis, which might involve γδ T cell-mediated tumor cell killing via NKG2D [38]. However, since γδ T cells are abundantly present in murine skin and only occasionally found in human skin, and the impact of these cells on human skin cancer development is less clear.
9. T Cell Responses
T cell responses are primed upon antigen presentation by activated dendritic cells in the lymph nodes. CD8+ T cells can exert direct antitumor activity by secreting Granzyme B and perforin (Figure 1). CD8+ T cells also secrete several cytokines, including TNFα and interferon-γ (IFNγ), which can activate other immune cells [39]. The role of CD8+ T cells in controlling cSCC was shown in CD8+ T cell knockout mice that developed more cSCC tumors upon UVB irradiation than wild type mice [40]. In a murine model of transplanted SCC that grow under immunosuppression by tacrolimus, it was found that tumor rejection upon tacrolimus withdrawal was dependent on the presence CD8+ T cells. This effect was mediated by IFNγ, since neutralizing IFNγ resulted in tumor progression and decreased survival [41]. However, within the TME, tumor-infiltrating CD8+ T cells often show an exhausted phenotype, characterized by decreased cytokine production and cytotoxic activity coinciding with the upregulation of inhibitory receptors PD-1 and CTLA-4, which decreases their antitumor activity [42][43].
In a study of BCC patients, patients with recurrent episodes had a significantly lower number of infiltrating CD8+ T cells and dendritic cells in the primary tumor than patients without recurrence, suggesting that their involvement in the chance of BCC recurrence [44]. Likewise, progressive head and neck cSCC displayed lower CD8+ and CD4+ T cell responses and more regulatory T cell (Treg) infiltration in primary tumors than in non-progressing cSCC [45]. CD4+ T-helper 1 (Th1) cells are considered to promote antitumor activity by producing cytokine interleukin-2 (IL-2) and IFNγ, which recruit and activate immune cells, and by stimulating the cytotoxic CD8+ T cell response. T helper 2 (Th2) cells express IL-4, IL-5 and IL-13, which have been associated with immune tolerance in transplantation. The gene expression analysis of cSSC and the surrounding skin in immunosuppressed, solid organ transplant recipients (SOTRs) showed that reduced CD4 T cell infiltration has a predominant Th2 expression profile, as compared to immuno-competent SSC patients. This reveals that the perineoplastic microenvironment in the adjacent non-tumor-bearing skin of SOTRs differs from immunocompetent individuals in suppressing Th1 responses and favoring Th2 polarization, thereby facilitating more SCC recurrence in SOTRs [46].
T-helper 17 (Th17) cells primarily express IL-17, an inflammatory cytokine that has been implicated in the proliferation of keratinocytes and the development of cSCC in the murine DMBA/TPA-induced cSCC model [47]. IL-17-mediated inflammation was also shown to be required for UVB-induced immunosuppression in the skin by inducing regulatory T cells (Tregs) and tolerogenic myeloid cells [48]. UV-induced skin damage decreases the antigen-presenting function of skin DC and causes Tregs to migrate to the skin, thereby dampening immunosurveillance and indirectly promoting tumor establishment. Tregs suppress the activity of other lymphocytes as immune regulation to limit the damaging effects of prolonged inflammation, whereas in cancer they shape the immunosuppressive TME [49]. Loser et al. (2007) [50] demonstrated that IL-10 knockout mice were protected from skin carcinogenesis after UV radiation. In these mice, the suppressive function of UV-induced Tregs was impaired, resulting in the enhanced antitumor activity of CD4+ and CD8+ T cell responses. In human cSCC tissues, more Tregs expressing immunosuppressive cytokines, including IL-10, and promoting T cell exhaustion were found in poorly differentiated G2–G3 stages than in well-differentiated G1 stages, indicating their correlation to tumor aggressiveness [51].
Th17 cells also secrete IL-23, which promotes chronic inflammation within the TME, which, in turn, results in increased tumorigenesis in the DMBA/TPA chemical murine skin carcinogenesis model [52]. In contrast to IL-12, which promotes antitumor immunity, IL-23 was shown to induce an inflammatory response, characterized by MMP9 expression and increased angiogenesis, while reducing CD8+ T cell infiltration. This effect could be reversed by the elimination of IL-23, resulting in protection against tumor development [52].
