1. Phase Transition Near the Compound B8C
Reliable information on the electronic structure of more boron-rich boron carbide is obtained from electrical conductivity, measured between 5 and 2100 K by Werheit et al.
[1], by Wood
[2][3] and by Aselage et al.
[4]. Results obtained by Amulele et al.
[5] up to 9 GPa pressure are compatible.
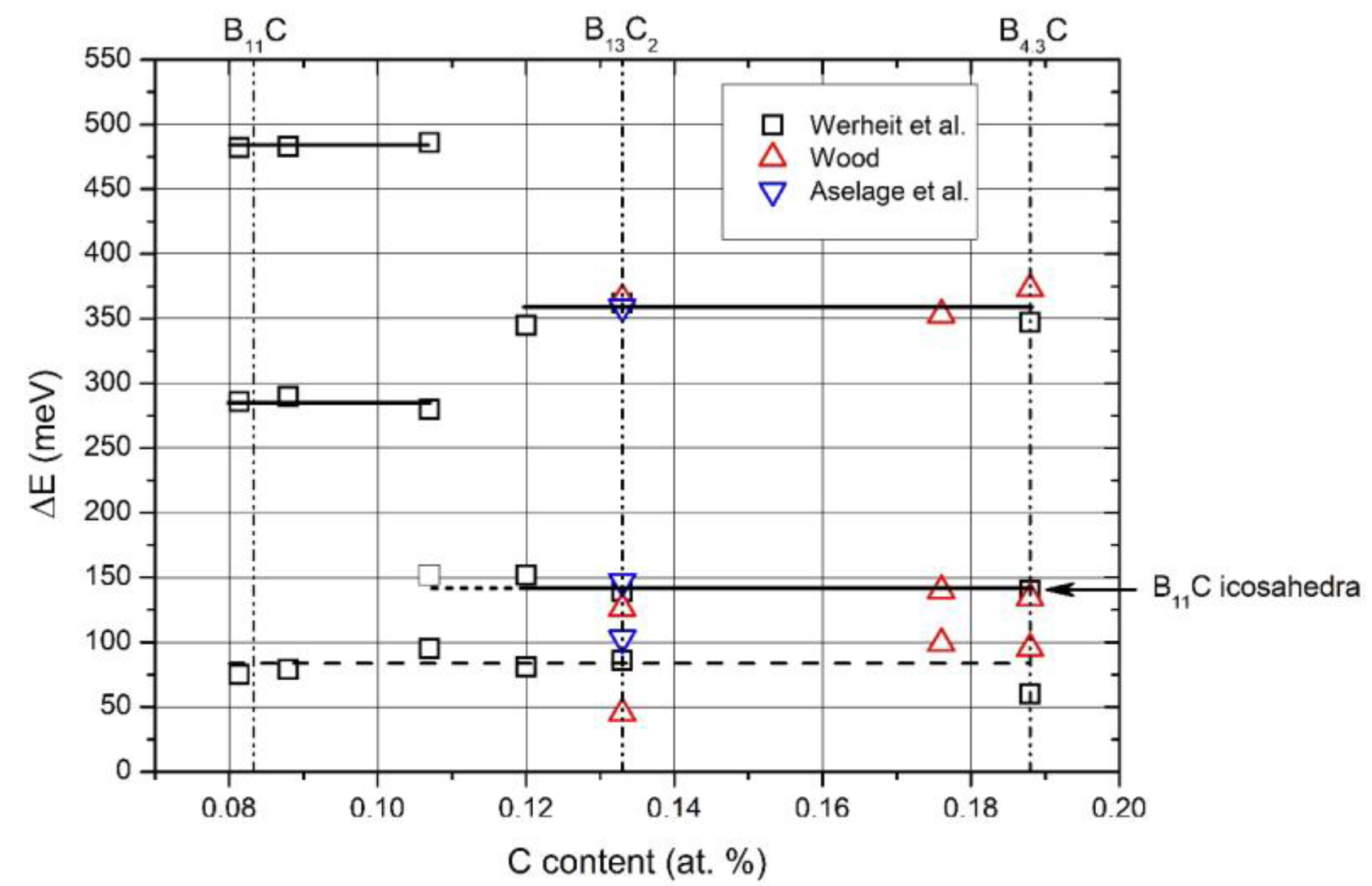
The activation energies obtained from the electrical conductivity are displayed in
Figure 1, showing that at ~B
8C, the homogeneity range is divided into two parts with significantly different electronic characteristics. The activation energy at about 80 meV found in both regions is uncertain as obtained in that range of temperature, where a continuous transition from thermal excitation to variable-range hopping takes place (see
[1]).
Figure 1. Phase transition near the compound B8C.
It is expected that this phase transition is correlated with a structural change. This should be reflected in the phonon spectra.
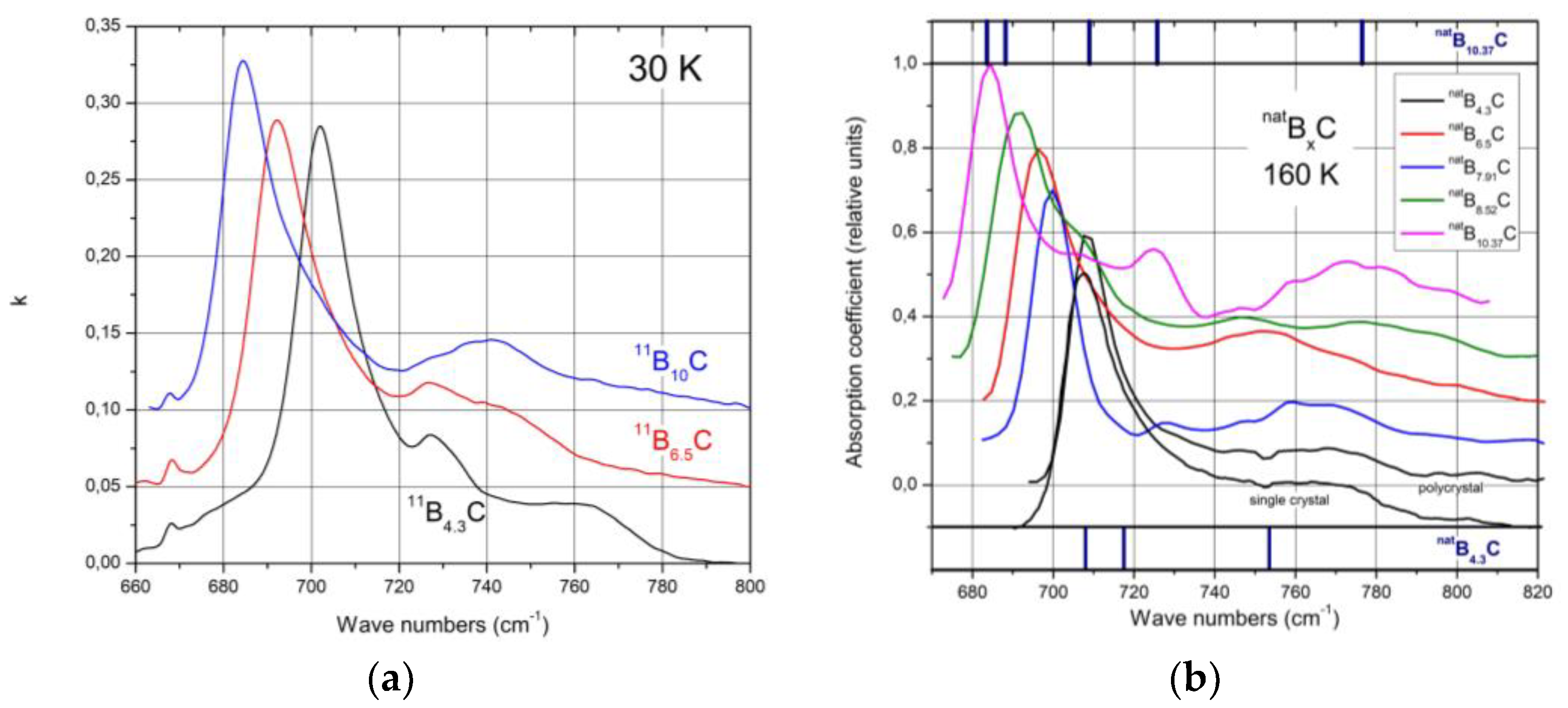
Figure 2 shows the IR- and Raman-active phonons of
11B
xC boron carbide
[6][7].
