Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Bo Wang | -- | 2401 | 2023-10-22 15:29:51 | | | |
| 2 | Lindsay Dong | Meta information modification | 2401 | 2023-10-23 05:15:04 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Liang, P.; Zhang, J.; Wang, B. Emerging Roles of Ubiquitination in Biomolecular Condensates. Encyclopedia. Available online: https://encyclopedia.pub/entry/50648 (accessed on 07 February 2026).
Liang P, Zhang J, Wang B. Emerging Roles of Ubiquitination in Biomolecular Condensates. Encyclopedia. Available at: https://encyclopedia.pub/entry/50648. Accessed February 07, 2026.
Liang, Peigang, Jiaqi Zhang, Bo Wang. "Emerging Roles of Ubiquitination in Biomolecular Condensates" Encyclopedia, https://encyclopedia.pub/entry/50648 (accessed February 07, 2026).
Liang, P., Zhang, J., & Wang, B. (2023, October 22). Emerging Roles of Ubiquitination in Biomolecular Condensates. In Encyclopedia. https://encyclopedia.pub/entry/50648
Liang, Peigang, et al. "Emerging Roles of Ubiquitination in Biomolecular Condensates." Encyclopedia. Web. 22 October, 2023.
Copy Citation
Biomolecular condensates are dynamic non-membrane-bound macromolecular high-order assemblies that participate in a growing list of cellular processes, such as transcription, the cell cycle, etc. Disturbed dynamics of biomolecular condensates are associated with many diseases, including cancer and neurodegeneration. Extensive efforts have been devoted to uncovering the molecular and biochemical grammar governing the dynamics of biomolecular condensates and establishing the critical roles of protein posttranslational modifications (PTMs) in this process.
ubiquitin
biomolecular condensates
liquid–liquid phase separation
stress granules
autophagy
1. Introduction
Eukaryotic cells have evolved prominent compartments to fulfill more efficient and sophisticated regulation of biochemical reactions and signaling. A considerable proportion of the cellular compartments are not membrane-bound; these are now collectively known as biomolecular condensates. The thermodynamics of biomolecular condensates is fundamentally distinct from those of membrane-bound compartments, such as mitochondria. For instance, the dynamics of biomolecular condensates is significantly faster than those of membrane-bound organelles, allowing biomolecular condensates to rapidly respond to acute environmental or intracellular stimuli [1]. Biomolecular condensates are implicated in an ever-growing list of biological pathways, such as transcription, translation, etc. [2]. Aberrant dynamics of biomolecular condensates is deemed an important contributor to many diseases, such as cancer and neurodegeneration [2][3].
Work from the last decade has established the widely appreciated role of liquid–liquid phase separation (LLPS) in the dynamics of biomolecular condensates. LLPS refers to the spontaneous demixing of biomolecular solutions, including protein and/or nucleic acid, into a condensed liquid phase that is biophysically and biochemically distinct from the surroundings [4]. Often, protein LLPS is dependent on unstructured regions—also known as intrinsically disordered regions (IDRs)—that engage proteins in either weak intra- or inter-molecular promiscuous interactions. Alternatively, protein interaction motifs facilitate LLPS and provide multivalency to membrane-less compartments by stoichiometrically interacting with binding partners [5]. These two mechanisms, however, are not mutually exclusive. Instead, both can cooperatively govern biomolecular LLPS and, therefore, the dynamics of complex membrane-less networks in a context-dependent fashion. While the mechanisms of how a protein’s primary amino-acid sequence dictates the material properties of biomolecular condensates are understood to a great extent [6], how posttranslational modifications (PTMs) in proteins, such as phosphorylation and ubiquitination, function as a molecular switch to fine-tune the dynamics of biomolecular condensates is less well understood.
Ubiquitination is a widespread and reversible PTM that covalently attaches ubiquitin, a small conserved regulatory protein (76 residues), to the target proteins (most commonly lysine residues). It involves stepwise catalyzation by a cascade of enzymes that are categorized as E1, E2, and E3. This modification involves either a single ubiquitin protein (mono-ubiquitylation) or a chain of ubiquitin (poly-ubiquitylation and multi-ubiquitination). In the case of poly-ubiquitination, the secondary ubiquitin molecules are always attached to one of the seven lysine residues (known as K6, K11, K27, K29, K33, K48, and K63 ubiquitination) or the N-terminal methionine (known as M1 ubiquitination) of the previous ubiquitin molecule.