10. B Cell Responses
The role of B cells in cancer is not fully clear and sometimes even contradictory. B cells have been shown to both inhibit tumor growth by promoting NK cells and macrophages but also to stimulate tumors by secreting tumor growth factors. The underlying mechanisms that drive B cells to be either anti-tumor or pro-tumor are not fully understood [53]. In cancer, vascularized tertiary lymphoid structures (TLS) comprising B cells, DC and T cells can arise in non-lymphoid tissues. TLS density has been associated with improved response to immunotherapy in various cancer types [54]. In BCC, TLS were more abundantly found in the nodular BCC subtype than in BCC without a nodular component, and more mature TLS numbers were associated with increased tumor-infiltrating T cell levels and tumor cell killing [55]. Moreover, the presence of memory B cells was correlated with a response of BCC to immunotherapy and was negatively correlated to macrophage presence. The balance between the antitumor activity of B cells and the inhibition of B cells by macrophages determines immunotherapy responsiveness [19].
High numbers of memory B cells in the peritumoral stroma were also associated with the improved progression-free survival of cSCC patients [56]. In a murine multistage cSSC model of HPV16 mice, de Visser et al. (2005) [57] demonstrated that chronic inflammation promotes epithelial hyperproliferation, tissue remodeling and angiogenesis during premalignancy. The local deposition of immunoglobulin was observed during chronic skin inflammation even though B cells did not infiltrate the premalignant skin. Through the adoptive transfer of B cells or serum into immunodeficient HPV16/RAG-1 knock out mice, they showed that peripheral B cell activation and B cell-derived factors, including antibodies, play a major role in driving chronic inflammation associated with carcinogenesis [57]. B cells were also described to promote TNFα-dependent carcinogenesis in the murine DMBA/TPA-induced cSCC model. This effect was mediated by regulatory B cells that produce TNFα and IL-10 and suppress autoimmune Th1 responses during chronic inflammation. As a result, malignant cells arising during chronic inflammation are not effectively eliminated [58]. The pro-tumorigenic role of B cells during skin carcinogenesis was further demonstrated in a murine model of UV-induced cSCC [59]. B cell depletion prior to tumor establishment did not affect tumor development, but the absence of B cells in the established tumor phase diminished tumor growth and metastasis.
11. Discussion
The involvement of the immune system in the development and progression of cSCC, and to a lesser extent BCC, has been well established, even though the exact underlying mechanisms remain unclear. The UV-induced gene mutations, found in cSCC, commonly occur in sun-exposed skin, indicating that immunosurveillance is crucial to prevent these mutated cells from undergoing malignant transformation. Moreover, the increased mutational load in UV-exposed skin cells enhances the frequency of neoantigens that can be recognized by the adaptive immune system, thereby empowering antitumor immunity. Most knowledge of antitumor immunity in NMSC is based on cSCC, being the most prevalent tumor in SOTRs, and since the preclinical models of chemical or UV-induced skin cancer generally involve cSCC development. However, cSCC and BCC are tumors of different pathogeneses and clinical behaviors, which justifies further studies to explore immunity against BCC in more detail.
References
- Cives, M.; Mannavola, F.; Lospalluti, L.; Sergi, M.C.; Cazzato, G.; Filoni, E.; Cavallo, F.; Giudice, G.; Stucci, L.S.; Porta, C.; et al. Non-Melanoma Skin Cancers: Biological and Clinical Features. Int. J. Mol. Sci. 2020, 21, 5394.
- Coghill, A.E.; Johnson, L.G.; Berg, D.; Resler, A.J.; Leca, N.; Madeleine, M.M. Immunosuppressive Medications and Squamous Cell Skin Carcinoma: Nested Case-Control Study Within the Skin Cancer after Organ Transplant (SCOT) Cohort. Am. J. Transpl. 2016, 16, 565–573.
- Zhao, H.; Shu, G.; Wang, S. The risk of non-melanoma skin cancer in HIV-infected patients: New data and meta-analysis. Int. J. STD AIDS 2016, 27, 568–575.
- Yanofsky, V.R.; Mitsui, H.; Felsen, D.; Carucci, J.A. Understanding Dendritic Cells and Their Role in Cutaneous Carcinoma and Cancer Immunotherapy. Clin. Dev. Immunol. 2013, 2013, 624123.
- Bostanci, S.; Kocyigit, P.; Alp, A.; Erdem, C.; Gürgey, E. Treatment of Basal Cell Carcinoma Located in the Head and Neck Region with Intralesional Interferon α-2: Evaluation of Long-Term Follow-Up Results. Clin. Drug Investig. 2005, 25, 661–667.