Figure 2. Phonon spectra of
11B
xC at 30K
[4][8]. (
a), IR-active phonons; (
b), Raman-active phonons.
1.1. IR-Active Phonon Near 400 cm−1
The phonon near 400 cm
−1 is composed of two components (see
Figure 3a), where the frequencies (
Figure 3b) and phonon strengths vary depending on the C content. Shirai et al.
[9][10] and Vast et al.
[11][12] attribute the IR phonon mode near 400 cm
−1 to the central B atom in the chain vibrating perpendicularly to the chain axis
[13]. Experimentally, this is confirmed for the component Ph 1, whose strength, depending on the C content, precisely matches the share of CBC chains (
Figure 3b). The considerably weaker component Ph 2 of this phonon belongs to the icosahedral structure, accordingly with its counterpart in the spectrum of α-rhombohedral boron. This phonon was already mentioned by Beckel et al.
[14] but not described in detail (see
[15]).
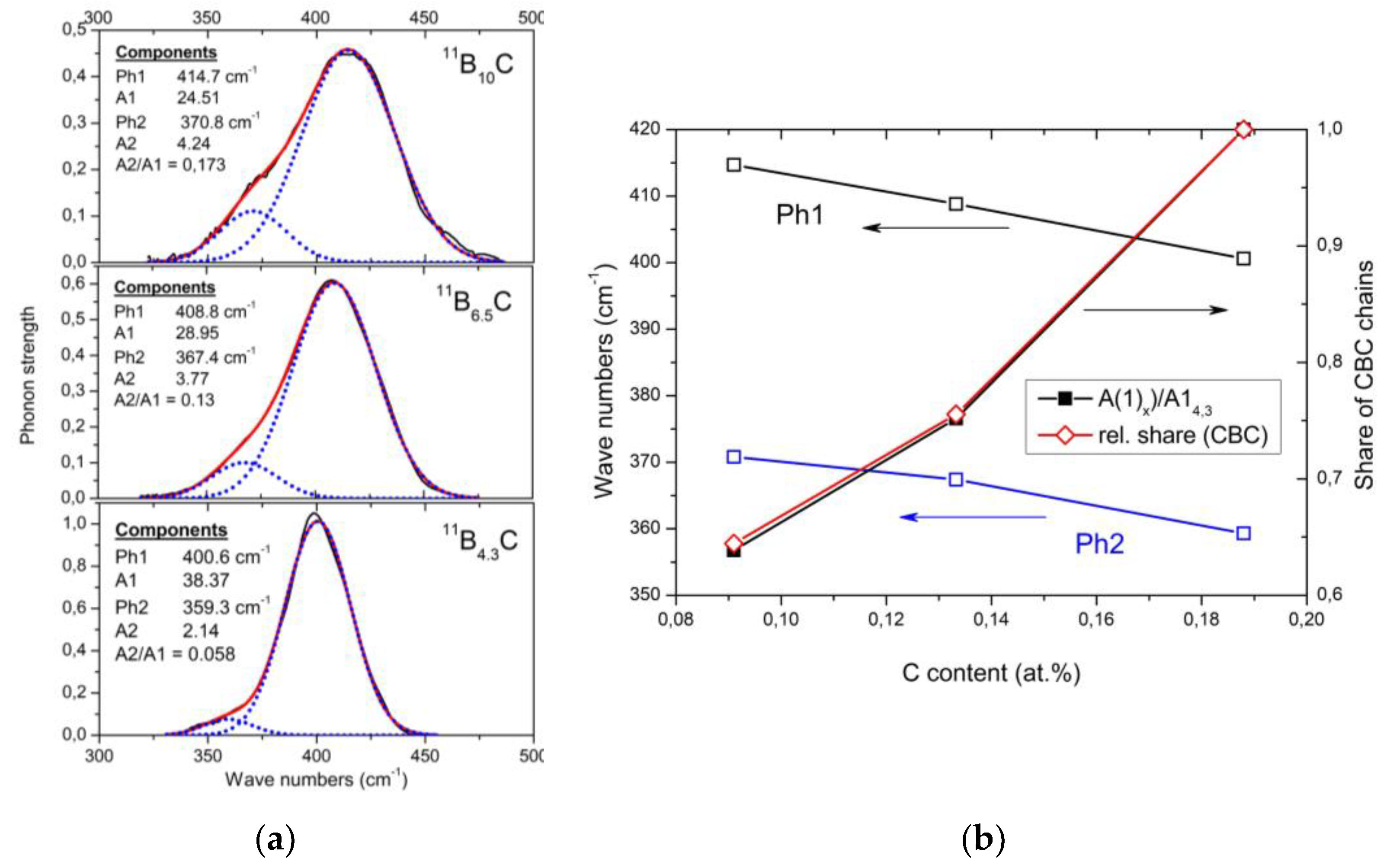
Figure 3. (a). IR-active phonon of 11BxC near 400 cm−1 (All fits were obtained using the Origin Pro software). (b). Phonon shift depending on C content and comparison between the reduction of phonon strength (components 1 and 2) and relation of the phonon strength of component #1 depending on C content compared with the relative share of CBC chains in the structure (obtained from the IR-active stretching vibration near 1600 cm−1).
In
Figure 3, no apparent change suggests a relation of this phonon to the phase transition at B
8.1C. However, the mode Grüneisen parameter γ of the IR-phonon (−3.21 at low and +6.5 at high temperatures) deviating strikingly from all other phonons
[16] suggests a peculiarity. The previously assumed involvement of this phonon in the T-dependent transition at 712 K
[17] is checked below.
