Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Madalina Paraschiv | -- | 2365 | 2023-10-23 10:42:08 | | | |
| 2 | Jason Zhu | Meta information modification | 2365 | 2023-10-25 03:37:46 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Postaru, M.; Tucaliuc, A.; Cascaval, D.; Galaction, A. Types of Cellular Stress. Encyclopedia. Available online: https://encyclopedia.pub/entry/50669 (accessed on 04 March 2026).
Postaru M, Tucaliuc A, Cascaval D, Galaction A. Types of Cellular Stress. Encyclopedia. Available at: https://encyclopedia.pub/entry/50669. Accessed March 04, 2026.
Postaru, Madalina, Alexandra Tucaliuc, Dan Cascaval, Anca-Irina Galaction. "Types of Cellular Stress" Encyclopedia, https://encyclopedia.pub/entry/50669 (accessed March 04, 2026).
Postaru, M., Tucaliuc, A., Cascaval, D., & Galaction, A. (2023, October 23). Types of Cellular Stress. In Encyclopedia. https://encyclopedia.pub/entry/50669
Postaru, Madalina, et al. "Types of Cellular Stress." Encyclopedia. Web. 23 October, 2023.
Copy Citation
With the start of the fermentation process, different stressful factors appear in the environment that directly affect the yeasts. Among them are osmotic, oxidative, and ethanol stresses, nitrogen starvation, low external pH, heat shock, prolonged anaerobiosis or the appearance of toxic molecules. As a shield against them, microorganisms have created defense responses specific to each type of stress, as well as a general environmental stress response (ESR).
Saccharomyces cerevisiae
yeast fermentation
oxidative stress
ethanol stress
osmotic stress
1. Oxidative stress
Oxidative stress results from the cells’ inability to reduce or eliminate reactive oxygen species and reactive nitrogen species (superoxide radical anion O2•−—the primordial reactive oxygen species, hydroxyl radicals, peroxynitrite, singlet oxygen, and hydrogen peroxide) or to repair the molecular damage produced by them [1][2][3][4][5]. In aerobic conditions, the generation of ROS takes place continuously (Figure 1), these being metabolic side products or the result of cellular control systems. Maintaining low concentrations of reactive oxygen species (ROS), below 10−8 M, is achieved by the generation and degradation mechanisms achieved through specific and non-specific cellular mechanisms [6].
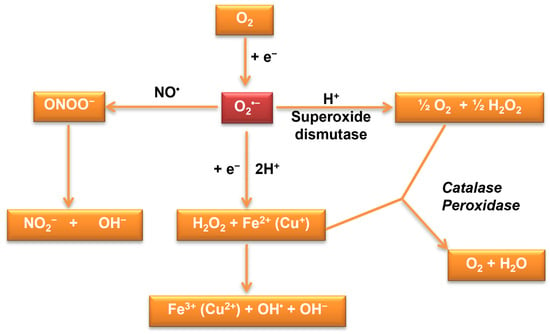
Figure 1. Reactive oxygen species production—antioxidant defense system regulating ROS levels to maintain physiological homeostasis [1].
Among the factors which determine oxidative stress, metabolism and aerobic respiration contribute the most to this process. A very recent study compared, by using an AI model and neural network algorithm, cellular morphology under basal and stress conditions and concluded that the changes induced by stress due to a high concentration of glucose in the medium were actually due to osmotic stress [7].
In the process of cell development, the microorganism culture goes through several stages, including the lag phase (adaptation), the exponential phase (active cell division), the stationary phase (in which the number of newly formed cells is equal to that of dead cells), and the phase of cell death. In the exponential phase of cell growth, the energy produced is the result of glycolysis. The number of mitochondria is reduced, oxygen consumption is minimal, and so, in this phase, the activity of antioxidant enzymes is also reduced. A responsible factor for oxidative stress is ethanol, by acting on the reactive oxygen species present in the mitochondria [8][9]. In the stationary phase, the cells use the ethanol obtained in the previous phase as an energy source. The number of mitochondria is increased, as a result of their need to oxidize ethanol, so, in this phase, the generation of reactive oxygen species intensifies. Thus, the transition of the cell culture to the stationary phase can cause the occurrence of oxidative stress [9]. The transformation of molecular oxygen into ROS leads to certain phenomena, such as protein oxidation, lipid peroxidation or DNA mutations. These actions occur even though the cells contain antioxidant defense mechanisms [10].
