Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Lilia Sabantina | -- | 3883 | 2023-10-25 18:56:16 | | | |
| 2 | Rita Xu | Meta information modification | 3883 | 2023-10-26 03:18:11 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Mamun, A.; Kiari, M.; Sabantina, L. Electrospun Porous Carbon Nanofiber Mats. Encyclopedia. Available online: https://encyclopedia.pub/entry/50799 (accessed on 08 February 2026).
Mamun A, Kiari M, Sabantina L. Electrospun Porous Carbon Nanofiber Mats. Encyclopedia. Available at: https://encyclopedia.pub/entry/50799. Accessed February 08, 2026.
Mamun, Al, Mohamed Kiari, Lilia Sabantina. "Electrospun Porous Carbon Nanofiber Mats" Encyclopedia, https://encyclopedia.pub/entry/50799 (accessed February 08, 2026).
Mamun, A., Kiari, M., & Sabantina, L. (2023, October 25). Electrospun Porous Carbon Nanofiber Mats. In Encyclopedia. https://encyclopedia.pub/entry/50799
Mamun, Al, et al. "Electrospun Porous Carbon Nanofiber Mats." Encyclopedia. Web. 25 October, 2023.
Copy Citation
Electrospun porous carbon nanofiber mats have excellent properties, such as a large surface area, tunable porosity, and excellent electrical conductivity, and have attracted great attention in energy storage and power generation applications. Moreover, due to their exceptional properties, they can be used in dye-sensitized solar cells (DSSCs), membrane electrodes for fuel cells, catalytic applications such as oxygen reduction reactions (ORRs), hydrogen evolution reactions (HERs), and oxygen evolution reactions (OERs), and sensing applications such as biosensors, electrochemical sensors, and chemical sensors, providing a comprehensive insight into energy storage development and applications.
electrospinning
porous carbon nanofibers
energy storage
1. Introduction
The escalating energy demand, driven by global population growth and improved living standards, poses a paramount challenge. This strain on conventional fossil fuel reserves raises concerns about energy security and geopolitical tensions. Furthermore, fossil fuel combustion compounds environmental issues, emitting pollutants and greenhouse gases and contributing to climate change. Addressing these challenges hinges on research and innovation. Progress in renewable energy technologies, energy storage, smart grids, and energy-efficient materials can foster a more sustainable energy landscape. The development of efficient energy storage and generation technologies is of paramount importance in meeting our society’s growing energy needs while reducing environmental impact [1][2][3]. In this context, electrospun nanofibers play an important role as they offer promising solutions to these challenges due to their unique structure and chemical properties. Due to the exceptional physical and chemical properties of electrospun nanofibers, their potential in this field is considerable. These properties include an intricate network of nano- and microporous structures, a high surface-to-volume ratio, tunable porosity, and a variety of surface functionalities and electrical conductivities. These nanofibers can be fabricated from a wide range of polymers, including ceramics. In addition, these nanofiber mats can be electrospun from bio- or natural polymer solutions. After that, they can be easily converted into carbon nanofiber mats through stabilization and carbonization processes [4][5][6]. For example, in the study conducted by Jeon et al., they successfully produced porous carbon nanofibers with excellent energy storage capabilities using a single precursor polymer, polyimides containing hexafluoroisopropylidene diphthalic anhydride and 2-2′-bis(trifluoromethyl)benzidine (6FDA-TFMB), while bypassing the need for pore-forming substances. The process involved the synthesis of 6FDA-TFMB, followed by electrospinning and subsequent thermal treatment, resulting in binder-free carbon nanofiber electrodes for use in electrochemical double-layer capacitors [7].
2. Energy Storage and Power Generation Applications
2.1. Dye-Sensitized Solar Cells (DSSCs)
Incorporating electrospun porous carbon nanofibers into DSSCs can improve the efficiency, light absorption, charge transport, and overall performance of these solar cells. Their unique properties make them promising candidates for improving the performance of DSSCs and advancing the field of renewable energy technologies [8]. Here are some specific applications of electrospun carbon nanofibers in DSSCs, such as counter electrodes, conducting frameworks, dye absorption enhancement, light scattering layers, charge collection networks, electron transport media, etc. Electrospun carbon nanofibers are ideally suited as counter electrodes due to their high electrical conductivity and large surface area. The cross-linked nanofiber structure provides efficient charge transfer and enhances catalytic activity [9]. Figure 1 shows the CNFs decorated with Co2S4 and their performance in a (DSSC). The counter electrode for DSSCs with SiCo2O4 structures resembles petals grown on CNFs. CNFs promote the growth of petal-like miniature MoS2 structures for use in solar systems. CNFs with Ni2P and Pt nanoparticles can be used as counter electrodes in (DSSCs).
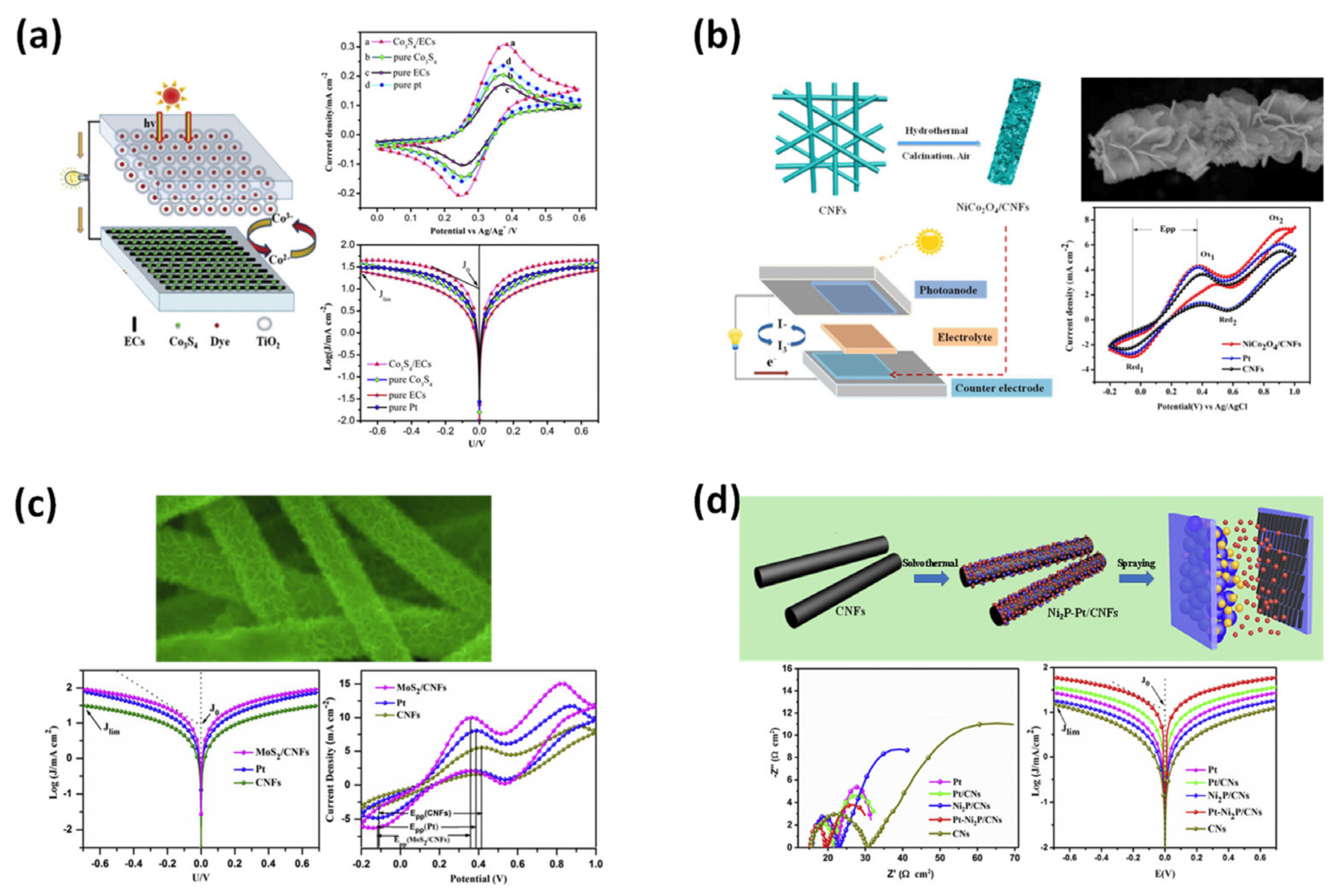
Figure 1. (a) CNFs decorated with Co2S4 and the performance in DSSC device; (b) flower-petal−like SiCo2O4 grown on CNFs used as counter electrode for DSS; (c) CNFs grow MoS2 mini−sized petals for solar systems; (d) CNFs decorated with Ni2P and Pt nanoparticles for use as the counter electrode in DSSCs.