1.2. IR-Active Phonons Near 700 cm−1
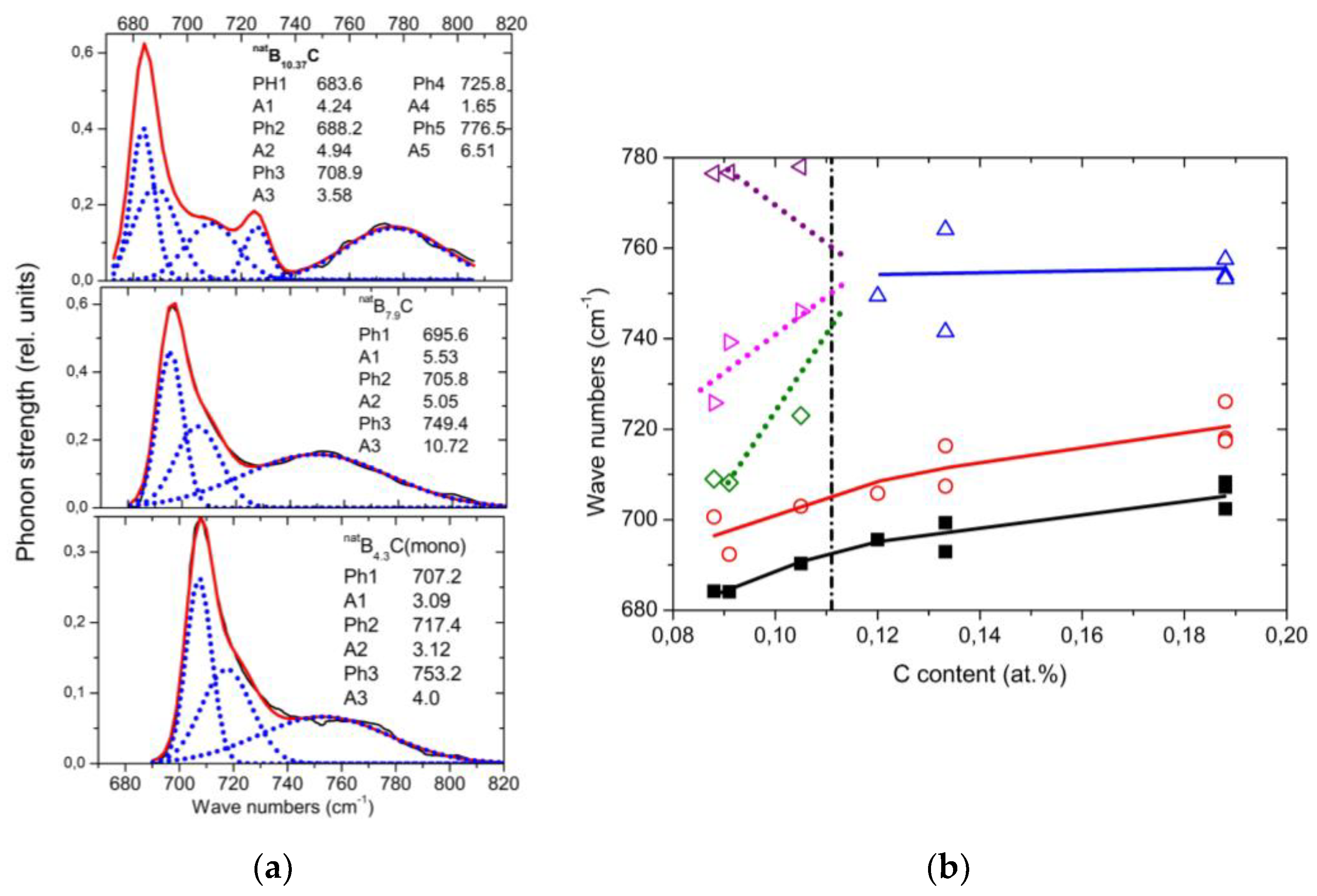
The spectra of isotope-enriched samples (
Figure 4a) exhibit clear changes depending on C content. For interpretation, a denser sequence of spectra is required. Data on
natB
xC are available (
[17] Figure 4b). The results of the analysis (examples in (
Figure 5a)) are displayed in
Figure 5b. Splitting of the component of this phonon near 760 cm
−1 into three branches indicates a structural change at the phase transition. This phonon is correlated with the strong 705 cm
−1 intra-icosahedral single-line A
2u mode in α-rhombohedral boron, described by Beckel et al.
[14], split in the boron carbide structure (see
[15][18]).
Figure 4. IR-active phonons near 700 cm−1. (a), 11BxC at 30 K; (b), natBxC at 160 K, phonon-wavenumbers of natB4.3C and natB10.37C.
Figure 5. IR-active phonons near 700 cm−1. (a) Fits obtained with OriginPro software. (b) Wave numbers of phonon components vs. C content. The results of the high-frequency components of B8.52C (C content 0.105) are rather uncertain.
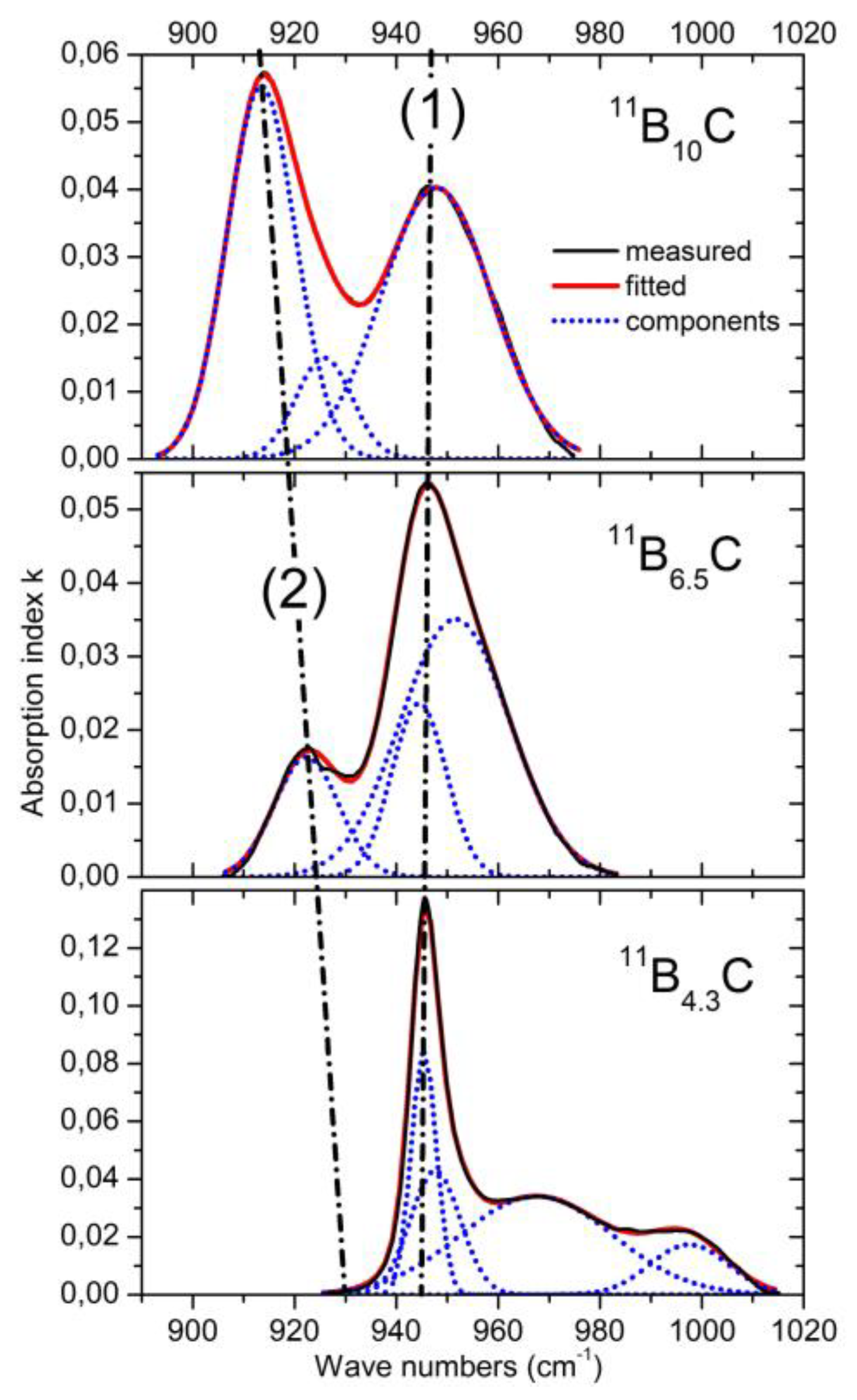
1.3. IR-Active Phonons Near 940 cm−1
The phonon near 940 cm
−1 (
Figure 6) represents an icosahedral vibration proved by the counterpart in the spectrum of α-rhombohedral boron. According to Beckel et al.