Oxidative stress can also be caused by compounds or substances such as tetrachlorobisphenol A or xylene [11][12]. In addition, through the autooxidation processes of some molecules, some oxidases generate reduced amounts of ROS, such as NADPH oxidase, xanthine oxidase, cyclooxygenases, and lipoxygenases [13].
The defense against oxidative stress is based on enzymatic and non-enzymatic mechanisms, which have the role of maintaining the level of reactive oxygen species within normal limits. The action of the enzymatic part in yeasts is based on the presence of two catalases (A and T) and two superoxide dismutases (Cu/Zn SOD and Mn/Zn SOD). Catalases have the role of breaking down H2O2 into H2O inside the peroxisome and cytosol. Superoxide dismutases convert the superoxide anion into oxygen and H2O2 in the cytoplasm and mitochondria. Enzymes that act against oxidative stress also play a very important role in the ethanol tolerance of yeasts. They eliminate the reactive oxygen species that appear in the presence of alcohol [8]. Studies have shown that S. cerevisiae develops the ability to survive in stress conditions that could become lethal, if it was previously exposed to low doses of cellular stress of the same or a different type [14].
Melatonin is a hormone that protects the human body from oxidative stress, but also from that caused by ethanol. It acts as an antioxidant both for S. cerevisiae and for non-Saccharomyces microorganisms. Directly, melatonin eliminates reactive oxygen species, and indirectly decreases the amount of oxidized glutathione and activates the genes involved in the response to oxidative stress. Given this information, some studies claim that S. cerevisiae produces melatonin to defend itself against the oxidative stress caused by the presence of ethanol and its consequences [8][15][16][17].
Other substances with an antioxidant role are carotenoids. In this case, their chemical structure plays an important role. The nature of the terminal group, the substituents they have in the composition or the length of the polyene chromophore are some of the aspects that determine the stopping of reactive oxygen species from producing unwanted effects [18].
2. Osmotic Stress—Stress Due to Environmental Salts
S. cerevisiae is a microorganism with a high sensitivity to environmental salts. High concentrations can limit water activity, thus leading to cell growth problems [19][20]. Over time, cells have developed a tolerance to high salt concentrations, thus being able to adapt to water fluctuations in the environment. At the same time, this tolerance has an important role in the processes of maintaining or restructuring biological, physiological or morphological functions [19][21][22].
Several strains of S. cerevisiae were analyzed to study salt resistance. Following exposure to different molar concentrations of K+ (from 0.025 M to 2 M), cells were found to lose viability at 1.5 M K+. It was also observed that S. cerevisiae strains exhibited a decrease in cell density ranging from 37 to 65% compared to the reference cultures, where they all had approximately the same density. Also, although the concentration of K+ increased, some strains of S. cerevisiae were able to consume all the glucose in the medium and convert ethanol to acetic acid. In addition to K+, the microorganisms were also exposed to different concentrations of Na+. Some S. cerevisiae strains showed a 79% decrease in growth rate at only 0.5 M Na+, while others had a 59% decrease at 2 M Na+. In the case of sodium use, the cell density of S. cerevisiae chains was between 25% and 68% compared to the reference values [19].
Some studies have shown, following the analysis of the microorganism Deinococcus radiodurans, that it has a gene which increases its tolerance to environmental stress. This was isolated and then combined with the genetic material of a strand of S. cerevisiae. After exposure to different salt concentrations (2%, 3%, 4%, 5%, and 7%), it was observed that normal strands of S. cerevisiae can withstand a maximum salt content in the medium of 5%. However, the strand of S. cerevisiae that contained the pprI gene, originating from the microorganism Deinococcus radiodurans, tolerated the salt concentration of 7% very well [23].