The nanofiber network provides a three-dimensional structure that enhances dye absorption and electron transport, leading to higher device performance [10]. Carbon nanofibers can be functionalized with dyes or dye sensitizers to improve light absorption in DSSCs. The large surface area of electrospun carbon nanofibers enables effective immobilization of dye molecules, resulting in increased light collection efficiency for the device [11]. The high electrical conductivity of the carbon nanofibers facilitates efficient electron transport from the photoanode to the counter electrode, minimizing electron recombination losses and improving the overall efficiency of the device [12]. The random arrangement of the nanofibers helps to scatter and trap light, increasing the optical path length and improving light absorption in the photoactive layer of the solar cell. This network helps in the rapid collection and transport of photogenerated electrons, minimizing charge recombination and improving the efficiency of the device [13]. Sun et al. have fabricated MoS2 carbon nanofiber composites using the electrospinning technique from ammonium thiomolybdate (VI) and polyvinylpyrrolidone. They have shown that the carbon nanocomposites improve the DSSCefficiency by 5.7% when used as counter electrodes [14]. Joschi et al. have investigated electrospun carbon nanofibers as counter electrodes, finding that they have low charge-transfer resistance, large capacitance, and fast reaction rates for triiodide reduction as electrocatalysts and can be provided a cost-effective alternative to platinum (Pt) for triiodide reduction in (DSSCs), which was investigated by using electrochemical impedance spectroscopy and cyclic voltammetry [15]. López-Covarrubias et al. described that the use of electrospinning technology in combination with the use of metal compounds could provide a great approach for the development of DSSCs with superior efficiency and high stability and durability [16]. Zhao et al. successfully synthesized and investigated carbon nanofibers supported by Pt and Ni2P nanoparticles as counter electrodes for DSSCs for the first time. DSSCs using Pt-Ni2P carbon nanofibers as counter electrodes show excellent photovoltaic performance (efficiency of 9.11%), which is much higher than the conventional Pt carbon nanofiber counter electrode (efficiency of 8.35%) due to the collective effect of high electrical conductivity of carbon nanofibers [17]. Aboagye et al. electrospun polyacrylonitrile (PAN) nanofibers and subsequently demonstrated controllable growth of Pt nanoparticles on the surface of the obtained carbon nanofibers by redox reaction. The hierarchical carbon nanofibers with Pt nanoparticles on the surface were then used as a low-cost counter electrode in DSSCs by stabilizing and carbonizing the carbon nanofibers with Pt nanoparticles on the surface. Compared with the conventional counter electrode, the counter electrode fabricated from carbon nanocomposites exhibited a higher open circuit voltage [18].
While electrospun carbon nanofibers hold promise as counter electrodes in DSSCs due to their low cost, versatility, and sustainability, addressing challenges related to electrical conductivity, catalytic activity, durability, material purity, scalability, cost, and compatibility with tandem devices is crucial for their widespread adoption in commercial DSSC applications.
2.2. Fuel Cell Membrane Electrodes
Electrospun porous carbon nanofibers can improve the electrode performance of fuel cell membrane electrodes, increase reactant transport, and optimize the overall efficiency of fuel cell systems. These advances contribute to the development of more efficient and sustainable energy conversion technologies [19]. In addition, electrospun porous carbon nanofibers have been shown to have significant potential for membrane electrode applications, including proton-exchange membrane fuel cells and gas diffusion electrodes in fuel cells [20]. Figure 2 shows a schematic representation of Sm0.5Sr0.5CoO3−δ (SSC) and Gd0.2Ce0.8O1.9 (GDC) electrode/electrolyte nanofibers with increased hetero-interfaces, decreased grain size, and expanded unit cell volume.
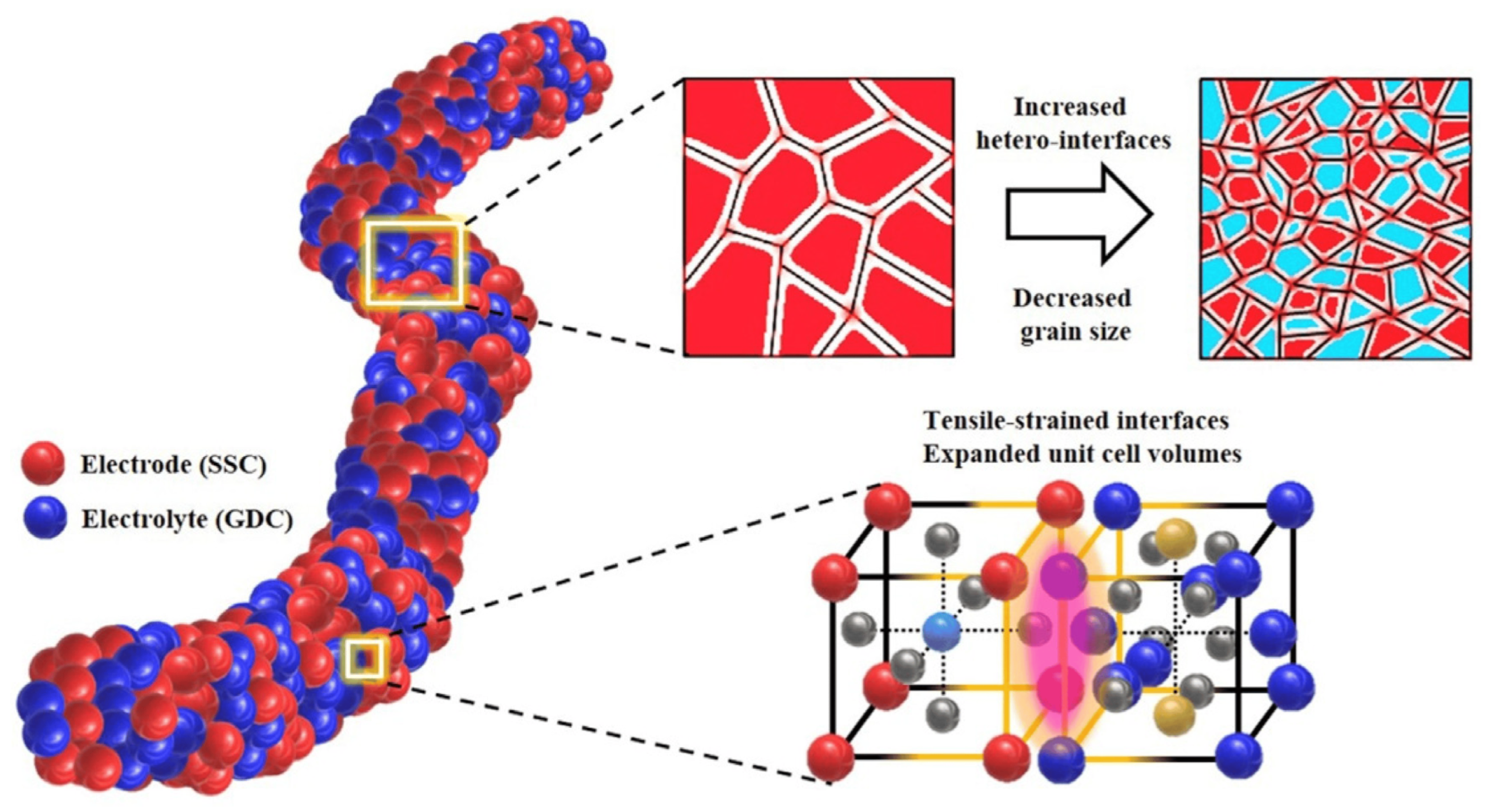
Figure 2. Schematic of electrode/electrolyte nanofiber from Sm0.5Sr0.5CoO3−δ (SSC) and Gd0.2Ce0.8O1.9 (GDC) with increased hetero-interfaces, decreased grain size, and expanded unit cell volumes.