K48- and K63-linked poly-ubiquitin chains represent the two most prevalent types of ubiquitin chains in cells [7][8]. K48-linked poly-ubiquitin serves as a crucial modification that mediates protein degradation [9]. A disruption in cellular redox balance leads to the extensive accumulation of oxidized proteins. K48-linked ubiquitination plays an essential role in the degradation pathway of these oxidized proteins [10]. In the event of DNA damage, ring finger protein 8 (RNF8), an E3 ubiquitin–protein ligase, applies K48-ubiquitin modifications to key regulatory factors at the damage sites. These modifications are subsequently removed, leading to a rearrangement of the signaling complex that facilitates the proper assembly of downstream factors at the damage site and, ultimately, driving DNA repair [11]. K63 ubiquitination is of significant importance as an initial event in signal transduction pathways associated with both innate and adaptive immunity [12]. K63-linked ubiquitination plays a central role in regulating NF-κB activation and inflammatory responses [13]. Moreover, K63-linked chains are known to promote autophagy within cells. When K63-linked ubiquitination is enhanced, the resulting ubiquitin-positive inclusions exhibit a higher degree of colocalization with the autophagy receptor p62 [14].
Various forms of non-canonical protein ubiquitination (such as K6, K11, and K27) exist in cells, and they play pivotal roles in the degradation of obsolete proteins across diverse signaling pathways [15]. K6-linked ubiquitination, for instance, is integral to mitophagy. When mitochondria become dysfunctional, PTEN-induced kinase 1 (PINK1) targets the mitochondrial outer membrane and phosphorylates ubiquitin associated with the outer membrane proteins [16]. This action recruits Parkin, an E3 ubiquitin ligase, which subsequently decorates damaged mitochondrial outer membrane proteins with K6- and K63-linked chains, thereby designating the mitochondria for autophagy [17]. In addition, K11- and K27-linked ubiquitination has been reported to control the cell cycle, DNA damage responses, and viral infections, among other cellular processes [18][19][20][21].
2. Ubiquitin in Stress Granule Dynamics
Stress granules are membrane-less organelles located in the cytoplasm, formed in response to various environmental threats, such as high temperature, oxidative stress, and viral infections [22]. Stress granules are messenger ribonucleoprotein complexes (mRNPs) containing stalled mRNA, RNA binding proteins (RBPs), translation initiation factors, and other proteins [23]. RBPs, such as Ras GTPase-activating protein (SH3 domain)-binding proteins 1 and 2 (G3BP1/2), interact with untranslated mRNAs and undergo LLPS, giving rise to stable “cores” [24]. These cores then recruit additional stress granule nucleators, resulting in the formation of more dynamic peripheral “shell”-like structures [24]. Once the external stimulus subsides, stress granules disassemble to release the RBPs and mRNAs, which coincides with translation recovery [25]. This cellular mechanism enables the efficient recycling of stress-granule components, including proteins and mRNAs, to avoid de novo synthesis [25]. The dynamics of stress granules is extensively regulated by PTMs, such as phosphorylation and methylation [26].
Various elements of ubiquitination-associated machinery, including E1 ubiquitin-activating enzyme, E3 ubiquitin ligases (the histone E3 ligase 2 (Hel2), anaphase promoting complex (APC), tripartite motif protein family members 21 (TRIM21), etc.), deubiquitylases (ubiquitin-specific-processing proteases USP5, USP10, and USP13), ubiquitin-binding proteins (the ubiquitin-binding proteins ubiquilin 2 (UBQLN2), histone deacetylase 6 (HDAC6)), the 26S proteasome, and valosin-containing protein (VCP/p97), have been found in the SG proteome [24][27][28][29][30][31][32][33][34][35].
Acute inhibition of protein ubiquitination by the E1 inhibitor, TAK243, does not appear to prevent stress granule formation in response to heat shock or arsenite [36][37], suggesting that active protein ubiquitination is likely not essential for stress granule formation, per se. On the contrary, several ubiquitin-binding proteins have been directly linked to stress-granule formation, including UBQLN2, which acts as a proteasome shuttle to deliver the ubiquitinated substrates for degradation [38]. UBQLN2 forms liquid condensates both in vitro and in vivo, which are inhibited with mono-ubiquitin or poly-ubiquitin chains [31]. UBQLN2 localizes to stress granules and interact with several stress granule proteins, including fused in sarcoma (FUS) [39][40]. By fluidizing the FUS-RNA complex, UBQLN2 negatively regulates stress granule assembly [39]. Therefore, ubiquitination may serve as a switch between UBQLN2 recruitment to stress granules and UBQLN2-dependent shuttling of ubiquitinated stress-granule components. Moreover, HDAC6, as a unique member of the class II deacetylase, containing a C-terminal zinc finger domain with a high binding affinity to free ubiquitin as well as mono- and poly-ubiquitinated proteins, is critical for stress-granule formation [30][41]. Pharmacological inhibition or genetic ablation of HDAC6 disrupts stress-granule assembly [30]. The seemingly conflicting findings from these studies likely stem from the context-dependent regulation of ubiquitination in stress-granule formation.