- Kim, K.H.; Yavel, R.M.; Gross, V.L.; Brody, N. Intralesional interferon α-2b in the treatment of basal cell carcinoma and squamous cell carcinoma: Revisited. Dermatol. Surg. 2004, 30, 116–120.
- Clifford, J.L.; Walch, E.; Yang, X.; Xu, X.; Alberts, D.S.; Clayman, G.L.; El-Naggar, A.K.; Lotan, R.; Lippman, S.M. Suppression of Type I Interferon Signaling Proteins Is an Early Event in Squamous Skin Carcinogenesis. Clin. Cancer Res. 2002, 8, 2067–2072.
- Kim, Y.; He, Y.Y. Ultraviolet radiation-induced non-melanoma skin cancer: Regulation of DNA damage repair and inflammation. Genes Dis. 2014, 1, 188–198.
- Hatakeyama, M.; Fukunaga, A.; Washio, K.; Taguchi, K.; Oda, Y.; Ogura, K.; Nishigori, C. Anti-Inflammatory Role of Langerhans Cells and Apoptotic Keratinocytes in Ultraviolet-B-Induced Cutaneous Inflammation. J. Immunol. 2017, 199, 2937–2947.
- Ortner, D.; Tripp, C.H.; Komenda, K.; Dubrac, S.; Zelger, B.; Hermann, M.; Doppler, W.; Tymoszuk, P.Z.; Boon, L.; Clausen, B.E.; et al. Langerhans cells and NK cells cooperate in the inhibition of chemical skin carcinogenesis. Oncoimmunology 2017, 6, e1260215.
- Furio, L.; Berthier-Vergnes, O.; Ducarre, B.; Schmitt, D.; Peguet-Navarro, J. UVA radiation impairs phenotypic and functional maturation of human dermal dendritic cells. J. Investig. Dermatol. 2005, 125, 1032–1038.
- Bluth, M.J.; Zaba, L.C.; Moussai, D.; Suarez-Farinas, M.; Kaporis, H.; Fan, L.; Pierson, K.C.; White, T.R.; Pitts-Kiefer, A.; Fuentes-Duculan, J.; et al. Myeloid dendritic cells from human cutaneous squamous cell carcinoma are poor stimulators of T-cell proliferation. J. Investig. Dermatol. 2009, 129, 2451–2462.
- Georgescu, S.R.; Tampa, M.; Mitran, C.I.; Mitran, M.I.; Caruntu, C.; Caruntu, A.; Lupu, M.; Matei, C.; Constantin, C.; Neagu, M. Tumour Microenvironment in Skin Carcinogenesis. Adv. Exp. Med. Biol. 2020, 1226, 123–142.
- Jensen, T.O.; Schmidt, H.; Møller, H.J.; Høyer, M.; Maniecki, M.B.; Sjoegren, P.; Christensen, I.J.; Steiniche, T. Macrophage markers in serum and tumor have prognostic impact in American Joint Committee on Cancer stage I/II melanoma. J. Clin. Oncol. 2009, 27, 3330–3337.
- Tsutsui, S.; Yasuda, K.; Suzuki, K.; Tahara, K.; Higashi, H.; Era, S. Macrophage infiltration and its prognostic implications in breast cancer: The relationship with VEGF expression and microvessel density. Oncol. Rep. 2005, 14, 425–431.
- Chen, Y.; Song, Y.; Du, W.; Gong, L.; Chang, H.; Zou, Z. Tumor-associated macrophages: An accomplice in solid tumor progression. J. Biomed. Sci. 2019, 26, 78.
- Pettersen, J.S.; Fuentes-Duculan, J.; Suárez-Fariñas, M.; Pierson, K.C.; Pitts-Kiefer, A.; Fan, L.; Belkin, D.A.; Wang, C.Q.; Bhuvanendran, S.; Johnson-Huang, L.M.; et al. Tumor-associated macrophages in the cutaneous SCC microenvironment are heterogeneously activated. J. Investig. Dermatol. 2011, 131, 1322–1330.
- Moussai, D.; Mitsui, H.; Pettersen, J.S.; Pierson, K.C.; Shah, K.R.; Suárez-Fariñas, M.; Cardinale, I.R.; Bluth, M.J.; Krueger, J.G.; Carucci, J.A. The human cutaneous squamous cell carcinoma microenvironment is characterized by increased lymphatic density and enhanced expression of macrophage-derived VEGF-C. J. Investig. Dermatol. 2011, 131, 229–236.