Another recent study investigated how the adhesion and cytotoxicity of positively charged polystyrene nanoparticles from two yeast cultures are affected in the presence of the NaCl salt. The nanocarriers were exposed for 24 h to NaCl concentrations ranging from 5 to 600 mM and temperatures from 4 to 25 °C. The yeast strains used were Saccharomyces cerevisiae and Schizosaccharomyces pombe. At the end of the research, it was observed that, for the survival of the two cultures, at a temperature of 4 °C, NaCl concentrations of 100 mM for S. pombe and 150 mM for S. cerevisiae were necessary. In the case of S. cerevisiae, the degree of adhesion decreased with increasing NaCl concentration at a temperature of 25 °C and an exposure time of less than 4 h. Thus, at NaCl values higher than 150 mM, the adhesion became almost insignificant. In terms of cytotoxicity, the higher the degree of adhesion at the beginning, the more pronounced the cell death. Regarding S. pombe, the degree of adhesion increased at the same time as the NaCl concentration. Thus, it was observed that the cells coated with nanoparticles had a mortality reduced by 50% at an exposure time of less than 4 h [24].
Water activity describes the chemical yield of free water in a solution. One of its uses is to help classify microorganisms into: halophile, halotolerant, and halosensitive. Halophile microorganisms have the ability to grow in environments with high salinity, while halo-sensitive ones are drastically affected by such amounts. At their core, halo-tolerant microorganisms are those that do not require certain amounts of salt to grow and can be viable over a wide range of salinity values in the environment. In order to be classified into one of the categories above, yeasts must have a certain value for water activity. Thus, for a value lower than 0.70, microorganisms are considered halophile. However, most of them develop at values between 0.90 and 0.95 [25].
Stress caused by environmental salts can lead to hyperosmotic stress, but also to specific cation toxicity. Its action on S. cerevisiae causes the microorganism to activate certain protective steps. First, some mechanisms are activated that prevent cell death following the change in osmolarity. Processes ensue that are intended to protect, repair, and recover from the osmotic effects and sodium toxicity. Finally, cells resume their growth process through readaptation conditions. However, S. cerevisiae has a long-term defense process that relies on osmotic changes driven by osmolyte synthesis and cation transport to remove sodium [26].
The waste water produced in the pharmaceutical, textile, chemical or food industries is very harmful to the environment. It contains large amounts of pollutants and salts that make the biodegradation process difficult. Although there are certain treatment processes, they involve the appearance of new substances at the end. For this reason, bioaugmentation was researched. The technology accelerates the destruction of refractory pollutants by involving microorganisms in a classic biological treatment system. Thus, it was found that halophile yeasts are effective in cleaning polluted waters. An example is Meyerozyma guilliermondii W2, which has a high degree of survivability and growth in high salinity environments. In one study, the use of microorganisms increased the chemical oxygen demand (COD) removal percentage from 67% to 94% after the bioaugmentation process [27].
Related to the salt tolerance of yeasts, a study was carried out with the aim to determine the effects of carotenoid substances in the defense against salt stress. Thus, strains of Sporidiobolus pararoseus NGR were exposed to different concentrations of NaCl. HPLC results showed that carotenoid products released by Sporidiobolus pararoseus NGR protect microorganisms against salt stress. Separately, amounts of diphenylamine were added to some samples to evaluate the behavior of carotenoids. It was observed that the synthesis of the substances was inhibited, thus no longer able to protect the cells of Sporidiobolus pararoseus NGR against NaCl in the environment [18].
3. Stress Due to Ethanol Accumulation
Ethanol is both the product resulting from the alcoholic fermentation of yeasts, but also one of the factors that lead to the appearance of stress at the cellular level. As the fermentation process progresses, more and more ethanol is produced. This leads to harmful actions against the yeast cells, such as blocking cell proliferation, depolarizing the cytoskeleton, or altering the activity of transport systems [28]. To respond effectively to stress conditions, yeast reprograms its cellular activity to protect important cell components for normal cellular activity and a higher degree of survival [29].