[14], this phonon consists of superimposing E
u and A
2u modes. In boron carbide, the additional effect of polar C atoms in the icosahedra must be considered. This explains, in principle, the complexity of the phonon and of its change depending on C content.
Figure 6. IR-active phonons near 950 cm−1. (1) and (2) mark the apparent shift and strength of specific components.
However, there is a significant change between B4.3C and B6.5C: two components of the phonon vanish completely. Unfortunately, there are no spectra in the intermediate range available, which could help explain this behavior.
1.4. FIR-Absorption
As indicated in the low-frequency region of
Figure 2a, the FIR optical absorption increases towards very low wave numbers.
Figure 7 shows this range extended down to 10 cm
−1. This absorption is caused by the plasma-like absorption of free charge carriers, which is heavily damped in boron carbide (for details, see
[19]). With respect to the electric structure,
Figure 7 shows that the concentration of free carriers increases with the C content decreasing. However, there is no effect of the phase transition at B
~8C on the concentration of free holes discernable.
Figure 7. Heavily damped plasma absorption in
11B
xC at 30 K
[19].
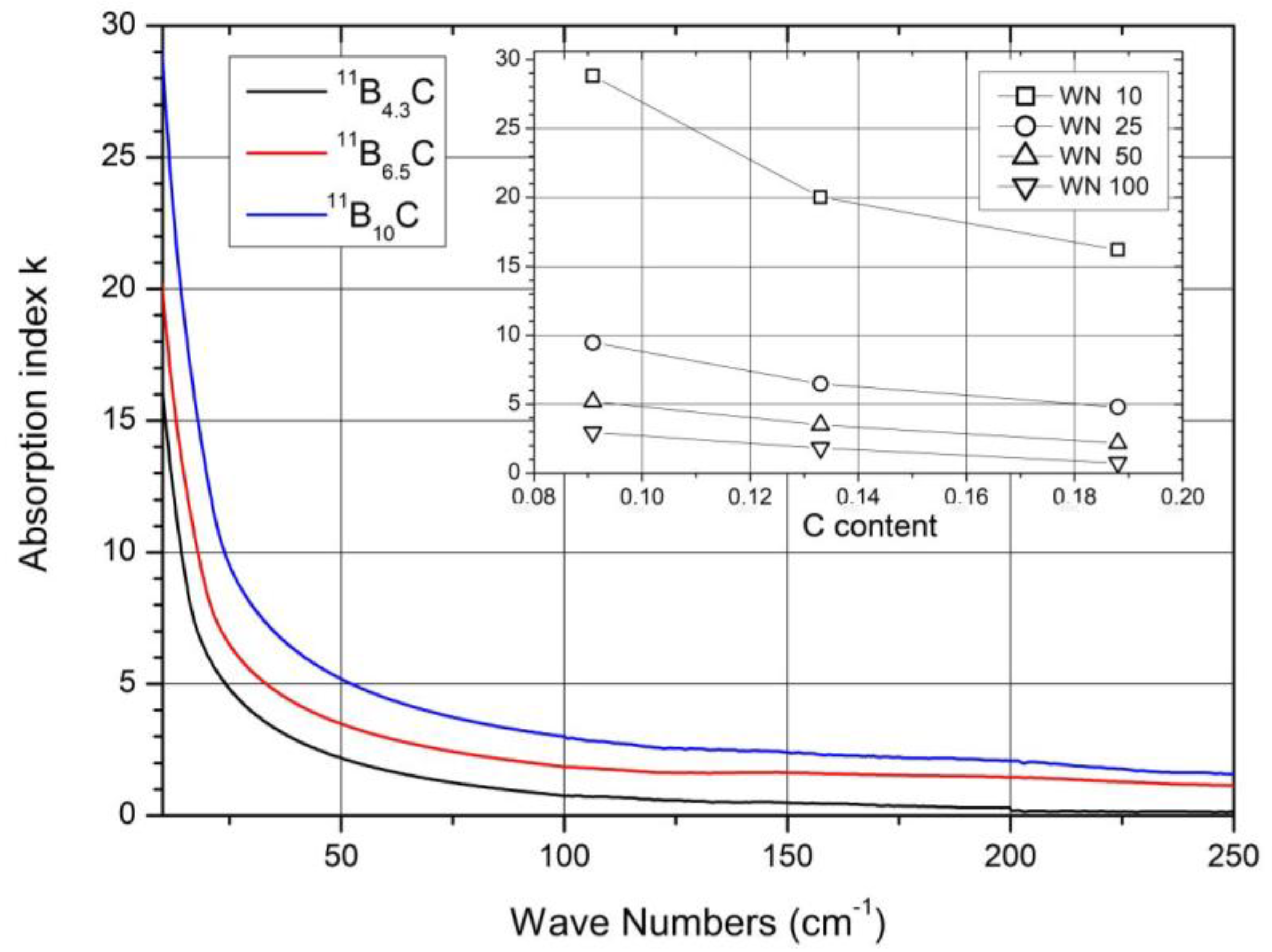
The share of B11C icosahedra decreases from ~1 in B4.3C to ~0.1 in B10C 9. The main components (1) and (2) seem to vary continuously depending on the C content. There is no indication of a sudden change near B8C. Therefore, no involvement of this phonon in the phase transition at B~8C is discernible.
1.5. Raman-Active Phonons
The resolution of the Raman spectra in Figure 2b is restricted. Therefore, variations dependence on the C content cannot be doubtlessly identified, apart from the strong phonon doublet at 270/320 cm−1 (Figure 8). This has no counterpart in the spectrum of α-rhombohedral boron. Therefore, it is to be attributed to the structural changes in boron carbide by C atoms: B11C icosahedra, CBC, or CBB chains.
Figure 8. Raman-active phonons of 11BxC at 270/320 cm−1.
2. Phase Transition at 712 K
The T-dependent specific heat (
Figure 9) shows an exothermic phase transition at 712 K, half-width 82 K
[20]. The electrical conductivity proves the correlation of this phase transition with a structural change, where the activation energy of 140 meV is correlated with the content of the B
11C icosahedra and polar C atoms, respectively (see above). At carbon contents below the phase transition B
~8C, it is no longer detectable
[11].
Figure 9. Specific heat of B4.3C boron carbide vs. T. Black circles, measured; red dashed line, polynomial fit; blue, difference between measured data and fitted line; purple dotted line, 1st derivative.
Yao et al.
[21] described theoretically in this range an endothermic phase transition that is related to the site exchange of carbon atoms within the icosahedra. Obviously, this process is not compatible.