Different stressors elicit distinct ubiquitination patterns within the stress-granule proteome. Specifically, heat shock results in prominent ubiquitination of the stress-granule constituents, whereas arsenite, another common inducer of stress granules, does not [36]. This heat-shock-induced ubiquitination plays a crucial role in the resumption of cellular activities and stress-granule disassembly [36]. One of the stress-granule scaffold proteins, G3BP1, is ubiquitinated under heat-shock conditions. The ubiquitination of G3BP1 weakens the stress-granule-specific interaction network, and in the meantime, enhances its interaction with the endoplasmic-reticulum-associated protein and engages the ubiquitin-dependent segregase VCP/p97. As a segregase, VCP/p97 efficiently extracts the ubiquitinated G3BP1 from the stress-granule network and, ultimately, leading to stress-granule disassembly [42].
The fate of stress granules and the mechanism of their elimination are context-dependent. The ubiquitination of stress-granule components not only impacts stress-granule assembly/disassembly but also elimination under different conditions. It is demonstrated that stress granules induced by short-term heat stress (30 or 60-min) can be dissolved via disassembly, whereas stress granules formed under prolonged 90-min heat stress require autophagy for clearance [42]. In both cases, the segregase VCP is essential for stress-granule dissolution, either through direct disassembly or autophagy-dependent degradation, which may involve differential regulation of VCP via cofactors that are capable of directly binding to ubiquitin [32][42]. While the VCP-FAF2 (FAS-associated factor 2) complex is relatively well characterized in stress-granule disassembly, the molecular mechanisms underlying the VCP-mediated degradation of stress granules through autophagy are yet to be fully uncovered (Figure 1).
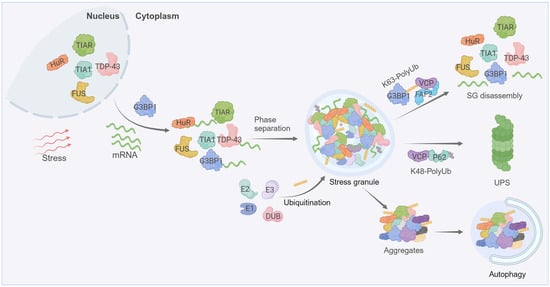
Figure 1. Stress-granule dynamics are tightly regulated by ubiquitination. Cells under stress inhibit the translation initiation of mRNAs, leading to stress granules’ formation that involves the phase separation of RBPs (such as G3BP1, T-cell intracellular antigen 1 (TIA-1), ELAV-like protein 1 (HuR), TIA1-related protein (TIAR) etc.) and mRNAs. Both the assembly and disassembly of stress granules are regulated by PTMs, including ubiquitination. The disassembly of stress granules is enhanced through the K63-linked ubiquitination of G3BP1, subsequently fostering the interaction between ubiquitin chains and VCP. Mutations in RBPs or prolonged stress cause the transition of stress granules into pathological aggregates. Ubiquitination governs the clearance of these aggregates, with K48-linked ubiquitin chains directing degradation through the ubiquitin-proteasome system (UPS), whereas K63-linked ubiquitination is associated with the autophagic degradation pathways. Created with BioRender.com.
3. Ubiquitin in Autophagy
Autophagy, an evolutionarily conserved process mediated by lysosomes, encapsulates a segment of cytoplasmic constituents by creating a double-membrane autophagosome, which is then targeted to the lysosome for degradation and subsequently macromolecule recycling [43][44]. Serving as a vital source of materials and energy for cells under stress, autophagy selectively eliminates the misfolded or surplus proteins to maintain cellular equilibrium [44][45]. Protein ubiquitination plays a pivotal role in this process. The initiation and nucleation phases of autophagosome formation are typically governed by ubiquitination [46].
Recent research has revealed that protein LLPS plays a crucial role in the assembly of autophagosomes and the condensation of autophagy substrates [47][48][49]. In budding yeast, the regulation of the cytoplasm-to-vacuole targeting (Cvt) pathway, an autophagy-related pathway trafficking proteolytic enzymes into vacuoles, is influenced by condensates formed through Ape1 phase separation. Ape1 utilizes phase separation to create a pliable assembly structure capable of engaging with the endoplasmic reticulum. This interaction is made possible by the strategic high-density positioning of Atg19 on its surface. Such interaction culminates in the sequestration of the Ape1 condensate into the Cvt vesicle [50]. The formation of a pre-autophagosomal structure (PAS), a dynamic and transient structure that regulates autophagosome formation on the vacuole, involves interactions between Atg1 and several proteins, including Atg13, Atg17, Atg29, and Atg31 [51]. When dephosphorylated, Atg13 forms an Atg1 complex with Atg1 and Atg17. This cross-linking activity prompts the Atg1 complex to form liquid-like condensates in vitro, which is responsible for organizing the PAS in vivo [52].