On the one hand, the interconnected porous structure of nanofibers enables efficient gas diffusion and transport and provides uniform distribution of reactants and products within the fuel cell [21]. Electrospun carbon nanofibers can be integrated into the electrodes of proton-exchange membrane fuel cells (PEMFCs). The porous nature of the nanofibers facilitates proton transport, which ensures efficient ion exchange through the fuel cell membrane and promotes high-performance fuel cell operation. Waldrop et al. investigated electrospun materials that are of great interest to the energy sector, especially for proton-exchange membrane fuel cells, due to their tunability and durability. Conventional PEMFC electrodes produced by methods such as spraying or coating are unsuitable for large-scale use. Electrospun fiber materials are a promising alternative because they carry catalysts and serve directly as electrodes. This approach improves PEMFC durability and performance with lower catalyst loading, which is critical for the commercialization of PEMFC electric vehicles [22]. Delikaya et al. obtained porous carbon felt structures by phase separation in the shell followed by carbonization treatment and investigated in fuel cell tests. Full cell tests (0.6 mg Pt cm−2) show a 21% increase in power density normalized to platinum content compared to the spray-coated reference (1 mg Pt cm−2) [23]. Iskandarani et al. developed new materials and strategies to improve the performance of PEM fuel cells at low humidity, and platinum loading is becoming increasingly important. In this study, the fabrication of electrospun sulfonated silica (S-SiO2) as a flexible, free-standing, and highly effective novel cathode structure based on poly(vinylidene fluoride-co-trifluoroethylene) for PEM fuel cells was presented [24]. Yusoff and Shaari fabricated nanofibers using electrospinning technology and provided an overview of the electrospinning process by explaining the operating principle and parameters that specifically affect the fabrication of electrospun nanofiber membranes and fiber materials as electrochemical catalysts in fuel cell applications [25]. The ethanolamine (MEA)s with Nafion in salt form (Na+, Li+, or Cs+ as sulfonic acid counterion) were prepared by Waldrop et al. They showed little or no change in maximum power density when the relative humidity (RH) of the feed gas was lowered from 100% to 40%, while the eMEAwith H+-Nafion/peroxyacetic acid (PAA) fibers showed a 33% power loss at 40% RH. The higher power densities at low humidity were attributed to capillary condensation of water in porous fibers with a pore diameter of 1.25 nm or less [26].
2.3. Catalysts
The unique properties of electrospun porous carbon nanofibers, such as their large surface area, excellent electrical conductivity, and tunable porosity, make them attractive candidates for catalytic applications [27]. For example, electrospun porous carbon nanofibers can be used as catalysts in various electrochemical reactions such as oxygen reduction reactions (ORRs), hydrogen evolution reactions (HERs), and oxygen evolution reactions (OERs) [28]. The high electrical conductivity and large surface area of nanofibers facilitate efficient charge transfer and enhance catalytic activity. Carbon nanofibers can be functionalized with metal nanoparticles such as platinum, palladium, or other transition metals to enhance the catalytic activity for fuel oxidation and oxygen reduction reactions and create heterogeneous catalysts for various chemical reactions that provide a high number of active sites for catalytic reactions, resulting in enhanced reaction rates and selectivity leading to improved fuel cell performance [29]. Functionalized nanofibers can promote the activation of persulfate or other oxidants, leading to the efficient degradation of pollutants in water or air [30]. As catalysts in energy conversion and storage devices, such as supercapacitors or lithium-ion batteries, porous carbon nanofibers can improve electrochemical performance by improving charge transfer kinetics and increasing electroactive surface area [31]. Also, as catalysts in various chemical synthesis reactions, including organic transformations, hydrogenations, oxidations, and coupling reactions, the large surface area and catalytic activity of nanofibers facilitate efficient reaction pathways and promote the formation of the desired product in chemical industries [32]. Due to their unique properties, electrospun porous carbon nanofibers are attractive candidates for a variety of catalytic processes, offering potential advances in areas of energy generation and storage applications [33]. Guerrero-Pérez et al. discussed the use of carbon nanofibers in catalysis because catalytic support is needed for electrocatalytic applications required for the development of fuel cells, water treatment systems, and batteries [34]. In the Liu et al. study, base carbon catalysts are investigated as the most promising alternatives to modern Pt/C catalysts for the oxygen reduction reaction. The Fe2C/CNF catalyst shows uniform dispersion and narrow size distribution of Fe2C nanoparticles embedded in CNFs. The obtained catalyst exhibits a positive onset potential (0.87 V vs. RHE) and a large kinetic current density (1.9 mA cm−2) and almost follows the effective four-electron pathway, suggesting excellent electrocatalytic activity for ORRs in 0.1 M KOH solution. Moreover, its stability is better than that of the commercial Pt/C catalyst, which can be attributed to the strong binding force between Fe2C particles and CNFs. This strategy opens new avenues for the design and efficient production of promising electrocatalysts for ORRs [35]. Woo et al. integrated the widely used Ni2Fe catalyst with high oxygen evolution activity (OER) into carbon nanofibers with Ni2Fe. To evaluate their overall electrochemical properties for water splitting, Co-CeO2 carbon nanofibers and Ni2Fe carbon nanofibers were used as HER and OER electrocatalysts in an alkaline electrolyzer. Due to the conformal incorporation of the nanoparticles into the carbon nanofiber, the electrocatalysts exhibit considerable long-term stability of over 70 h of total water splitting [36]. Ponomarev et al. electrospun nanofibers in formic acid with further treatment in the H2 stream at 500 °C, which resulted in intense sintering of the platinum particles, forming conglomerates of 50 nm in size, but the individual particles were still less than 10 nm in size. The electrochemically active surface area of the Pt/C catalyst was measured by electrochemical hydrogen adsorption/desorption measurements in 0.5 M H2SO4 [37].
2.4. Sensors Applications
The sensor applications of electrospun carbon nanofibers are diverse. Their unique properties make them versatile and suitable for various sensing applications in fields such as environmental monitoring, healthcare, industrial sensing, and many others. Electrospun carbon nanofibers have various sensor applications due to their unique properties, such as high surface area, excellent electrical conductivity, mechanical strength, and chemical stability [38]. Figure 3 depicts the primary sensing mechanisms employed by nanofiber-based sensors. It showcases analyte-specific recognition (ASR), where fiber colors match the functional group color of the bound analyte, highlighting specificity. In the center is electrocatalysis (EC), where nanofibers facilitate electron flow for catalyzing reduction or oxidation reactions. To the right, adsorption (AD) is illustrated, where molecules adhere to nanofiber surfaces due to their high porosity and extensive specific surface area.