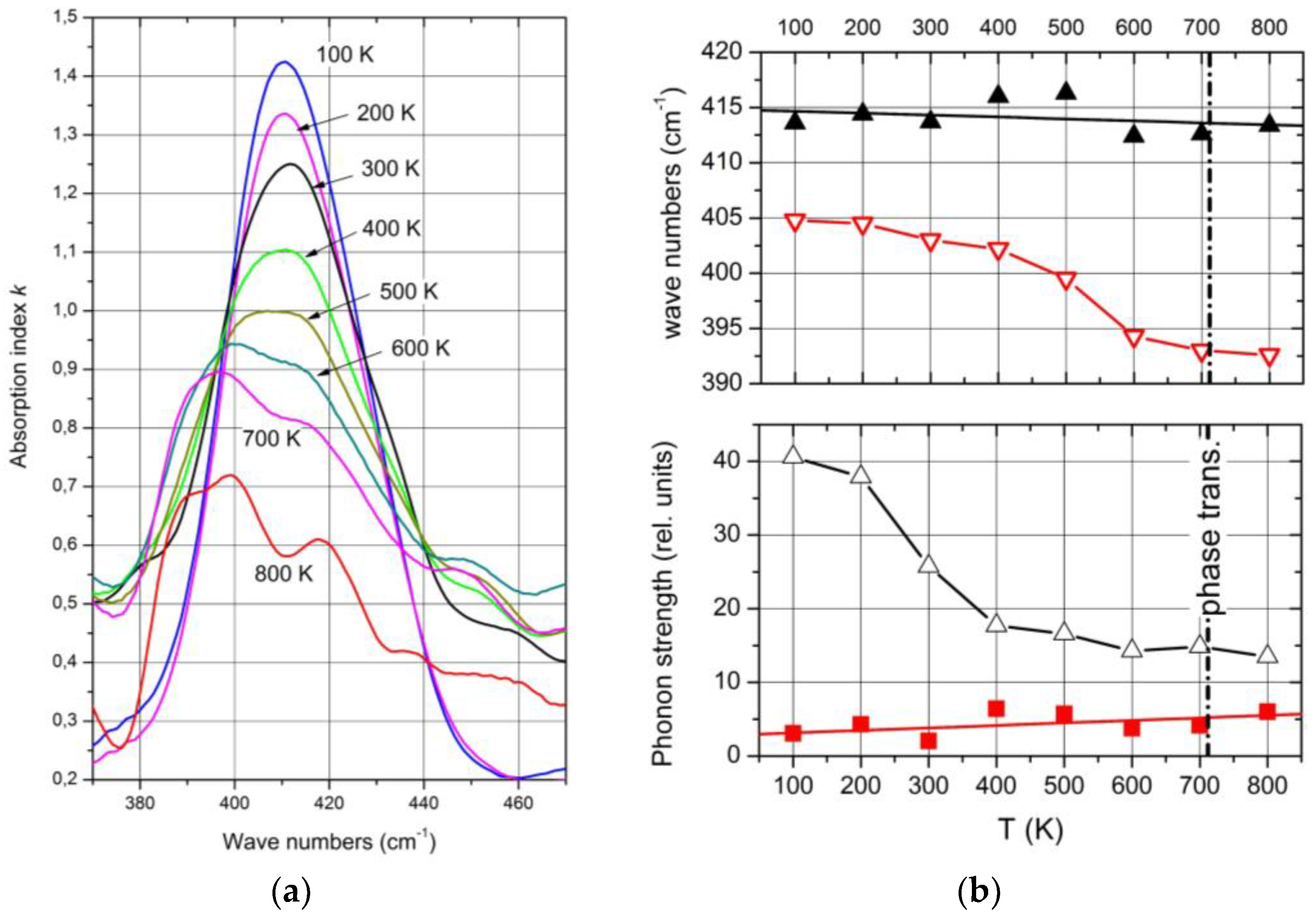
Complete temperature-dependent phonon spectra of boron carbide are available below 450 K only. The exception is the IR-active phonon mode near 400 cm
−1, available between 100 and 800 K (
Figure 10a)
[19]. The spectra suggested a structural change by mode splitting near the phase transition. However, this splitting is feigned.
Figure 10. IR-active phonon of natB4.3C boron carbide near 400 cm−1. (a) Absorption index k vs. wave number between 100 and 800 K; (b) wave number and phonon strength of the components vs. T. Black, bending mode of the CBC chain; red, icosahedral vibration.
As shown above, the IR-active phonon mode near 400 cm
−1 consists of two components. One of them is an icosahedral vibration identified by the equivalent vibration in α-rhombohedral boron. As such, it is mentioned by Beckel et al.
[14] but not described in detail. Shirai et al.
[9][10] and Vast et al.
[12][22] attribute the second component to the central B atom in the CBC chain vibrating perpendicularly to the chain axis.
Passing the phase transition, the frequency of the component representing the icosahedral vibration decreases clearly from 405 cm−1 at low T to 395 cm−1. Simultaneously, the phonon strength concerned increases marginally, showing that the number of vibrating elements remains nearly unchanged. The atomic bonding force concerned falls slightly in the range of phase transition but is nevertheless clearly measurable. It is surprising that the range of decrease is wider than the half-width of the phase transition (Figure 6). A certain effect of the phase transition on the electronic properties is clearly established.
The vibration of the central B atom in the chain behaves differently. The frequency remains nearly constant, showing that the bonds within the CBC chain are hardly affected by T. In contrast, the phonon strength representing the number of vibrating elements decreases considerably.
A certain difference between the structures below and above the phase transition is indicated by the frequency of the icosahedral phonon near 400 cm−1 (Figure 8b), which decreases from 405 cm−1 at low T to 392 cm−1 at high T. In contrast, the frequency of the bending mode of the central B atom in the CBC chain decreases at most marginally from ~414 to ~407 cm−1. The redistribution of polar C atoms in the phase transition affects the bonding force within the icosahedron and, consequently, the electronic structure as well.
An important further characteristic of this phase transition can be obtained from the electrical conductivity at pressures up to 9 GPa
[5]. The phase transition is reversible, however, with considerable relaxation time.