Under conditions of proteotoxic stress, such as the inactivation of chaperone proteins or proteasomes, proteins tagged with ubiquitin rapidly conglomerate into aggregates [53]. The creation of these aggregates is largely reliant on p62, as its depletion results in ubiquitinated proteins being more scattered within cells [54]. p62, as one of the first selective autophagy receptors discovered in mammals, is believed to mediate the selective autophagic degradation of poly-ubiquitinated protein aggregates [55]. p62 consists of a self-association PB1 domain, a UBA domain, and a LC3/Atg8 interaction region [56].
Under physiological conditions, the ubiquitination-mediated phase separation of the autophagic cargoes is regulated by multiple components [57]. The collaboration of p62, NBR1, and TAX1BP1 cargo receptors plays a crucial role in the formation and autophagic degradation of ubiquitin-enriched condensates [58][59]. Specifically, the phase separation of p62 and ubiquitin chains is considered as the primary driving force for the formation of autophagic cargo condensates [60][61]. NBR1 directly boosts condensation by offering a high-affinity UBA domain and simultaneously recruits TAX1BP1 and FIP200 to the p62-ubiquitin condensates. Moreover, the NBR1 UBA domain has a stronger affinity for ubiquitin compared to the UBA domain of p62, suggesting that hetero-oligomeric NBR1–p62 complexes may have a stronger affinity for ubiquitinated substrates than homo-oligomeric p62 [62]. Mitophagy is a specialized type of selective autophagy that eliminates damaged mitochondria. During mitophagy, mitochondrial proteins are often poly-ubiquitinated, which is recognized by p62 and several other receptors such as OPTN, NBR1, etc. [63]. The function and regulation of p62 in mitophagy can vary with the upstream inducers of mitophagy. In the context of celastrol-induced mitophagy, p62 cooperates with Nur77 to promote the efficient clearance of mitochondria. Nur77, a nuclear receptor, is ubiquitinated and translocated to the damaged mitochondria due to celastrol treatment. Ubiquitinated mitochondrial Nur77 interacts with the UBA domain of p62, and together they may form membrane-less condensates which can isolate damaged mitochondria.
4. Ubiquitin in Other Biomolecular Condensates
In addition to the relatively well-defined roles of ubiquitination in autophagosome formation and stress-granule dynamics, ubiquitination has also been implicated in several other biomolecular condensates.
Nuclear speckles are typical membrane-less biomolecular condensates located in the interchromatin-space of the nucleus, serving as the reservoir for RNA processing and splicing factors for mRNA alternative splicing [64]. Nuclear speckle assembly is driven by two large scaffold proteins, SRRM2 and SON [65][66]. Direct evidence that nuclear speckles arise through LLPS is lacking, which is at least partially due to technical challenges. Nevertheless, the existing evidence supports an active role of ubiquitination in nuclear speckle dynamics, likely through modulating LLPS. Several ubiquitin-associated proteins are detected in the nuclear speckles. Specifically, speckle-type POZ protein (SPOP) is a substrate adaptor of the cullin3-RING ubiquitin ligase that is frequently mutated in various solid tumors [67]. SPOP localizes to nuclear speckles, which is reliant on high-order oligomerization and substrate binding [68][69]. Cancer-related SPOP mutations disrupt its oligomerization, LLPS, nuclear speckle localization, and ubiquitin signaling [68]. The biological consequences of these SPOP mutants on nuclear speckle dynamics have yet to be explored. USP42 is a deubiquitylase bearing a positively-charged C-terminus that is sufficient for USP42 LLPS and nuclear speckle formation [70].
Cells assemble dynamic and transient biomolecular condensates in response to external stress. An interesting type of nuclear condensate that contains proteasomal constituents and poly-ubiquitinated proteins is recently demonstrated to be triggered by acute hyperosmotic stress [71]. The formation of such nuclear proteasomal condensates is driven by the ubiquitin-binding shuttle protein RAD23B. The binding of RAD23B to poly-ubiquitin chains via its two UBA domains promotes RAD23B condensation. Subsequently, RAD23B recruits various proteasome-associated proteins, including the E3 ligase E6-AP and VCP, into these nuclear condensates via its ubiquitin-like (UBL) domain. RAD23B condensates are proteolytic centers that actively degrade specific ribosomal proteins.