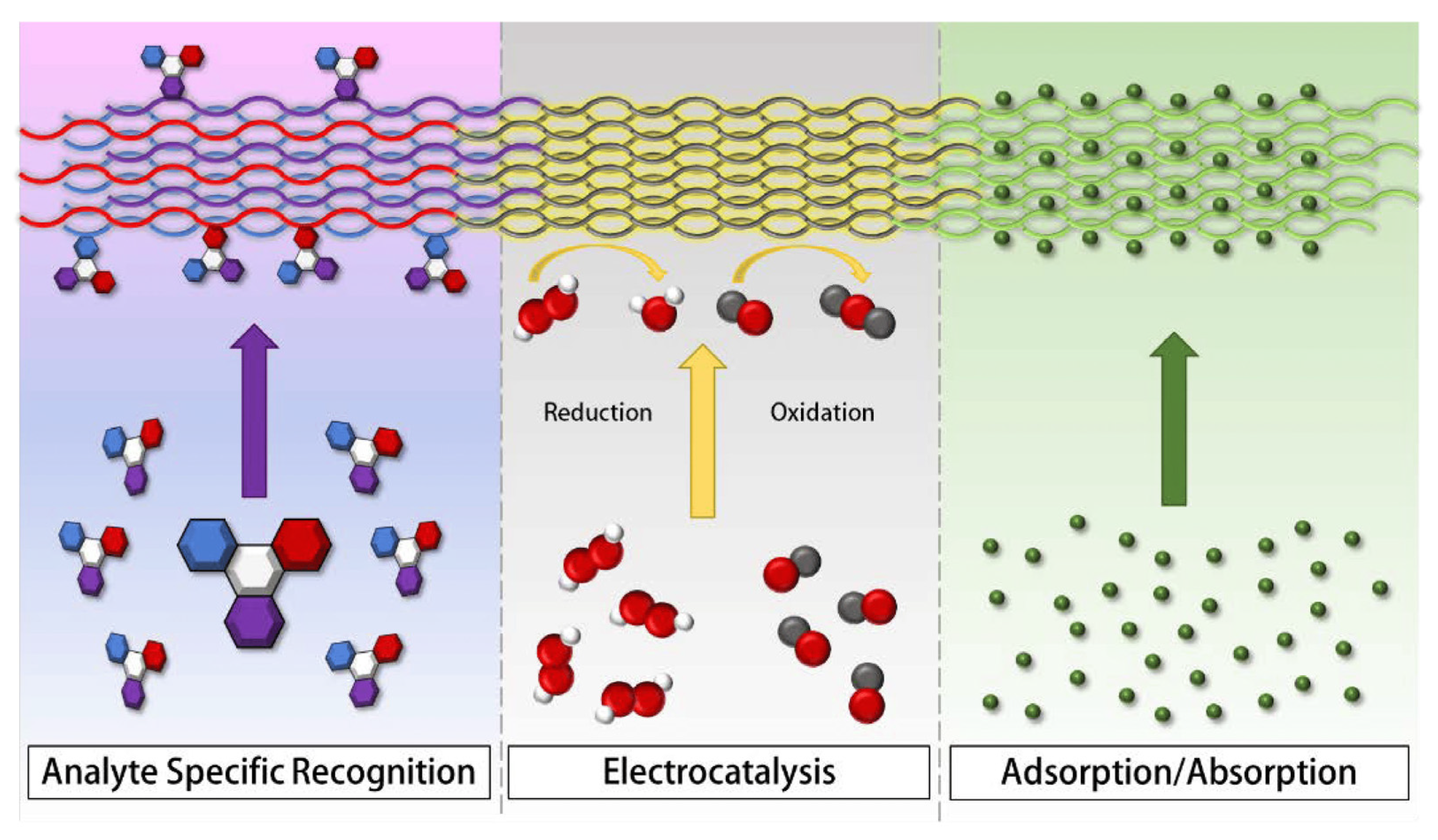
Figure 3. Chart depicting the main detection mechanisms employed by nanofiber-based sensors. On the left: analyte-specific recognition (ASR)—the fibers’ colors correspond to the functional group color of the analyte they adhere to, highlighting specificity. In the center: electrocatalysis (EC)—nanofibers facilitate electron flow, catalyzing reduction or oxidation reactions. On the right: adsorption (AD)—molecules are captured on the nanofiber surfaces, facilitated by their high porosity and extensive specific surface area.
2.5. Biosensors
Electrospun carbon nanofibers can be used as biosensors due to their high sensitivity, fast response, and biocompatibility, making them promising candidates for various biomedical and diagnostic applications [39]. Electrospun carbon nanofibers can be functionalized with specific biomolecules such as enzymes or antibodies, glucose, DNA, proteins, or other biomarkers by measuring changes in electrical conductivity or other electrical properties to create biosensors [40]. These biosensors can detect biological analytes. For example, electrospun carbon nanofibers can be functionalized with the enzyme glucose oxidase to produce glucose sensors. Sensors based on electrospun carbon nanofibers can detect and quantify glucose levels in biological samples such as blood or saliva by measuring the changes in electrical conductivity that result from the enzymatic reaction [41]. In addition, electrospun carbon nanofibers functionalized with single-stranded DNA probes can be used as DNA sensors. The complementary DNA strands in the sample hybridize with the immobilized DNA probes on the nanofibers, resulting in changes in electrical properties that can be measured. This approach enables the detection and identification of specific DNA sequences [42]. Moreover, electrospun carbon nanofibers can be functionalized with specific antibodies or proteins to produce protein sensors. The antibodies immobilized on the nanofibers selectively bind to the target protein analytes, resulting in changes in electrical conductivity that can be measured to detect and quantify proteins [43]. In the same way, electrospun carbon nanofibers can be modified with various enzymes to produce enzymatic biosensors. The catalytic activity of the enzymes on the nanofiber surface triggers specific chemical reactions that lead to measurable changes in electrical properties. Enzymatic biosensors can be used for the detection of various analytes, including metabolites, toxins, or environmental pollutants [44]. Hu et al. have described a polarized poly(vinylidene fluoride-trifluoroethylene)/barium titanate nanofiber mat as the sensing layer, a polyimide film with arrays of circular cavities as the substrate, and a poly(methyl methacrylate) column as the cilium. This bioinspired flexible lateral line sensor with hydrodynamic sensing capability shows promising applications in underwater robotics for real-time flow analysis [45]. Sengupta et al. reported a cilia-inspired high aspect ratio titanium column on an electrospun carbon nanofiber sensing membrane. Calibration experiments on flow sensing demonstrate the feasibility of the proposed method to develop low-cost yet sensitive flow sensors that will be useful for applications such as precise flow monitoring in microfluidic devices, accurate monitoring of air/oxygen delivery in hypoxic patients, and other biomedical devices for monitoring intravenous infusions or urine flow [46]. Kim et al. presented electrospun polyvinylidene fluoride–graphene oxide hybrid nanofibers in aligned mode, which were tested for their multiple efficacies as a locomotion detector, bio-e-skin, smart chair, etc., using the proposed system [47]. Devarajet al. presented that the actual M13 bacteriophage-based multilayer biofilm with porous nanostructures with a diameter of about 150–500 nm and a depth of about 15–30 nm agrees well with the experimental and simulation results, showing that the fabricated multilayer biofilms can be used in sensing, filtering, plasmonics, and biomimetics [48]. Zheng et al. presented a new class of strain sensors fabricated from cellulose nanofibers and graphene in a poly(vinyl alcohol)borax hydrogel with promising applications in the field of wearable sensing [49].