3. Phase Transition at 32 GPa
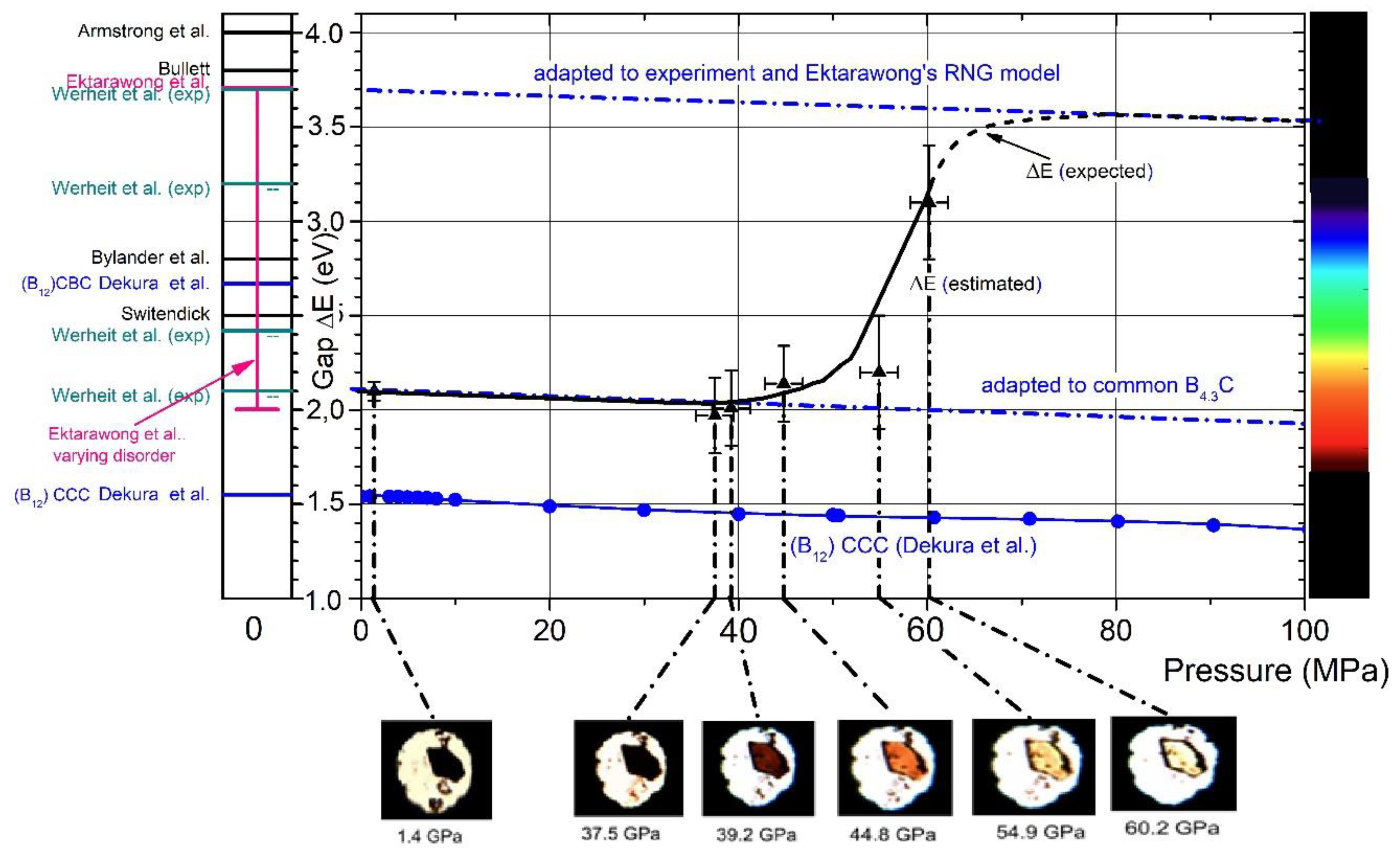
The drastic change from opacity to glasslike transparency is the visual appearance of the phase transition in boron carbide at 32 GPa. Hushur et al.
[23] used the photos shown in
Figure 11 to estimate the band gap increasing at increasing pressure roughly. This implies that the electronic structure of boron carbide known from ambient conditions
[24] changes fundamentally at high pressure.
Figure 11. The gap width of boron carbide depending on pressure. Black triangles, gap width, roughly estimated from transient-light photos
[23]. Compared with experimental and theoretical results
[25][26][27][28]; pink, ordered structure
[27]; pink vertical bar, varying configurational disorder
[27]; blue filled circles, pressure-dependence
[23]. Experimental results: cyan, ambient conditions
[29][30]; dash-dotted lines, adapted to relevant gap widths at 0 GPa for distorted and undistorted B
4.3C (Ektarawong’s RNG model), respectively; pressure-dependence according to the calculation for (B
12)CCC
[28].
In
Figure 11 [23], the estimated shift of the band gap is compared with theoretical model calculations on undistorted and distorted boron carbides
[25][26][27][28] and experimental results
[29][30][31]. Obviously, this phase transition converts boron carbide, as it is commercially available or prepared at a laboratory scale and is highly distorted, into a largely undistorted state, which is at least close to the idealized models used in theoretical calculations.
At rising pressure, the band gap increases from values near 2 eV, experimentally determined
[27] and theoretically attributed to a strongly distorted boron carbide
[25] to gap widths between 3 and 4 eV, theoretically predicted for idealized meaning defect-free boron carbide.
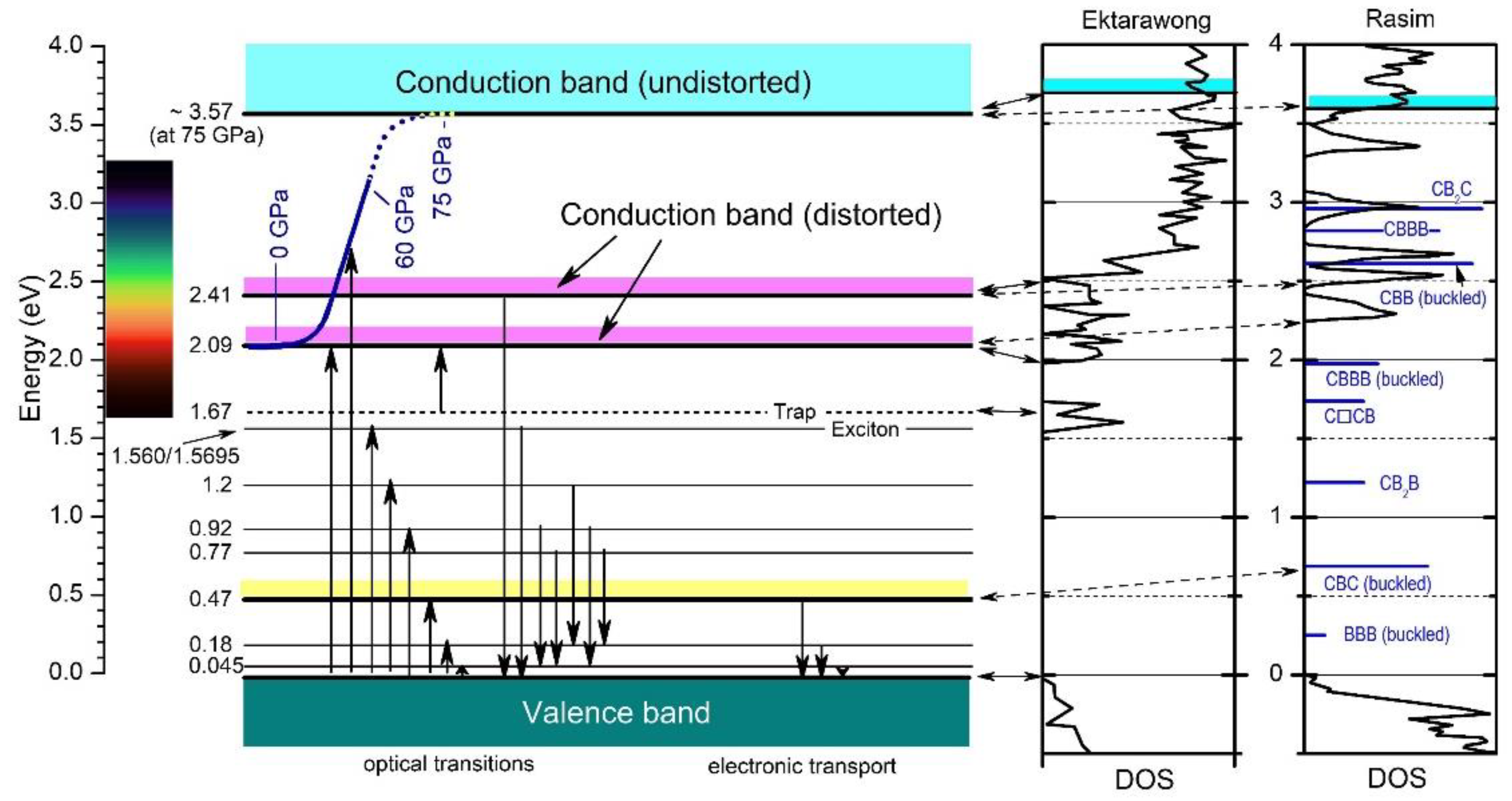
Both approaches mark significant achievements compared with the previous theoretical studies based on idealized and, therefore, unrealistic structure models. In
Figure 12 [32], the researchers check to what extent the DOS of both model calculations are compatible with the electronic transitions in the empirical band scheme based on experimental results. These fit excellently with Ektarawong’s RNG model, assuming a random distribution of icosahedral C atoms over all the polar sites
[25].