References
- Banani, S.F.; Lee, H.O.; Hyman, A.A.; Rosen, M.K. Biomolecular condensates: Organizers of cellular biochemistry. Nat. Rev. Mol. Cell Biol. 2017, 18, 285–298.
- Boeynaems, S.; Alberti, S.; Fawzi, N.L.; Mittag, T.; Polymenidou, M.; Rousseau, F.; Schymkowitz, J.; Shorter, J.; Wolozin, B.; Van Den Bosch, L.; et al. Protein Phase Separation: A New Phase in Cell Biology. Trends Cell Biol. 2018, 28, 420–435.
- Alberti, S.; Dormann, D. Liquid-Liquid Phase Separation in Disease. Annu. Rev. Genet. 2019, 53, 171–194.
- Shin, Y.; Brangwynne, C.P. Liquid phase condensation in cell physiology and disease. Science 2017, 357, eaaf4382.
- Zhang, H.; Ji, X.; Li, P.; Liu, C.; Lou, J.; Wang, Z.; Wen, W.; Xiao, Y.; Zhang, M.; Zhu, X. Liquid-liquid phase separation in biology: Mechanisms, physiological functions and human diseases. Sci. China Life Sci. 2020, 63, 953–985.
- Li, J.; Zhang, M.; Ma, W.; Yang, B.; Lu, H.; Zhou, F.; Zhang, L. Post-translational modifications in liquid-liquid phase separation: A comprehensive review. Mol. Biomed. 2022, 3, 13.
- Swatek, K.N.; Komander, D. Ubiquitin modifications. Cell Res. 2016, 26, 399–422.
- Husnjak, K.; Dikic, I. Ubiquitin-binding proteins: Decoders of ubiquitin-mediated cellular functions. Annu. Rev. Biochem. 2012, 81, 291–322.
- Yau, R.; Rape, M. The increasing complexity of the ubiquitin code. Nat. Cell Biol. 2016, 18, 579–586.
- Manohar, S.; Jacob, S.; Wang, J.; Wiechecki, K.A.; Koh, H.W.L.; Simoes, V.; Choi, H.; Vogel, C.; Silva, G.M. Polyubiquitin Chains Linked by Lysine Residue 48 (K48) Selectively Target Oxidized Proteins In Vivo. Antioxid. Redox Signal 2019, 31, 1133–1149.
- Meerang, M.; Ritz, D.; Paliwal, S.; Garajova, Z.; Bosshard, M.; Mailand, N.; Janscak, P.; Hubscher, U.; Meyer, H.; Ramadan, K. The ubiquitin-selective segregase VCP/p97 orchestrates the response to DNA double-strand breaks. Nat. Cell Biol. 2011, 13, 1376–1382.
- Madiraju, C.; Novack, J.P.; Reed, J.C.; Matsuzawa, S.I. K63 ubiquitination in immune signaling. Trends Immunol. 2022, 43, 148–162.
- Chen, J.; Chen, Z.J. Regulation of NF-kappaB by ubiquitination. Curr. Opin. Immunol. 2013, 25, 4–12.
- Dosa, A.; Csizmadia, T. The role of K63-linked polyubiquitin in several types of autophagy. Biol. Futur. 2022, 73, 137–148.
- Tracz, M.; Bialek, W. Beyond K48 and K63: Non-canonical protein ubiquitination. Cell Mol. Biol. Lett. 2021, 26, 1.
- Pickrell, A.M.; Youle, R.J. The roles of PINK1, parkin, and mitochondrial fidelity in Parkinson’s disease. Neuron 2015, 85, 257–273.
- Ordureau, A.; Heo, J.M.; Duda, D.M.; Paulo, J.A.; Olszewski, J.L.; Yanishevski, D.; Rinehart, J.; Schulman, B.A.; Harper, J.W. Defining roles of PARKIN and ubiquitin phosphorylation by PINK1 in mitochondrial quality control using a ubiquitin replacement strategy. Proc. Natl. Acad. Sci. USA 2015, 112, 6637–6642.
- Castaneda, C.A.; Kashyap, T.R.; Nakasone, M.A.; Krueger, S.; Fushman, D. Unique structural, dynamical, and functional properties of k11-linked polyubiquitin chains. Structure 2013, 21, 1168–1181.
- Wu-Baer, F.; Lagrazon, K.; Yuan, W.; Baer, R. The BRCA1/BARD1 heterodimer assembles polyubiquitin chains through an unconventional linkage involving lysine residue K6 of ubiquitin. J. Biol. Chem. 2003, 278, 34743–34746.