2.6. Electrochemical and Chemical Sensors
Electrospun carbon nanofibers find extensive applications in the development of electrochemical and chemical sensors. Their unique properties, such as high surface area, excellent electrical conductivity, and chemical stability, make them suitable for various sensing applications [50].
Electrospun carbon nanofibers can be used as electrode materials in electrochemical sensors. They provide a large surface area for efficient electrochemical reactions and electron transfer [51]. Due to their specific properties and porous structure, they can be used to develop pH sensors, gas sensors, biosensors, etc. For example, electrospun carbon nanofibers can be used as pH sensors by measuring the changes in electrochemical potential resulting from the fluctuations in proton concentration [52]. Functionalization of the nanofibers with pH-sensitive materials increases their pH-sensing capability. In the same way, electrospun carbon nanofibers can be used as electrode materials for gas sensors. Adsorption of gas molecules on the nanofiber surface changes the electrochemical properties and enables the detection and quantification of certain gases [53].
Electrospun carbon nanofibers can be used for chemical sensing applications by detecting changes in electrical conductivity or other electrical properties when exposed to certain chemicals [54]. Examples include volatile organic compound sensors, heavy metal ions, environmental sensors, etc. [55]. Electrospun carbon nanofibers can detect and measure volatile organic compounds commonly found in environmental pollutants or in industry. Adsorption of volatile organic compounds on the surface of nanofibers changes the conductivity and enables their detection and quantification. In addition, the functionalization of carbon nanofibers with specific ligands or chelating agents enables selective detection and quantification of heavy metal ions. The binding of metal ions to the functionalized nanofiber surface leads to changes in electrical conductivity or other electrical properties [56]. In the same way, electrospun carbon nanofibers can be used for monitoring and detection of various environmental pollutants, such as water or air pollutants. The high sensitivity and selectivity of nanofibers enable the detection and quantification of certain chemical analytes [57]. The applications of electrospun carbon nanofibers offer enhanced value in the development of electrochemical and chemical sensors for a wide range of applications, including environmental monitoring, industrial sensing, and biomedical diagnostics [58]. Rashid et al. fabricated silver nanofibers. These silver nanofibers were used in sensors to measure relative humidity (RH), ammonia (NH3), and temperature. The sensor was of the resistive type and exhibited 4.3 kΩ for the relative humidity (RH%) range of 30–90%, 400 kΩ for NH3 (40,000 ppm), and 5 MΩ for temperature detection (69 °C). The durability and speed of the sensor were verified by repeat, response, and recovery tests of the sensor in a humidity and gas chamber [59]. Yoo et al. have fabricated mesoporous electrospun nanofibers by electrospinning with TiO2, which exhibit the superior chemical and electrical properties of TiO2 and are considered suitable materials for various applications, such as photoelectrodes, photocatalysts, and semiconductor gas sensors [60]. Mehrabi et al. have fabricated nanofibers based on tin dioxide and poly(ethylene oxide) by electrospinning and improved them by calcination and gold doping. The electrospun composite nanofibers were investigated with different doping thicknesses and different calcination temperatures to determine the optimal fabrication parameters leading to high sensitivity [61]. Liu et al. fabricated nanofibers based on oriented polypyrrole (PPy)-coated polyacrylonitrile nanofiber yarn using an electrospinning technique. The electrical responses of the gas sensor based on the PPy-PAN nanofiber yarn to ammonia were studied at room temperature, and the response time was less than 1 s. The above excellent sensor characteristics offer good application potential in the field of ammonia sensors [62].
2.7. Batteries
It is imperative to create energy storage devices that can store electrical energy [63]. Due to its effective storage and distribution of electrical energy, secondary batteries have emerged as one of the most well-known and quickly growing energy storage technologies. There are few publications concerning the creation of porous CNFs and their use as anode materials for rechargeable lithium-ion batteries (LIBs), despite the fact that several possible uses have been envisioned [64][65][66]. Carbon nanofibers are the perfect one-dimensional conductive additives for electrodes due to their high electrical conductivity and great mechanical stability [67]. By electrospinning a bicomponent polymer solution, followed by thermal processing in various atmospheres, porous carbon nanofibers were created. When utilized as anodes for rechargeable lithium-ion batteries, these porous carbon nanofibers’ distinctive structure led to outstanding electrochemical performance, including high reversible capacity and strong cycle stability [68]. Lithium-ion batteries are the most popular rechargeable batteries because of their numerous benefits, including high energy density, safety, lengthy lifespan, and environmental friendliness [69][70]. LIBs are crucial power sources for power tools and contemporary electronics, such as computers and cellphones, and are increasingly employed in drones, electric vehicles, and hybrid electric vehicles [71][72][73][74][75]. The specific energy and power of LIBs, which are essentially constrained by the voltage and cathode capacity, are two of their most crucial properties. The majority of batteries on the market today use complex metal oxides or LiCoO2 cathodes [76], which have a variety of drawbacks, including safety concerns. Therefore, finding novel materials with great dependability is very crucial. Zhang et al. extensively investigated the use of MnO2 as a cathode material for zinc ion batteries, emphasizing its exceptional electrochemical capabilities. They also investigated the promise of the plasma-induced method for fabricating advanced metal oxide electrode materials optimized for high-performance aqueous zinc ion batteries. In addition, the study addressed the intricacies of plasma-induced ε-MnO2 in the field of aqueous zinc ion batteries and shed light on the mechanisms underlying the dissolution and deposition processes of this material [77]. Han et al. explained the process of integrating zinc into nanostructured δ-MnO2 in the context of non-aqueous rechargeable zinc metal batteries [78].
2.8. Supercapacitors
A supercapacitor is an electrochemical energy storage device with high power capabilities [79], quick charge propagation and charge–discharge operations (in seconds), extended cycle life (greater than 100,000 cycles), low maintenance requirements, and little self-discharging [80][81]. Supercapacitors show potential for various applications due to their advantages of being environmentally friendly, high safety, and ability to operate in a wide temperature range with a nearly infinitely long cycling life. Potential applications include portable electronics (cell phones), memory backup systems, and hybrid cars, where incredibly fast charging is a useful feature [82]. They can be used as load-levelers (backup power for memory, microcomputers, clocks, system boards, etc.) and uninterruptible power supplies (backup supplies intended to defend against power disruption). Due to the mentioned advantages of carbon nanofibers, they are receiving more and more interest as electrode materials for use in supercapacitors [83].
References
- Moriarty, P.; Honnery, D. Review: Renewable Energy in an Increasingly Uncertain Future. Appl. Sci. 2023, 13, 388.
- Makešová, M.; Valentová, M. The Concept of Multiple Impacts of Renewable Energy Sources: A Critical Review. Energies 2021, 14, 3183.
- Łukasiewicz, K.; Pietrzak, P.; Kraciuk, J.; Kacperska, E.; Cieciora, M. Sustainable Energy Development—A Systematic Literature Review. Energies 2022, 15, 8284.
- Dubal, S.; Chavan, S.; Jadhav, P.; Kadam, S.; Dhotre, S. Effects of stabilization on structures and properties of Electrospun Polyacrylonitrile based carbon nanofibers as a binder free electrode for supercapacitor application. Mater. Today Proc. 2023, 72, 2841–2845.
- Hussain, A.; Bahi, A.; Ko, F.; Abdin, Y. Development of low-cost electrospun carbon nanofibers using asphaltene precursor. Adv. Nat. Sci. Nanosci. Nanotechnol. 2023, 14, 025012.