- Dollinger, E.; Bergman, D.; Zhou, P.; Atwood, S.X.; Nie, Q. Divergent Resistance Mechanisms to Immunotherapy Explain Responses in Different Skin Cancers. Cancers 2020, 12, 2946.
- Saeidi, V.; Doudican, N.; Carucci, J.A. Understanding the squamous cell carcinoma immune microenvironment. Front. Immunol. 2023, 14, 1084873.
- Katoh, M. Genomic testing, tumor microenvironment and targeted therapy of Hedgehog-related human cancers. Clin. Sci. 2019, 133, 953–970.
- Weide, B.; Martens, A.; Zelba, H.; Stutz, C.; Derhovanessian, E.; Di Giacomo, A.M.; Maio, M.; Sucker, A.; Schilling, B.; Schadendorf, D.; et al. Myeloid-derived suppressor cells predict survival of patients with advanced melanoma: Comparison with regulatory T cells and NY-ESO-1- or melan-A-specific T cells. Clin. Cancer Res. 2014, 20, 1601–1609.
- Mao, Y.; Sarhan, D.; Steven, A.; Seliger, B.; Kiessling, R.; Lundqvist, A. Inhibition of tumor-derived prostaglandin-e2 blocks the induction of myeloid-derived suppressor cells and recovers natural killer cell activity. Clin. Cancer Res. 2014, 20, 4096–4106.
- Pan, P.Y.; Ma, G.; Weber, K.J.; Ozao-Choy, J.; Wang, G.; Yin, B.; Divino, C.M.; Chen, S.H. Immune stimulatory receptor CD40 is required for T-cell suppression and T regulatory cell activation mediated by myeloid-derived suppressor cells in cancer. Cancer Res. 2010, 70, 99–108.
- Bai, X.; Shan, F.; Qu, N.; Huang, H.; Handley, M.; Griffin, N.; Zhang, S.; Cao, X. Regulatory role of methionine enkephalin in myeloid-derived suppressor cells and macrophages in human cutaneous squamous cell carcinoma. Int. Immunopharmacol. 2021, 99, 107996.
- Seddon, A.; Hock, B.; Miller, A.; Frei, L.; Pearson, J.; McKenzie, J.; Simcock, J.; Currie, M. Cutaneous squamous cell carcinomas with markers of increased metastatic risk are associated with elevated numbers of neutrophils and/or granulocytic myeloid derived suppressor cells. J. Dermatol. Sci. 2016, 83, 124–130.
- Uribe-Querol, E.; Rosales, C. Neutrophils in Cancer: Two Sides of the Same Coin. J. Immunol. Res. 2015, 2015, 983698.
- Khou, S.; Popa, A.; Luci, C.; Bihl, F.; Meghraoui-Kheddar, A.; Bourdely, P.; Salavagione, E.; Cosson, E.; Rubod, A.; Cazareth, J.; et al. Tumor-Associated Neutrophils Dampen Adaptive Immunity and Promote Cutaneous Squamous Cell Carcinoma Development. Cancers 2020, 12, 1860.
- Hart, P.H.; Grimbaldeston, M.A.; Swift, G.J.; Jaksic, A.; Noonan, F.P.; Finlay-Jones, J.J. Dermal mast cells determine susceptibility to ultraviolet B-induced systemic suppression of contact hypersensitivity responses in mice. J. Exp. Med. 1998, 187, 2045–2053.
- Grimbaldeston, M.A.; Skov, L.; Baadsgaard, O.; Skov, B.G.; Marshman, G.; Finlay-Jones, J.J.; Hart, P.H. Communications: High dermal mast cell prevalence is a predisposing factor for basal cell carcinoma in humans. J. Investig. Dermatol. 2000, 115, 317–320.
- Wu, S.Y.; Fu, T.; Jiang, Y.Z.; Shao, Z.M. Natural killer cells in cancer biology and therapy. Mol. Cancer 2020, 19, 120.
- Wagner, M.; Koyasu, S. Cancer Immunoediting by Innate Lymphoid Cells. Trends Immunol. 2019, 40, 415–430.
- Luci, C.; Bihl, F.; Bourdely, P.; Khou, S.; Popa, A.; Meghraoui-Kheddar, A.; Vermeulen, O.; Elaldi, R.; Poissonnet, G.; Sudaka, A.; et al. Cutaneous Squamous Cell Carcinoma Development Is Associated with a Temporal Infiltration of ILC1 and NK Cells with Immune Dysfunctions. J. Investig. Dermatol. 2021, 141, 2369–2379.