- Wickliffe, K.E.; Williamson, A.; Meyer, H.J.; Kelly, A.; Rape, M. K11-linked ubiquitin chains as novel regulators of cell division. Trends Cell Biol. 2011, 21, 656–663.
- Wu, X.; Lei, C.; Xia, T.; Zhong, X.; Yang, Q.; Shu, H.B. Regulation of TRIF-mediated innate immune response by K27-linked polyubiquitination and deubiquitination. Nat. Commun. 2019, 10, 4115.
- Buchan, J.R.; Parker, R. Eukaryotic stress granules: The ins and outs of translation. Mol. Cell 2009, 36, 932–941.
- Kedersha, N.; Stoecklin, G.; Ayodele, M.; Yacono, P.; Lykke-Andersen, J.; Fritzler, M.J.; Scheuner, D.; Kaufman, R.J.; Golan, D.E.; Anderson, P. Stress granules and processing bodies are dynamically linked sites of mRNP remodeling. J. Cell Biol. 2005, 169, 871–884.
- Jain, S.; Wheeler, J.R.; Walters, R.W.; Agrawal, A.; Barsic, A.; Parker, R. ATPase-Modulated Stress Granules Contain a Diverse Proteome and Substructure. Cell 2016, 164, 487–498.
- Kedersha, N.L.; Gupta, M.; Li, W.; Miller, I.; Anderson, P. RNA-binding proteins TIA-1 and TIAR link the phosphorylation of eIF-2 alpha to the assembly of mammalian stress granules. J. Cell Biol. 1999, 147, 1431–1442.
- Ohn, T.; Anderson, P. The role of posttranslational modifications in the assembly of stress granules. Wiley Interdiscip. Rev. RNA 2010, 1, 486–493.
- Turakhiya, A.; Meyer, S.R.; Marincola, G.; Bohm, S.; Vanselow, J.T.; Schlosser, A.; Hofmann, K.; Buchberger, A. ZFAND1 Recruits p97 and the 26S Proteasome to Promote the Clearance of Arsenite-Induced Stress Granules. Mol. Cell 2018, 70, 906–919.e7.
- Xie, X.; Matsumoto, S.; Endo, A.; Fukushima, T.; Kawahara, H.; Saeki, Y.; Komada, M. Deubiquitylases USP5 and USP13 are recruited to and regulate heat-induced stress granules through their deubiquitylating activities. J. Cell Sci. 2018, 131, jcs210856.
- Takahashi, M.; Kitaura, H.; Kakita, A.; Kakihana, T.; Katsuragi, Y.; Onodera, O.; Iwakura, Y.; Nawa, H.; Komatsu, M.; Fujii, M. USP10 Inhibits Aberrant Cytoplasmic Aggregation of TDP-43 by Promoting Stress Granule Clearance. Mol. Cell Biol. 2022, 42, e0039321.
- Kwon, S.; Zhang, Y.; Matthias, P. The deacetylase HDAC6 is a novel critical component of stress granules involved in the stress response. Genes. Dev. 2007, 21, 3381–3394.
- Dao, T.P.; Kolaitis, R.M.; Kim, H.J.; O’Donovan, K.; Martyniak, B.; Colicino, E.; Hehnly, H.; Taylor, J.P.; Castaneda, C.A. Ubiquitin Modulates Liquid-Liquid Phase Separation of UBQLN2 via Disruption of Multivalent Interactions. Mol. Cell 2018, 69, 965–978.e6.
- Wang, B.; Maxwell, B.A.; Joo, J.H.; Gwon, Y.; Messing, J.; Mishra, A.; Shaw, T.I.; Ward, A.L.; Quan, H.; Sakurada, S.M.; et al. ULK1 and ULK2 Regulate Stress Granule Disassembly Through Phosphorylation and Activation of VCP/p97. Mol. Cell 2019, 74, 742–757.e8.
- Valdez-Sinon, A.N.; Lai, A.; Shi, L.; Lancaster, C.L.; Gokhale, A.; Faundez, V.; Bassell, G.J. Cdh1-APC Regulates Protein Synthesis and Stress Granules in Neurons through an FMRP-Dependent Mechanism. iScience 2020, 23, 101132.
- Yang, C.; Wang, Z.; Kang, Y.; Yi, Q.; Wang, T.; Bai, Y.; Liu, Y. Stress granule homeostasis is modulated by TRIM21-mediated ubiquitination of G3BP1 and autophagy-dependent elimination of stress granules. Autophagy 2023, 19, 1934–1951.
- Buchan, J.R.; Kolaitis, R.M.; Taylor, J.P.; Parker, R. Eukaryotic stress granules are cleared by autophagy and Cdc48/VCP function. Cell 2013, 153, 1461–1474.