- Bang, J.; Kim, J.H.; Park, S.W.; Kim, J.; Jung, M.; Jung, S.; Kim, J.C.; Choi, I.G.; Kwak, H.W. Effect of chemically modified lignin addition on the physicochemical properties of PCL nanofibers. Int. J. Biol. Macromol. 2023, 240, 124330.
- Jeon, B.; Ha, T.; Lee, D.Y.; Choi, M.S.; Lee, S.W.; Jung, K.-H. Preparation and Electrochemical Properties of Porous Carbon Nanofiber Electrodes Derived from New Precursor Polymer: 6FDA-TFMB. Polymers 2020, 12, 1851.
- Mamun, A.; Trabelsi, M.; Klöcker, M.; Sabantina, L.; Großerhode, C.; Blachowicz, T.; Grötsch, G.; Cornelißen, C.; Strei-tenberger, A.; Ehrmann, A. Electrospun nanofiber mats with embedded non-sintered TiO2 for dye-sensitized solar cells (DSSCs). Fibers 2019, 7, 60.
- Wen, J.; Chen, S.; Xu, Y.; Guan, T.; Zhang, X.; Bao, N. Synthesis of Single Crystal 2D Cu2FeSnS4 Nanosheets with High-Energy Facets (111) as a Pt-Free Counter Electrode for Dye-Sensitized Solar Cells. Materials 2023, 16, 4743.
- Nguyen, T.D.; Lee, J.S. Electrospinning-Based Carbon Nanofibers for Energy and Sensor Applications. Appl. Sci. 2022, 12, 6048.
- Kou, Y.; Oya, T. Unique Dye-Sensitized Solar Cell Using Carbon Nanotube Composite Papers with Gel Electrolyte. J. Compos. Sci. 2023, 7, 232.
- Montagni, T.; Rodríguez Chialanza, M.; Cerdá, M.F. Blueberries as a Source of Energy: Physical Chemistry Characterization of Their Anthocyanins as Dye-Sensitized Solar Cells’ Sensitizers. Solar 2023, 3, 283–297.
- Wu, T.C.; Huang, W.-M.; Meen, T.H.; Tsai, J.K. Performance Improvement of Dye-Sensitized Solar Cells with Pressed TiO2 Nanoparticles Layer. Coatings 2023, 13, 907.
- Sun, H.; Zhou, X.; Yang, W.; Gao, Y.; Zhang, R.; Liu, J.; Wang, H.; Guo, Y.; Wang, Z. High Performance Dye-Sensitized Solar Cells Based on Electrospun MoS2-Carbon Nanofiber Composite Counter Electrode. Front. Mater. 2020, 7, 593345.
- Joshi, P.; Zhang, L.; Chen, Q.; Galipeau, D.; Fong, F.; Qiao, Q. Electrospun Carbon Nanofibers as Low-Cost Counter Electrode for Dye-Sensitized Solar Cells. ACS Appl. Mater. Interfaces 2010, 2, 3572–3577.
- López-Covarrubias, J.G.; Soto-Muñoz, L.; Iglesias, A.L.; Villarreal-Gómez, L.J. Electrospun Nanofibers Applied to Dye Solar Sensitive Cells: A Review. Materials 2019, 12, 3190.
- Zhao, K.; Zhang, X.; Wang, M.; Zhang, W.; Li, X.; Wang, H.; Li, L. Electrospun carbon nanofibers decorated with Pt-Ni2P nanoparticles as high efficiency counter electrode for dye-sensitized solar cells. J. Alloys Compd. 2019, 786, 50–55.
- Aboagye, A.; Elbohy, H.; Kelkar, A.D.; Qiao, Q.; Zai, J.; Qian, X.; Zhang, X. Electrospun carbon nanofibers with surface-attached platinum nanoparticles as cost-effective and efficient counter electrode for dye-sensitized solar cells. Nano Energy 2015, 11, 550–556.
- Skupov, K.M.; Ponomarev, I.I.; Vtyurina, E.S.; Volkova, Y.A.; Ponomarev, I.I.; Zhigalina, O.M.; Khmelenin, D.N.; Cherkovskiy, E.N.; Modestov, A.D. Proton-Conducting Polymer-Coated Carbon Nanofiber Mats for Pt-Anodes of High-Temperature Polymer-Electrolyte Membrane Fuel Cell. Membranes 2023, 13, 479.
- Banitaba, S.N.; Ehrmann, A. Application of Electrospun Nanofibers for Fabrication of Versatile and Highly Efficient Electrochemical Devices: A Review. Polymers 2021, 13, 1741.
- Chandra Kishore, S.; Perumal, S.; Atchudan, R.; Alagan, M.; Wadaan, M.A.; Baabbad, A.; Manoj, D. Recent Advanced Synthesis Strategies for the Nanomaterial-Modified Proton Exchange Membrane in Fuel Cells. Membranes 2023, 13, 590.
- Waldrop, K.; Wycisk, R.; Pintauro, P.N. Application of electrospinning for the fabrication of proton-exchange membrane fuel cell electrodes. Curr. Opin. Electrochem. 2020, 21, 257–264.
- Delikaya, Ö.; Bevilacqua, N.; Eifert, L.; Kunz, U.; Zeis, R.; Roth, C. Porous electrospun carbon nanofibers network as an integrated electrode@gas diffusion layer for high temperature polymer electrolyte membrane fuel cells. Electrochim. Acta 2020, 345, 136192.
- Iskandarani, B.; Mojarrad, N.R.; Yürüm, A.; Gürsel, S.K.; Kaplan, B.Y. Electrospun Nanofiber Electrodes for Boosted Performance and Durability at Lower Humidity Operation of PEM Fuel Cells. Energy Fuels 2022, 36, 9282–9294.
- Yusoff, Y.N.; Shaari, N. An overview on the development of nanofiber-based as polymer electrolyte membrane and electrocatalyst in fuel cell application. Int. J. Energy Res. 2021, 45, 18441–18472.
- Waldrop, K.; Slack, J.J.; Gumeci, C.; Parrondo, J.; Dale, N.; Reeves, K.S.; Cullen, D.A.; More, K.L.; Pintauro, P.N. Electrospun Nanofiber Electrodes for High and Low Humidity PEMFC Operation. J. Electrochem. Soc. 2023, 170, 024507.
- Beltrán-Gastélum, M.; Portillo-Fuentes, S.G.; Flores-Hernández, J.R.; Salazar-Gastélum, M.I.; Trujillo-Navarrete, B.; Romero-Castañón, T.; Silva-Carrillo, C.; Reynoso-Soto, E.A.; Félix-Navarro, R.M. Ag-Cu Nanoparticles as Cathodic Catalysts for an Anion Exchange Membrane Fuel Cell. Catalysts 2023, 13, 1050.
- Maafa, I.M.; Zouli, N.; Abutaleb, A.; Yousef, A.; Qudsieh, I.Y.; Matar, S.M.; Adam, A.S.M.; El-Halwany, M.M. Synthesis of Ilmenite Nickel Titanite-Supported Carbon Nanofibers Derived from Polyvinylpyrrolidone as Photocatalyst for H2 Production from Ammonia Borane Photohydrolysis. Polymers 2023, 15, 3262.
- Schossig, J.; Gandotra, A.; Arizapana, K.; Weber, D.; Wildy, M.; Wei, W.; Xu, K.; Yu, L.; Chimenti, R.; Mantawy, I.; et al. CO2 to Value-Added Chemicals: Synthesis and Performance of Mono- and Bimetallic Nickel–Cobalt Nanofiber Catalysts. Catalysts 2023, 13, 1017.