- Amôr, N.G.; de Oliveira, C.E.; Gasparoto, T.H.; Vilas Boas, V.G.; Perri, G.; Kaneno, R.; Lara, V.S.; Garlet, G.P.; da Silva, J.S.; Martins, G.A.; et al. ST2/IL-33 signaling promotes malignant development of experimental squamous cell carcinoma by decreasing NK cells cytotoxicity and modulating the intratumoral cell infiltrate. Oncotarget 2018, 9, 30894–30904.
- Hope, C.M.; Troelnikov, A.; Hanf, W.; Jesudason, S.; Coates, P.T.; Heeger, P.S.; Carroll, R.P. Peripheral natural killer cell and allo-stimulated T-cell function in kidney transplant recipients associate with cancer risk and immunosuppression-related complications. Kidney Int. 2015, 88, 1374–1382.
- Devillier, R.; Chrétien, A.S.; Pagliardini, T.; Salem, N.; Blaise, D.; Olive, D. Mechanisms of NK cell dysfunction in the tumor microenvironment and current clinical approaches to harness NK cell potential for immunotherapy. J. Leukoc. Biol. 2021, 109, 1071–1088.
- McKee, S.J.; Mattarollo, S.R.; Leggatt, G.R. Immunosuppressive roles of natural killer T (NKT) cells in the skin. J. Leukoc. Biol. 2014, 96, 49–54.
- Girardi, M.; Oppenheim, D.E.; Steele, C.R.; Lewis, J.M.; Glusac, E.; Filler, R.; Hobby, P.; Sutton, B.; Tigelaar, R.E.; Hayday, A.C. Regulation of cutaneous malignancy by γδ T cells. Science 2001, 294, 605–609.
- Pardo, J.; Aguilo, J.I.; Anel, A.; Martin, P.; Joeckel, L.; Borner, C.; Wallich, R.; Müllbacher, A.; Froelich, C.J.; Simon, M.M. The biology of cytotoxic cell granule exocytosis pathway: Granzymes have evolved to induce cell death and inflammation. Microbes Infect. 2009, 11, 452–459.
- Nasti, T.H.; Iqbal, O.; Tamimi, I.A.; Geise, J.T.; Katiyar, S.K.; Yusuf, N. Differential roles of T-cell subsets in regulation of ultraviolet radiation induced cutaneous photocarcinogenesis. Photochem. Photobiol. 2011, 87, 387–398.
- Zeng, Z.; Veitch, M.; Kelly, G.A.; Tuong, Z.K.; Cruz, J.G.; Frazer, I.H.; Wells, J.W. IFN-γ Critically Enables the Intratumoural Infiltration of CXCR3+ CD8+ T Cells to Drive Squamous Cell Carcinoma Regression. Cancers 2021, 13, 2131.
- Linedale, R.; Schmidt, C.; King, B.T.; Ganko, A.G.; Simpson, F.; Panizza, B.J.; Leggatt, G.R. Elevated frequencies of CD8 T cells expressing PD-1, CTLA-4 and Tim-3 within tumour from perineural squamous cell carcinoma patients. PLoS ONE 2017, 12, e0175755.
- Sen, D.R.; Kaminski, J.; Barnitz, R.A.; Kurachi, M.; Gerdemann, U.; Yates, K.B.; Tsao, H.W.; Godec, J.; LaFleur, M.W.; Brown, F.D.; et al. The epigenetic landscape of T cell exhaustion. Science 2016, 354, 1165–1169.
- Beksaç, B.; İlter, N.; Erdem, Ö.; Çakmak, P.; Çenetoğlu, S.; Yapar, D. Sparsity of dendritic cells and cytotoxic T cells in tumor microenvironment may lead to recurrence in basal cell carcinoma. Int. J. Dermatol. 2020, 59, 1258–1263.
- Ferguson, A.L.; Sharman, A.R.; Allen, R.O.; Ye, T.; Lee, J.H.; Low, T.H.; Ch’ng, S.; Palme, C.E.; Ashford, B.; Ranson, M.; et al. High-Dimensional and Spatial Analysis Reveals Immune Landscape-Dependent Progression in Cutaneous Squamous Cell Carcinoma. Clin. Cancer Res. 2022, 28, 4677–4688.