- Maxwell, B.A.; Gwon, Y.; Mishra, A.; Peng, J.; Nakamura, H.; Zhang, K.; Kim, H.J.; Taylor, J.P. Ubiquitination is essential for recovery of cellular activities after heat shock. Science 2021, 372, eabc3593.
- Tolay, N.; Buchberger, A. Comparative profiling of stress granule clearance reveals differential contributions of the ubiquitin system. Life Sci. Alliance 2021, 4, e202000927.
- Itakura, E.; Zavodszky, E.; Shao, S.; Wohlever, M.L.; Keenan, R.J.; Hegde, R.S. Ubiquilins Chaperone and Triage Mitochondrial Membrane Proteins for Degradation. Mol. Cell 2016, 63, 21–33.
- Alexander, E.J.; Ghanbari Niaki, A.; Zhang, T.; Sarkar, J.; Liu, Y.; Nirujogi, R.S.; Pandey, A.; Myong, S.; Wang, J. Ubiquilin 2 modulates ALS/FTD-linked FUS-RNA complex dynamics and stress granule formation. Proc. Natl. Acad. Sci. USA 2018, 115, E11485–E11494.
- Peng, G.; Gu, A.; Niu, H.; Chen, L.; Chen, Y.; Zhou, M.; Zhang, Y.; Liu, J.; Cai, L.; Liang, D.; et al. Amyotrophic lateral sclerosis (ALS) linked mutation in Ubiquilin 2 affects stress granule assembly via TIA-1. CNS Neurosci. Ther. 2022, 28, 105–115.
- Hook, S.S.; Orian, A.; Cowley, S.M.; Eisenman, R.N. Histone deacetylase 6 binds polyubiquitin through its zinc finger (PAZ domain) and copurifies with deubiquitinating enzymes. Proc. Natl. Acad. Sci. USA 2002, 99, 13425–13430.
- Gwon, Y.; Maxwell, B.A.; Kolaitis, R.M.; Zhang, P.; Kim, H.J.; Taylor, J.P. Ubiquitination of G3BP1 mediates stress granule disassembly in a context-specific manner. Science 2021, 372, eabf6548.
- Dikic, I.; Elazar, Z. Mechanism and medical implications of mammalian autophagy. Nat. Rev. Mol. Cell Biol. 2018, 19, 349–364.
- Mizushima, N.; Komatsu, M. Autophagy: Renovation of cells and tissues. Cell 2011, 147, 728–741.
- Levine, B.; Kroemer, G. Autophagy in the pathogenesis of disease. Cell 2008, 132, 27–42.
- Goodall, E.A.; Kraus, F.; Harper, J.W. Mechanisms underlying ubiquitin-driven selective mitochondrial and bacterial autophagy. Mol. Cell 2022, 82, 1501–1513.
- Fujioka, Y.; Noda, N.N. Biomolecular condensates in autophagy regulation. Curr. Opin. Cell Biol. 2021, 69, 23–29.
- Noda, N.N.; Wang, Z.; Zhang, H. Liquid-liquid phase separation in autophagy. J. Cell Biol. 2020, 219, e202004062.
- Lu, Y.; Chang, C. Phase Separation in Regulation of Autophagy. Front. Cell Dev. Biol. 2022, 10, 910640.
- Yamasaki, A.; Alam, J.M.; Noshiro, D.; Hirata, E.; Fujioka, Y.; Suzuki, K.; Ohsumi, Y.; Noda, N.N. Liquidity Is a Critical Determinant for Selective Autophagy of Protein Condensates. Mol. Cell 2020, 77, 1163–1175.e9.
- Feng, Y.; He, D.; Yao, Z.; Klionsky, D.J. The machinery of macroautophagy. Cell Res. 2014, 24, 24–41.
- Fujioka, Y.; Alam, J.M.; Noshiro, D.; Mouri, K.; Ando, T.; Okada, Y.; May, A.I.; Knorr, R.L.; Suzuki, K.; Ohsumi, Y.; et al. Phase separation organizes the site of autophagosome formation. Nature 2020, 578, 301–305.
- Pankiv, S.; Clausen, T.H.; Lamark, T.; Brech, A.; Bruun, J.A.; Outzen, H.; Overvatn, A.; Bjorkoy, G.; Johansen, T. p62/SQSTM1 binds directly to Atg8/LC3 to facilitate degradation of ubiquitinated protein aggregates by autophagy. J. Biol. Chem. 2007, 282, 24131–24145.
- Danieli, A.; Martens, S. p62-mediated phase separation at the intersection of the ubiquitin-proteasome system and autophagy. J. Cell Sci. 2018, 131, jcs214304.