- Stonkus, O.; Kibis, L.; Slavinskaya, E.; Zadesenets, A.; Garkul, I.; Kardash, T.; Stadnichenko, A.; Korenev, S.; Podyacheva, O.; Boronin, A. Pd-Ceria/CNMs Composites as Catalysts for CO and CH4 Oxidation. Materials 2023, 16, 4257.
- Abdel-Aty, M.M.; Gomaa, H.E.; Abdu, H.M.; Almasri, R.A.; Irfan, O.M.; Barakat, N.A.M. Molybdenum Carbide/Ni Nanoparticles Embedded into Carbon Nanofibers as an Effective Non-Precious Catalyst for Green Hydrogen Production from Methanol Electrooxidation. Polymers 2023, 15, 2430.
- Ghosh, S.; Courthéoux, L.; Brunet, S.; Lacroix-Desmazes, P.; Pradel, A.; Girard, E.; Uzio, D. Effect of the Microstructure of Composite CoMoS/Carbon Catalysts on Hydrotreatment Performances. Catalysts 2023, 13, 862.
- Popov, A.A.; Afonnikova, S.D.; Varygin, A.D.; Bauman, Y.I.; Trenikhin, M.V.; Plyusnin, P.E.; Shubin, Y.V.; Vedyagin, A.A.; Mishakov, I.V. Pt1−xNix Alloy Nanoparticles Embedded in Self-Grown Carbon Nanofibers: Synthesis, Properties and Catalytic Activity in HER. Catalysts 2023, 13, 599.
- Guerrero-Pérez, M.O. Research Progress on the Applications of Electrospun Nanofibers in Catalysis. Catalysts 2022, 12, 9.
- Liu, Y.; Li, T.; Cao, X.; Liu, J.; Zhang, J.; Jia, J.; Wang, F.; Pan, K. Electrospun Fe2C-loaded carbon nanofibers as efficient electrocatalysts for oxygen reduction reaction. Nanotechnology 2019, 30, 32.
- Woo, S.; Lee, J.; Lee, D.S.; Kim, J.K.; Lim, B. Electrospun Carbon Nanofibers with Embedded Co-Ceria Nanoparticles for Efficient Hydrogen Evolution and Overall Water Splitting. Materials 2020, 13, 856.
- Ponomarev, I.I.; Zhigalina, M.O.; Skupov, M.K.; Modestov, D.A.; Basu, G.V.; Sufiyanova, E.A.; Ponomareva, I.I.; Dmitry, Y. Preparation and thermal treatment influence on Pt-decorated electrospun carbon nanofiber electrocatalysts. RSC Adv. 2019, 9, 27406–27418.
- Kanjwal, M.A.; Ghaferi, A.A. Graphene Incorporated Electrospun Nanofiber for Electrochemical Sensing and Biomedical Applications: A Critical Review. Sensors 2022, 22, 8661.
- Alagarsamy, S.; Sundaresan, R.; Chen, S.-M.; Devi, J.M.; Chandrasekar, N.; Ramachandran, B. Fabrication of an Electrocatalyst Based on Rare Earth Manganites Incorporated with Carbon Nanofiber Hybrids: An Efficient Electrochemical Biosensor for the Detection of Anti-Inflammatory Drug Mefenamic Acid. C 2023, 9, 47.
- Rathinasamy, S.K.; Maheswar, R.; Lorincz, J. Silk Fibroin-Based Piezoelectric Sensor with Carbon Nanofibers for Wearable Health Monitoring Applications. Sensors 2023, 23, 1373.
- Wang, F.; Li, Y.; Yan, C.; Ma, Q.; Yang, X.; Peng, H.; Wang, H.; Du, J.; Zheng, B.; Guo, Y. Bismuth-Decorated Honeycomb-like Carbon Nanofibers: An Active Electrocatalyst for the Construction of a Sensitive Nitrite Sensor. Molecules 2023, 28, 3881.
- Karlapudi, M.C.; Vahdani, M.; Bandari, S.M.; Peng, S.; Wu, S. A Comparative Study on the Effects of Spray Coating Methods and Substrates on Polyurethane/Carbon Nanofiber Sensors. Sensors 2023, 23, 3245.
- Ketmen, S.; Er Zeybekler, S.; Gelen, S.S.; Odaci, D. Graphene Oxide-Magnetic Nanoparticles Loaded Polystyrene-Polydopamine Electrospun Nanofibers Based Nanocomposites for Immunosensing Application of C-Reactive Protein. Biosensors 2022, 12, 1175.
- Li, M.; Dong, J.; Deng, D.; Ouyang, X.; Yan, X.; Liu, S.; Luo, L. Mn3O4/NiO Nanoparticles Decorated on Carbon Nanofibers as an Enzyme-Free Electrochemical Sensor for Glucose Detection. Biosensors 2023, 13, 264.
- Hu, X.; Jiang, Y.; Ma, Z.; Xu, Y.; Zhang, D. Bio-inspired Flexible Lateral Line Sensor Based on P(VDF-TrFE)/BTO Nanofiber Mat for Hydrodynamic Perception. Sensors 2019, 19, 5384.
- Sengupta, D.; Trap, D.; Kottapalli, A.G.P. Piezoresistive Carbon Nanofiber-Based Cilia-Inspired Flow Sensor. Nanomaterials 2020, 10, 211.
- Kim, M.; Kaliannagounder, V.K.; Unnithan, A.R.; Park, C.H.; Kim, C.S.; Ramachandra Kurup Sasikala, A. Development of In-Situ Poled Nanofiber Based Flexible Piezoelectric Nanogenerators for Self-Powered Motion Monitoring. Appl. Sci. 2020, 10, 3493.
- Devaraj, V.; Han, J.; Kim, C.; Kang, Y.C.; Oh, J.W. Self-Assembled Nanoporous Biofilms from Functionalized Nanofibrous M13 Bacteriophage. Viruses 2018, 10, 322.
- Zheng, C.; Yue, Y.; Gan, L.; Xu, X.; Mei, C.; Han, J. Highly Stretchable and Self-Healing Strain Sensors Based on Nanocellulose-Supported Graphene Dispersed in Electro-Conductive Hydrogels. Nanomaterials 2019, 9, 937.
- Khan, A.; Alamry, K.A.; Althomali, R.H. Chemically Modified Carbon Nanotubes for Electrochemical Sensors. In Chemically Modified Carbon Nanotubes for Commercial Applications; Aslam, J., Hussain, C.M., Aslam, R., Eds.; Wiley: Hoboken, NJ, USA, 2023.
- Scroccarello, A.; Pelle, F.D.; Bukhari, Q.U.A.; Silveri, F.; Zappi, D.; Cozzoni, E.; Compagnone, D. Eucalyptus Biochar as a Sustainable Nanomaterial for Electrochemical Sensors. Chem. Proc. 2021, 5, 13.
- da Silva, V.A.O.P.; Stefano, J.S.; Kalinke, C.; Bonacin, J.A.; Janegitz, B.C. Additive Manufacturing Sensor for Stress Biomarker Detection. Chemosensors 2023, 11, 306.
- Galvão, J.C.R.; Araujo, M.d.S.; Prete, M.C.; Neto, V.L.; Dall’Antonia, L.H.; Matos, R.; Tarley, C.R.T.; Medeiros, R.A. Electrochemical Determination of 17-β-Estradiol Using a Glassy Carbon Electrode Modified with α-Fe2O3 Nanoparticles Supported on Carbon Nanotubes. Molecules 2023, 28, 6372.
- Chen, J.; Rong, F.; Xie, Y. Fabrication, Microstructures and Sensor Applications of Highly Ordered Electrospun Nanofibers: A Review. Materials 2023, 16, 3310.
- Al-Abduljabbar, A.; Farooq, I. Electrospun Polymer Nanofibers: Processing, Properties, and Applications. Polymers 2023, 15, 65.