The structure of the yeast cell wall protects it against permanent changes in the external environment and it prevents cell lysis resulting from changes in osmotic conditions. The alcohol causes the disruption of cell integrity by intercalation in the hydrophilic layer inside the lipid bilayer of the membrane, thus increasing cell permeability [14][30]. The presence of ethanol during fermentation subjects yeasts to continuous stress. Thus, most of the time, cell viability is reduced, followed by the full blockage of the process for obtaining a certain product [8]. To prevent this, cells use several protective mechanisms. Among them are the activation of some heat shock proteins, the increase in the level of unsaturated fatty acids and ergosterol in the plasma membrane or the accumulation of the intracellular trehalose (known to replace the water molecule to stabilize proteins and membranes from desiccation and to protect yeast cells from thermal denaturation) content in the cells [31][32][33][34]. Additionally, studies demonstrate that ATPase in the plasma membrane also controls the tolerance of yeast cells to the stress due to ethanol, as this enzyme is reported to be activated by the alcohol [35]. Vacuolar and membrane ATPase are involved in the recovery after the acidification at a cytosol level induced by the presence of alcohol, by pumping protons into the vacuole and outside the cell, due the effect of alcohol in order to induce a membrane permeabilization effect through a passive influx of protons [29][36][37].
As for the concentration of ethanol in the environment, it produces certain effects both at low and high levels. At a low percentage, the yeast cells undergo a slowdown in division, resulting in slower growth. At high concentration, the fluidity of the cell membrane is improved, but cell viability decreases, the electrochemical gradient decreases, and the activity of glycolytic enzymes is affected by protein denaturation and the increase in the percentage of insoluble proteins [34]. By analyzing the effect of ethanol concentration on yeast proteins, it was observed that the inhibition of the process is dependent on it. To support the statement, a study was carried out on two cultures of Saccharomyces cerevisiae, one commercial, for wine production, and one of laboratory type. Both were subjected to alcohol concentrations from 6% to 14%. Cell growth was completely stopped at a concentration of 12% for laboratory strain and 14% for the commercial one. In another study, one set of cells was subjected to 10% ethanol. As a result, protein aggregates formed and the translation of the genetic material was repressed. Thus, it can be said that, at values higher than 10%, the degradation of the proteasome is strongly affected [8][28].
The ethanol resulting from fermentation has harmful effects on the yeast cell membrane. To avoid cell damage, some studies have shown that Mg2+ ions exhibit protective actions against ethanol stress. Other researchers report that maintaining a balance of K+ ions in the cell membrane lessens ethanol stress, increasing fermentative activity. Resveratrol is a polyphenol capable of increasing ethanol tolerance. This increase occurs by decreasing lipid peroxidation and superoxide dismutase activity [8][38].
Sometimes, the exposure of yeast cells to ethanol stress may have positive effects. For example, the activity of the ubiquitin–proteasome proteolytic system can be analyzed during this process. For certain proteins, ethanol leads to the suppression of proteolysis, but after its removal, the degradation resumes. In other words, the inhibition of proteasomal degradation is a reversible process [28].
References
- Eleutherio, E.; Brasil, A.A.; França, M.B.; de Almeida, D.S.G.; Rona, G.B.; Magalhães, S.S. Oxidative stress and aging: Learning from yeast lessons. Fungal Biol. 2018, 122, 514–525.
- Herrero, E.; Ros, J.; Belli, G.; Cabiscol, E. Redox control and oxidative stress in yeast cells. Biochim. Biophys. Acta 2008, 1780, 1217–1235.
- Moreno-Garcia, J.; Mauricio, J.C.; Moreno, J.; Garcia-Martinez, T. Stress responsive proteins of a flor yeast strain during the early stages of biofilm formation. Process Biochem. 2016, 51, 578–588.
- Vázquez, J.; Grillitsch, K.; Daum, G.; Mas, A.; Beltran, G.; Torija, M.J. The role of the membrane lipid composition in the oxidative stress tolerance of different wine yeasts. Food Microbiol. 2019, 78, 143–154.
- Ayer, A.; Fazakerley, D.J.; James, D.E.; Stocker, R. The role of mitochondrial reactive oxygen species in insulin resistance. Free Radic. Biol. Med. 2022, 179, 339–362.