- Kumar, A.V.; Mills, J.; Lapierre, L.R. Selective Autophagy Receptor p62/SQSTM1, a Pivotal Player in Stress and Aging. Front. Cell Dev. Biol. 2022, 10, 793328.
- Zaffagnini, G.; Savova, A.; Danieli, A.; Romanov, J.; Tremel, S.; Ebner, M.; Peterbauer, T.; Sztacho, M.; Trapannone, R.; Tarafder, A.K.; et al. p62 filaments capture and present ubiquitinated cargos for autophagy. EMBO J. 2018, 37, e98308.
- Peng, H.; Yang, J.; Li, G.; You, Q.; Han, W.; Li, T.; Gao, D.; Xie, X.; Lee, B.H.; Du, J.; et al. Ubiquitylation of p62/sequestosome1 activates its autophagy receptor function and controls selective autophagy upon ubiquitin stress. Cell Res. 2017, 27, 657–674.
- Rasmussen, N.L.; Kournoutis, A.; Lamark, T.; Johansen, T. NBR1: The archetypal selective autophagy receptor. J. Cell Biol. 2022, 221, e202208092.
- Adriaenssens, E.; Ferrari, L.; Martens, S. Orchestration of selective autophagy by cargo receptors. Curr. Biol. 2022, 32, R1357–R1371.
- Sun, D.; Wu, R.; Zheng, J.; Li, P.; Yu, L. Polyubiquitin chain-induced p62 phase separation drives autophagic cargo segregation. Cell Res. 2018, 28, 405–415.
- Herhaus, L.; Dikic, I. Ubiquitin-induced phase separation of p62/SQSTM1. Cell Res. 2018, 28, 389–390.
- Turco, E.; Savova, A.; Gere, F.; Ferrari, L.; Romanov, J.; Schuschnig, M.; Martens, S. Reconstitution defines the roles of p62, NBR1 and TAX1BP1 in ubiquitin condensate formation and autophagy initiation. Nat. Commun. 2021, 12, 5212.
- Vargas, J.N.S.; Hamasaki, M.; Kawabata, T.; Youle, R.J.; Yoshimori, T. The mechanisms and roles of selective autophagy in mammals. Nat. Rev. Mol. Cell Biol. 2023, 24, 167–185.
- Spector, D.L.; Lamond, A.I. Nuclear speckles. Cold Spring Harb. Perspect. Biol. 2011, 3, a000646.
- Ilik, I.A.; Malszycki, M.; Lubke, A.K.; Schade, C.; Meierhofer, D.; Aktas, T. SON and SRRM2 are essential for nuclear speckle formation. Elife 2020, 9, e60579.
- Xu, S.; Lai, S.K.; Sim, D.Y.; Ang, W.S.L.; Li, H.Y.; Roca, X. SRRM2 organizes splicing condensates to regulate alternative splicing. Nucleic Acids Res. 2022, 50, 8599–8614.
- Cuneo, M.J.; Mittag, T. The ubiquitin ligase adaptor SPOP in cancer. FEBS J. 2019, 286, 3946–3958.
- Bouchard, J.J.; Otero, J.H.; Scott, D.C.; Szulc, E.; Martin, E.W.; Sabri, N.; Granata, D.; Marzahn, M.R.; Lindorff-Larsen, K.; Salvatella, X.; et al. Cancer Mutations of the Tumor Suppressor SPOP Disrupt the Formation of Active, Phase-Separated Compartments. Mol. Cell 2018, 72, 19–36.e8.
- Marzahn, M.R.; Marada, S.; Lee, J.; Nourse, A.; Kenrick, S.; Zhao, H.; Ben-Nissan, G.; Kolaitis, R.M.; Peters, J.L.; Pounds, S.; et al. Higher-order oligomerization promotes localization of SPOP to liquid nuclear speckles. EMBO J. 2016, 35, 1254–1275.
- Liu, S.; Wang, T.; Shi, Y.; Bai, L.; Wang, S.; Guo, D.; Zhang, Y.; Qi, Y.; Chen, C.; Zhang, J.; et al. USP42 drives nuclear speckle mRNA splicing via directing dynamic phase separation to promote tumorigenesis. Cell Death Differ. 2021, 28, 2482–2498.
- Yasuda, S.; Tsuchiya, H.; Kaiho, A.; Guo, Q.; Ikeuchi, K.; Endo, A.; Arai, N.; Ohtake, F.; Murata, S.; Inada, T.; et al. Stress- and ubiquitylation-dependent phase separation of the proteasome. Nature 2020, 578, 296–300.
More
Information
Subjects:
Cell Biology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
398
Revisions:
2 times
(View History)
Update Date:
23 Oct 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