Figure 12. Electronic band scheme of B
4.3C boron carbide
[32] derived from optical and electrical measurements; DOS calculated by Ektarawong et al. (RNG model)
[25] and Rasim et al.
[33] for reference (data taken from diagram each). Vertical arrows show absorption (upward) and emission (downward) processes; the visible range is marked. Reprinted from Ref.
[32] published by Elsevier Masson SAS. All rights reserved.
Considering these results, the strong optically excited transitions between ~2 and ~3.5 eV, which are responsible for the opacity of boron carbide at ambient pressure, are evoked in any way by the polar C atoms in boron carbide. The strong optical absorption concerned fulfills the requirements of electronic interband transitions and has been interpreted accordingly. Compared with classical semiconductors, the polar C atoms in boron carbide differ decisively from dopants having one excess electron compared with the basic lattice atoms and form low-density states in the gap. In boron carbide, the distribution of polar C atoms at room temperature induces high-density electronic states. A “simple” redistribution of the polar C atoms at high pressure, apparently to a higher degree of order, makes these high-density states completely disappear. This redistribution is reversible. That means the largely defect-free structure at high pressure
[34] is metastable only, while the distorted structure is the thermodynamically stable one, at least under ambient conditions.
The reversibility of the structural distortion induced by the C atoms randomly distributed over all six polar sites stands in fundamental contrast to a sometimes-used model based on the assumption that the actual distorted structure of boron carbide is a non-equilibrium state only
[19].
The DOS of boron carbide affected by structural elements replacing the CBC chain, calculated by Rasim et al.
[33], is obviously not compatible with the high-pressure phase transition. Indeed, some experimentally determined narrow levels in the band scheme may correlate with specific structural elements assumed to replace the CBC chain. However, the related optical absorption is far too small to explain the strong absorption in this range at ambient conditions.
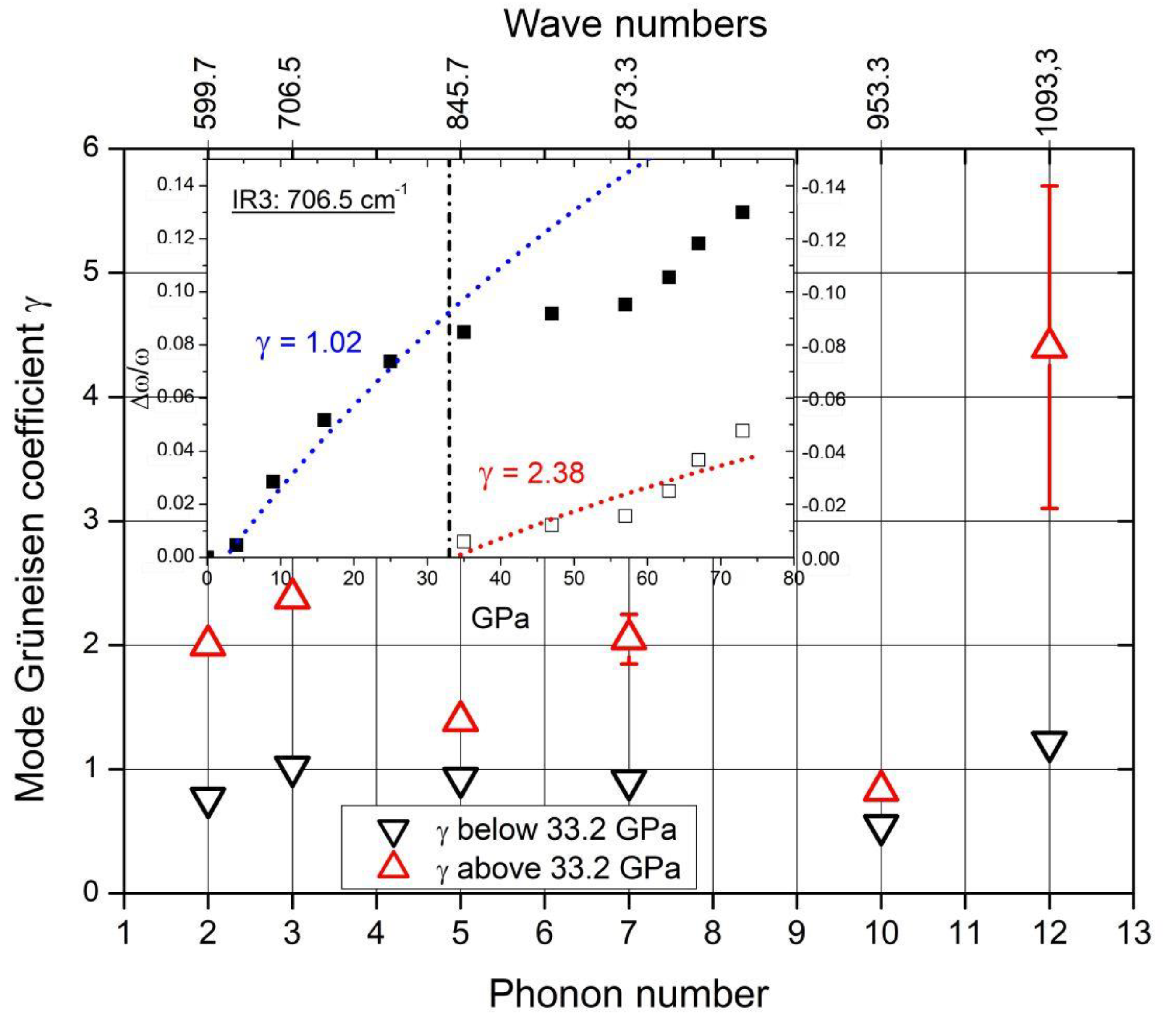
Therefore, the rearrangement of polar C atoms means a structural change and should be verifiable in the phonon spectra. The whole structure is affected by the phase transition at 33 GPa. This is proved by the phonon mode Grüneisen parameters γ. In the case of all IR- and Raman-active phonons, γ differs more or less considerably at pressure below and above the phase transition
[16][35][36]. Unfortunately, in the pressure-dependent IR-phonon spectra of Chuvashova et al.
[36], accordingly commented in
[18] (some results are shown in
Figure 13), the IR-active phonon near 400 cm
−1 has been neglected. As shown above, a component of this phonon is sensitive regarding polar C atoms in B
11C icosahedra.
Figure 13. IR phonon mode Grüneisen parameters below (▽) and above (△) the phase transition at 33.2 GPa. Insert: an example of determining γ.
The spectra of IR- and Raman-active phonons (Figure 4) exhibit strong, probably partially split peaks near 1100 cm−1. These are at least partially attributed to intra- and inter-icosahedral vibrations of polar atoms in the icosahedra. Hence, they should be affected by the rearrangement of polar C atoms.
In Figure 13, the Grüneisen coefficient of the 1093.3 cm−1 IR phonon above the phase transition considerably exceeds that of the other phonons. Moreover, the difference in the value below the phase transition is especially large. This suggests that the redistribution of polar C atoms increases the bonding force considerably.