- Lushchak, V.I. Adaptive response to oxidative stress: Bacteria, fungi, plants and animals. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2011, 153, 175–190.
- Itto-Nakama, K.; Watanabe, S.; Ohnuki, S.; Kondo, N.; Kikuchi, R.; Nakamura, T.; Ogasawara, W.; Kasahara, K.; Ohya, Y. Prediction of ethanol fermentation under stressed conditions using yeast morphological data. J. Biosci. Bioeng. 2023, 135, 210–216.
- Sunyer-Figueres, M.; Mas, A.; Beltran, G.; Torija, M.J. Protective Effects of Melatonin on Saccharomyces cerevisiae under Ethanol Stress. Antioxidants 2021, 10, 1735.
- Lushchak, V.I. Oxidative stress in yeast. Biochemistry 2010, 75, 346–364.
- Inderpal, M. Oxidative Stress in Saccharomyces cerevisiae. Doctoral Dissertation, University of KwaZulu-Natal, Durban, South Africa, 2017.
- Ji, Z.; Zhang, Y.; Tian, J.; Wang, F.; Song, M.; Li, H. Oxidative stress and cytotoxicity induced by tetrachlorobisphenol A in Saccharomyces cerevisiae cells. Ecotoxicol. Environ. Saf. 2018, 161, 1–7.
- Yashimoto, N.; Kawai, T.; Yoshida, M.; Izawa, S. Xylene causes oxidative stress and pronounced translation repression in Saccharomyces cerevisiae. J. Biosci. Bioeng. 2019, 128, 697–703.
- Puddu, P.; Puddu, G.M.; Cravero, E.; Rosati, M.; Muscari, A. The molecular sources of reactive oxygen species in hypertension. Blood Press. 2008, 17, 70–77.
- Ndukwe, J.K.; Aliyu, G.O.; Onwosi, C.; Chukwu, K.O.; Ezugworie, F.N. Mechanisms of weak acid-induced stress tolerance in yeasts: Prospects for improved bioethanol production form lignocellulosic biomass. Process Biochem. 2020, 90, 118–130.
- Romero, A.; Ramos, E.; de Los Rios, C.; Egea, J.; del Pino, J.; Reiter, R.J. A review of metal-catalyzed molecular damage: Protection by melatonin. J. Pineal Res. 2014, 56, 343–370.
- Bisquert, R.; Muniz-Calvo, S.; Guillamon, J.M. Protective Role of Intracellular Melatonin Against Oxidative Stress and UV Radiation in Saccharomyces cerevisiae. Front. Microbiol. 2018, 9, 318.
- Morcillo-Parra, M.A.; Beltran, G.; Mas, A.; Torija, M.J. Effect of Several Nutrients and Environmental Conditions on Intracellular Melatonin Synthesis in Saccharomyces cerevisiae. Microorganims 2020, 8, 853.
- Li, C.; Zhang, N.; Li, B.; Xu, Q.; Song, J.; Wei, N.; Wang, W.; Zou, H. Increased torulene accumulation in red yeast Sporidiobolus pararoseus NGR as stress response to high salt conditions. Food Chem. 2017, 237, 1041–1047.
- Illarionov, A.; Lahtvee, P.-J.; Kumar, R. Potassium and Sodium Salt Stress Characterization in the Yeasts Saccharomyces cerevisiae, Kluyveromyces marxianus, and Rhodotorula toruloides. Appl. Environ. Microbiol. 2021, 87, e0310020.
- Dakal, T.C.; Solieri, L.; Giudici, P. Adaptive response and tolerance to sugar and salt stress in the food yeast Zygosaccharomyces rouxii. Int. J. Food Microbiol. 2014, 185, 140–157.
- Yancey, P.H. Organic osmolytes as compatible, metabolic and counteracting cytoprotectants in high osmolarity and other stresses. J. Exp. Biol. 2005, 208, 2819–2830.
- Klipp, E.; Nordlander, B.; Kruger, R.; Gennemark, P.; Hohmann, S. Integrative model of the response of yeast to osmotic shock. Nat. Biotechnol. 2005, 23, 975–982.