- Wang, P.; Liu, X.; You, Y.; Wang, M.; Huang, Y.; Li, Y.; Li, K.; Yang, Y.; Feng, W.; Liu, Q.; et al. Fabrication of High-Performance Colorimetric Membrane by Incorporation of Polydiacetylene into Polyarylene Ether Nitriles Electrospinning Nanofibrous Membranes. Nanomaterials 2022, 12, 4379.
- Chen, T.W.; Kalimuthu, P.; Veerakumar, P.; Lin, K.C.; Chen, S.M.; Ramachandran, R.; Mariyappan, V.; Chitra, S. Recent Developments in Carbon-Based Nanocomposites for Fuel Cell Applications: A Review. Molecules 2022, 27, 761.
- Zhang, L.; Qin, D.; Feng, J.; Tanga, T.; Cheng, H. Rapid quantitative detection of luteolin using an electrochemical sensor based on electrospinning of carbon nanofibers doped with single-walled carbon nanoangles. Anal. Methods 2023, 15, 3073–3083.
- Rashid, H.U.; Ali, M.; Sarker, M.R.; Md Ali, S.H.; Akhtar, N.; Khan, N.A.; Asif, M.; Shah, S. Synthesis, Characterization, and Applications of Silver Nano Fibers in Humidity, Ammonia, and Temperature Sensing. Micromachines 2021, 12, 682.
- Yoo, S.H.; Yoon, H.-S.; Han, H.; Na, K.H.; Choi, W.Y. Fabrications of Electrospun Mesoporous TiO2 Nanofibers with Various Amounts of PVP and Photocatalytic Properties on Methylene Blue (MB) Photodegradation. Polymers 2023, 15, 134.
- Mehrabi, P.; Hui, J.; Janfaza, S.; O’Brien, A.; Tasnim, N.; Najjaran, H.; Hoorfar, M. Fabrication of SnO2 Composite Nanofiber-Based Gas Sensor Using the Electrospinning Method for Tetrahydrocannabinol (THC) Detection. Micromachines 2020, 11, 190.
- Liu, P.; Wu, S.; Zhang, Y.; Zhang, H.; Qin, X. A Fast Response Ammonia Sensor Based on Coaxial PPy–PAN Nanofiber Yarn. Nanomaterials 2016, 6, 121.
- Yang, Z.; Zhang, J.; Kintner-meyer, M.C.W.; Lu, X.; Choi, D.; Lemmon, J.P. Electrochemical Energy Storage for Green Grid. Chem. Rev. 2011, 111, 3577–3613.
- Yang, S.; Zhang, L.; Sun, J.; Li, K.; Zhao, S.; Zhao, D.; Wang, J.; Yang, C.; Wang, X.; Cao, B. Corncob-derived hierarchical porous activated carbon for high-performance lithium-ion capacitors. Energy Fuels 2020, 34, 16885–16892.
- Sun, J.; Yang, S.; Yang, C.; Jia, Q.; Yang, X.; Cao, B. Corncob-derived hierarchical porous carbons constructed by re-activation for high-rate lithium-ion capacitors. New J. Chem. 2019, 43, 10103–10108.
- Yarin, A.L.; Zussman, E.; Wendorff, J.H.; Greiner, A. Material encapsulation and transport in core-shell micro/nanofibers, polymer and carbon nanotubes and micro/nanochannels. J. Mater. Chem. 2007, 17, 2585–2599.
- Stenina, I.; Minakova, P.; Kulova, T.; Yaroslavtsev, A. Electrochemical Properties of LiFePO4 Cathodes: The Effect of Carbon Additives. Batteries 2022, 8, 111.
- Ji, L.; Zhang, X. Fabrication of porous carbon nanofibers and their application as anode materials for rechargeable lithium-ion batteries. Nanotechnology 2009, 20, 155705.
- Nitta, N.; Wu, F.; Lee, J.T.; Yushin, G. Li-ion battery materials: Present and future. Mater. Today 2015, 18, 252–264.
- Grey, C.P.; Hall, D.S. Prospects for lithium-ion batteries and beyond—A 2030 vision. Nat. Commun. 2020, 11, 6279.
- Hanif, M.B.; Motola, M.; Rauf, S.; Li, C.J.; Li, C.X. Recent advancements, doping strategies and the future perspective of perovskite-based solid oxide fuel cells for energy conversion. Chem. Eng. J. 2022, 428, 132603.
- Cao, T.; Kwon, O.; Gorte, R.J.; Vohs, J.M. Metal exsolution to enhance the catalytic activity of electrodes in solid oxide fuel cells. Nanomaterials 2020, 10, 2445.
- Qiu, P.; Yang, X.; Wang, W.; Wei, T.; Lu, Y.; Lin, J.; Yuan, Z.; Jia, L.; Li, J.; Chen, F. Redox-reversible electrode material for direct hydrocarbon solid oxide fuel cells. ACS Appl. Mater. Interfaces 2020, 12, 13988–13995.
- Hanif, M.B.; Rauf, S.; ul Abadeen, Z.; Khan, K.; Tayyab, Z.; Qayyum, S.; Mosiałek, M.; Shao, Z.; Li, C.X.; Motola, M. Proton-conducting solid oxide electrolysis cells: Relationship of composition-structure-property, their challenges, and prospects. Matter 2023, 6, 1782–1830.
- Kang, Y.; Deng, C.; Chen, Y.; Liu, X.; Liang, Z.; Li, T.; Hu, Q.; Zhao, Y. Binder-Free Electrodes and Their Application for Li-Ion Batteries. Nanoscale Res. Lett. 2020, 15, 112.
- Chen, S.; Zhang, X.; Xia, M.; Wei, K.; Zhang, L.; Zhang, X.; Yang, C.C.; Jose, R. Issues and challenges of layered lithium nickel cobalt manganese oxides for lithium-ion batteries. J. Electroanal. Chem. 2021, 895, 115412.
- Zhang, L.; Yang, S.; Fu, W.; Cui, Y.; Wang, J.; Zhao, D.; Yang, C.; Wang, X.; Cao, B. Plasma-induced ε-MnO2 based aqueous zinc-ion batteries and their dissolution-deposition mechanism. J. Mater. Sci. Technol. 2022, 127, 206–213.
- Han, S.D.; Kim, S.; Li, D.; Petkov, V.; Yoo, H.D.; Phillips, P.J.; Wang, H.; Kim, J.J.; More, K.L.; Key, B.; et al. Mechanism of Zn insertion into nanostructured δ-MnO2: A nonaqueous rechargeable Zn metal battery. Chem. Mater. 2017, 29, 4874–4884.
- Herbert, W.; Kappauf, H.W. Metadata of the Chapter That Will Be Visualized Online Spontanremissionen; Springer: Cham, Switzerland, 2021; pp. 1–12.
- Burke, A. Ultracapacitors: Why, how, and where is the technology. J. Power Sources 2000, 91, 37–50.
- Burke, A. R & D considerations for the performance and application of electrochemical capacitors. Electrochim. Acta 2007, 53, 1083–1091.
- Ke, Q.; Wang, J. Graphene-based materials for supercapacitor electrodes—A review. J. Mater. 2016, 2, 37–54.
- Yang, S.; Cui, Y.; Yang, G.; Zhao, S.; Wang, J.; Zhao, D.; Yang, C.; Wang, X.; Cao, B. ZnCl2 induced hierarchical porous carbon for zinc-ion hybrid supercapacitors. J. Power Sources 2023, 554, 232347.
More
Information
Subjects:
Engineering, Chemical
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
511
Revisions:
2 times
(View History)
Update Date:
26 Oct 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