- Kosmidis, M.; Dziunycz, P.; Suarez-Farinas, M.; Muhleisen, B.; Scharer, L.; Lauchli, S.; Hafner, J.; French, L.E.; Schmidt-Weber, C.; Carucci, J.A.; et al. Immunosuppression affects CD4+ mRNA expression and induces Th2 dominance in the microenvironment of cutaneous squamous cell carcinoma in organ transplant recipients. J. Immunother. 2010, 33, 538–546.
- Wu, L.; Chen, X.; Zhao, J.; Martin, B.; Zepp, J.A.; Ko, J.S.; Gu, C.; Cai, G.; Ouyang, W.; Sen, G.; et al. A novel IL-17 signaling pathway controlling keratinocyte proliferation and tumorigenesis via the TRAF4-ERK5 axis. J. Exp. Med. 2015, 212, 1571–1587.
- Li, H.; Prasad, R.; Katiyar, S.K.; Yusuf, N.; Elmets, C.A.; Xu, H. Interleukin-17 mediated inflammatory responses are required for ultraviolet radiation-induced immune suppression. Photochem. Photobiol. 2015, 91, 235–241.
- Tanaka, A.; Sakaguchi, S. Targeting Treg cells in cancer immunotherapy. Eur. J. Immunol. 2019, 49, 1140–1146.
- Loser, K.; Apelt, J.; Voskort, M.; Mohaupt, M.; Balkow, S.; Schwarz, T.; Grabbe, S.; Beissert, S. IL-10 controls ultraviolet-induced carcinogenesis in mice. J. Immunol. 2007, 179, 365–371.
- Azzimonti, B.; Zavattaro, E.; Provasi, M.; Vidali, M.; Conca, A.; Catalano, E.; Rimondini, L.; Colombo, E.; Valente, G. Intense Foxp3+ CD25+ regulatory T-cell infiltration is associated with high-grade cutaneous squamous cell carcinoma and counterbalanced by CD8+/Foxp3+ CD25+ ratio. Br. J. Dermatol. 2015, 172, 64–73.
- Langowski, J.L.; Zhang, X.; Wu, L.; Mattson, J.D.; Chen, T.; Smith, K.; Basham, B.; McClanahan, T.; Kastelein, R.A.; Oft, M. IL-23 promotes tumour incidence and growth. Nature 2006, 442, 461–465.
- Yuen, G.J.; Demissie, E.; Pillai, S. B lymphocytes and cancer: A love-hate relationship. Trends Cancer 2016, 2, 747–757.
- Sautes-Fridman, C.; Petitprez, F.; Calderaro, J.; Fridman, W.H. Tertiary lymphoid structures in the era of cancer immunotherapy. Nat. Rev. Cancer 2019, 19, 307–325.
- Byers, C.; Gill, M.; Kurtansky, N.R.; Alessi-Fox, C.; Harman, M.; Cordova, M.; Gonzalez, S.; Guitera, P.; Rotemberg, V.; Marghoob, A.; et al. Tertiary lymphoid structures accompanied by fibrillary matrix morphology impact anti-tumor immunity in basal cell carcinomas. Front. Med. 2022, 9, 981074.
- Thai, A.A.; Young, R.J.; Bressel, M.; Angel, C.; McDowell, L.; Tiong, A.; Bucknell, N.W.; Fellowes, A.; Xu, H.; Trigos, A.; et al. Comprehensive profiling identifies tumour and immune-microenvironmental differences in clinical subsets of cutaneous squamous cell carcinoma. Br. J. Dermatol. 2023.
- de Visser, K.E.; Korets, L.V.; Coussens, L.M. De novo carcinogenesis promoted by chronic inflammation is B lymphocyte dependent. Cancer Cell 2005, 7, 411–423.
- Schioppa, T.; Moore, R.; Thompson, R.G.; Rosser, E.C.; Kulbe, H.; Nedospasov, S.; Mauri, C.; Coussens, L.M.; Balkwill, F.R. B regulatory cells and the tumor-promoting actions of TNF-α during squamous carcinogenesis. Proc. Natl. Acad. Sci. USA 2011, 108, 10662–10667.
- Kok, L.F.; Ferguson, A.L.; Marshall, J.E.; Tse, B.C.Y.; Halliday, G.M.; Byrne, S.N. B Cell-Targeted Immunotherapy Limits Tumor Growth, Enhances Survival, and Prevents Lymph Node Metastasis of UV-Induced Keratinocyte Cancers in Mice. J. Investig. Dermatol. 2020, 140, 1459–1463.
More
Information
Subjects:
Dermatology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
508
Revisions:
2 times
(View History)
Update Date:
31 Oct 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