- Helalat, S.H.; Bidaj, S.; Samani, S.; Moradi, M. Producing alcohol and salt stress tolerant strain of Saccharomyces cerevisiae by heterologous expression of pprI gene. Enzyme Microb. Technol. 2019, 124, 17–22.
- Shinto, H.; Kojima, M.; Shigaki, C.; Hirohashi, Y.; Seto, H. Effect of salt concentration and exposure temperature on adhesion and cytotoxicity of positively charged nanoparticles toward yeast cells. Adv. Powder Technol. 2022, 33, 103835.
- Tokuoka, K. Sugar- and salt-tolerant yeasts. J. Appl. Bacteriol. 1993, 74, 101–110.
- Dinu, L.D.; Craciun, T. Salt stress resistance in yeast is heterogeneous. Period. Mineral. 2022, 91, 18–29.
- Wen, H.; Xiong, K.; Yang, H.; Zhang, P.; Wang, X. Dynamic mechanism of the microbiota of high-salinity organic wastewater with salt-tolerant yeast and its application. J. Environ. Chem. Eng. 2022, 10, 107377.
- Nguyet, V.T.A.; Furutani, N.; Ando, R.; Izawa, S. Acquired resistance to severe ethanol stress-induced inhibition of proteasomal proteolysis in Saccharomyces cerevisiae. Biochim. Biophys. Acta Gen. Subj. 2022, 1866, 130241.
- Saini, P.; Beniwal, A.; Kokkiligadda, A.; Vij, S. Response and tolerance of yeast to changing environmental stress during ethanol fermentation. Process Biochem. 2018, 72, 1–12.
- Charoenbhakdi, S.; Dokpikur, T.; Burphan, T.; Techo, T.; Auesukaree, C. Vacuolar H+-ATPase protects Saccharomyces cerevisiae cells against ethanol-induced oxidative and cell wall stresses. Appl. Environ. Microbiol. 2016, 82, 3121–3130.
- Francois, J.M.; Walther, T.; Parrou, J.L. Genetics and Regulation of Glycogen and Trehalose Metabolism in Saccharomyces cerevisiae. In Microbial Stress Tolerance for Biofuels. Microbiology Monographs; Liu, Z., Ed.; Springer: Berlin/Heidelberg, Germany, 2012; Volume 22, pp. 29–54.
- Ciobanu, C.; Tucaliuc, A.; Cascaval, D.; Turnea, M.; Galaction, A.I. Correlation between aeration and ergosterol production by yeasts. Environ. Eng. Manag. J. 2019, 18, 2747–2756.
- Pupyshev, A.B.; Klyushnik, T.P.; Akopyan, A.A.; Singh, S.K.; Tikhonova, M.A. Disaccharide trehalose in experimental therapies for neurodegenerative disorders: Molecular targets and translational potential. Pharmacol. Res. 2022, 183, 106373.
- Singh, L.; Sharma, S.C.; Rai, J. Role of Hal5p protein kinase under ethanol stress in Saccharomyces cerevisiae. Appl. Biol. Chem. J. (TABCJ) 2023, 4, 44–53.
- Lei, J.; Zhao, X.; Ge, X.; Bai, F. Ethanol tolerance and the variation of plasma membrane composition of yeast floc populations with different size distribution. J. Biotechnol. 2007, 131, 270–275.
- Martinez-Munoz, G.A.; Kane, P. Vacuolar and Plasma Membrane Proton Pumps Collaborate to Achieve Cytosolic pH Homeostasis in Yeast. J. Biol. Chem. 2008, 283, 20309–20319.
- Auesukaree, C. Molecular mechanisms of the yeast adaptive response and tolerance to stresses encountered during ethanol fermentation. J. Biosci. Bioeng. 2017, 124, 133–142.
- Walker, G.M.; Basso, T.O. Mitigating stress in industrial yeasts. Fungal Biol. 2020, 124, 387–397.
More
Information
Subjects:
Biotechnology & Applied Microbiology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
981
Revisions:
2 times
(View History)
Update Date:
25 Oct 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