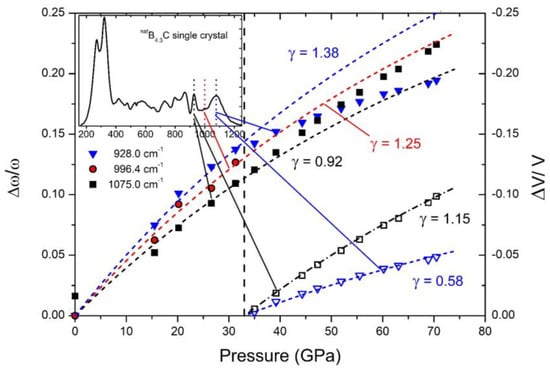
The Grüneisen coefficients concerning Raman-active phonons, determined in Figure 14, change significantly at the phase transition due to rearranging polar C atoms.
Figure 14. Relative shift of phonon frequencies (left ordinate) and unit cell volume (right ordinate) vs. pressure, determining mode γ of typical IR-active phonons of B
4.3C boron carbide
[35]. Insert: FT-Raman spectrum of single-crystal B
4.3C boron carbide at ambient conditions. Reprinted from Ref.
[35] published by Elsevier Masson SAS. All rights reserved.
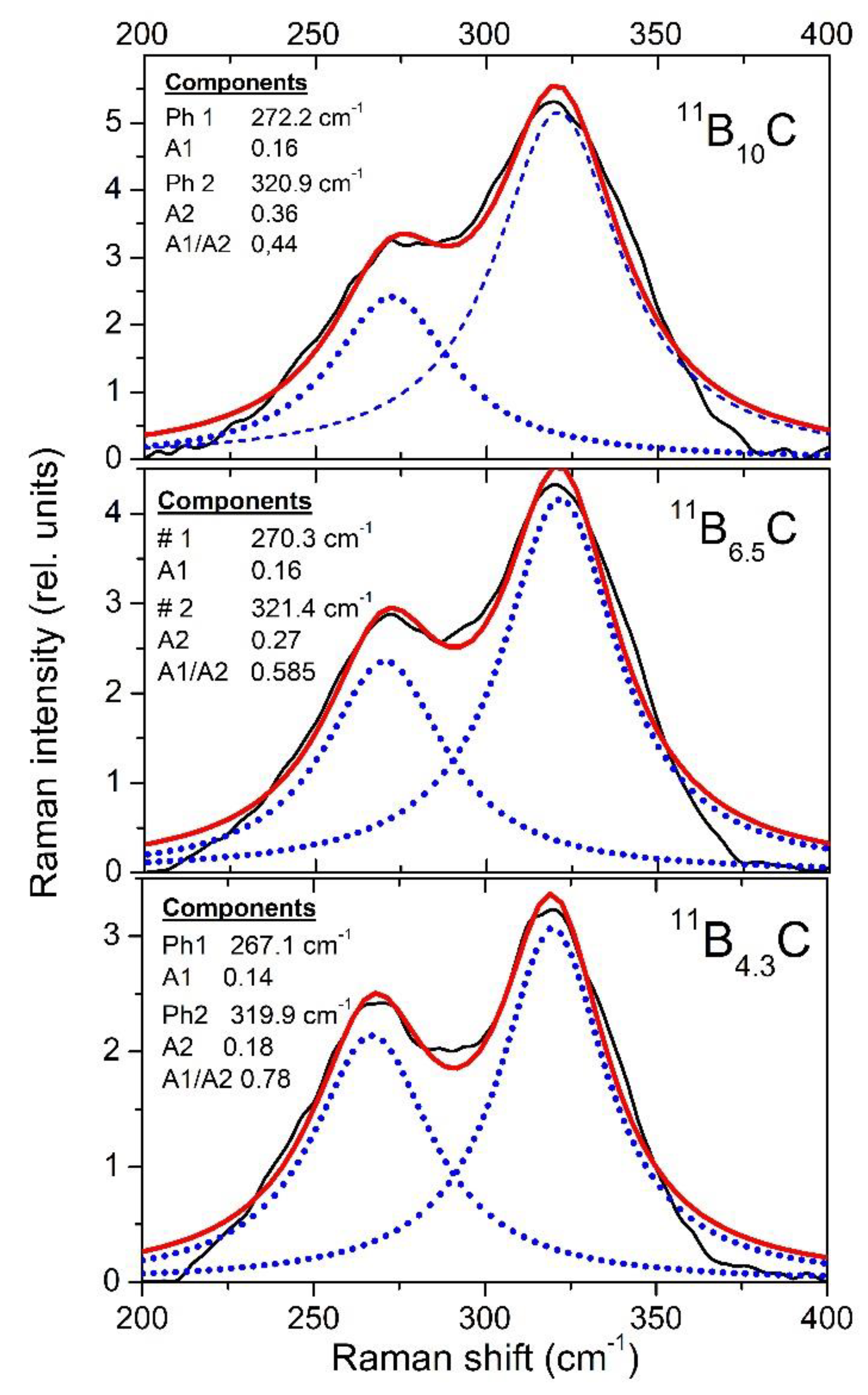
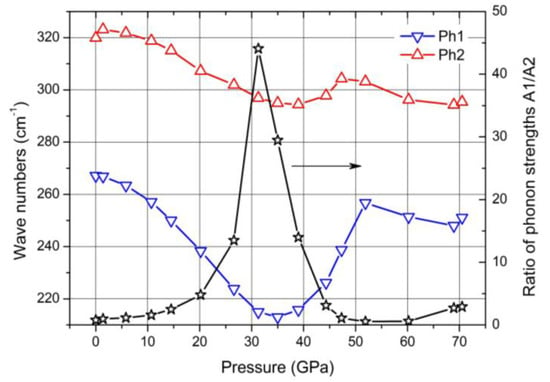
In the Raman spectra of boron carbide, the phonon doublet at 270/320 cm
−1 is the most striking feature. Werheit et al.
[37] assigned this doublet to the 335 cm
−1 Eg phonon that Shirai and Emura
[10] attributed to a rotation of the 3-atom chain combined with a wagging icosahedron. Firstly, it is surprising that this phonon splits, though there are only B
11C icosahedra in B
4.3C. This could be explained as follows. The translation vectors of atoms involved in this phonon (in Reference
[7]) show that 8 of 12 atoms in the icosahedra and 4 of 6 polar sites, respectively, are involved. Assuming that the C atom is randomly distributed over all polar sites of the icosahedra, there are B
11C icosahedra with C atoms on sites that are involved in this vibration, while others contain exclusively B atoms on such sites. Due to different m
red, the phonon mode splits and the components Ra 1 (~270 cm
−1; C on one of the relevant sites) and Ra 2 (~320 cm
−1; B atoms only) could be attributed accordingly.
The negative Grüneisen coefficients of both components differ fundamentally from all other phonons. Normally, compression reduces the lattice parameters, leading to a positive Grüneisen parameter, at least when the force constant is unaffected. However, that seems to be the case in boron carbide in connection with the rearrangement of polar C atoms at the high-pressure phase transition.
In
Figure 15, some parameters of both components of this doublet are displayed. For the evaluation, it had to be considered that the spectra were obtained using a laser with problematic excitation energy (for details, see
[32]). While the phonon frequencies are not affected this way, the phonon strengths A1 and A2 derived from the measured spectra depend uncontrollably on pressure. This influence is eliminated by determining the ratio A1/A2 of both components shown in
Figure 14. Based on the description of the 270/320 cm
−1 phonon doublet above,
Figure 15 indicates a strong site exchange of polar C atoms within the phase transition around 32 GPa.
Figure 15. Wave numbers of the Raman-active 270/320 cm−1 doublet and ratio of the phonon strengths of both components.