Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Nico Cellinese | -- | 2405 | 2023-10-19 17:18:01 | | | |
| 2 | Catherine Yang | -1 word(s) | 2404 | 2023-10-20 03:19:59 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Krupar, S.; Naranjo, A.A.; Godden, G.; Cellinese, N. Guzmania monostachia in Florida Rests with Humans. Encyclopedia. Available online: https://encyclopedia.pub/entry/50571 (accessed on 07 February 2026).
Krupar S, Naranjo AA, Godden G, Cellinese N. Guzmania monostachia in Florida Rests with Humans. Encyclopedia. Available at: https://encyclopedia.pub/entry/50571. Accessed February 07, 2026.
Krupar, Shelby, Andre A. Naranjo, Grant Godden, Nico Cellinese. "Guzmania monostachia in Florida Rests with Humans" Encyclopedia, https://encyclopedia.pub/entry/50571 (accessed February 07, 2026).
Krupar, S., Naranjo, A.A., Godden, G., & Cellinese, N. (2023, October 19). Guzmania monostachia in Florida Rests with Humans. In Encyclopedia. https://encyclopedia.pub/entry/50571
Krupar, Shelby, et al. "Guzmania monostachia in Florida Rests with Humans." Encyclopedia. Web. 19 October, 2023.
Copy Citation
Land use changes by humans have eliminated more than half of the wetlands in Florida over the last 200 years, and additional losses are anticipated as a consequence of climate change and ongoing development activities that will accommodate a rapidly growing human population. Both spell danger for the biodiversity and ecosystem services in Florida, and data are needed to inform conservation priorities and actions concerning threatened or endangered wetland species.
Bromeliaceae
Florida
Guzmania monostachia
climate change
1. Introduction
The wetlands of Florida are species-rich and home to a variety of rare and charismatic plant and animal species, including the locally endangered Guzmania monostachia (L.) Rusby ex Mez (West Indian tufted airplant). Unfortunately, over half of the original wetlands in Florida have already been lost to dramatic land use changes made by humans, and both anthropogenic climate change and rapidly growing human populations threaten additional losses to wetland biodiversity throughout the state. Given its already significantly contracted distribution, the risk of permanently losing the genetic diversity found within a few remaining, isolated populations of G. monostachia in Florida is dangerously high. Thus, it is imperative that the potential factors that may contribute to additional habitat losses, as well as ecological or microclimate changes within the existing habitats where G. monostachia persists. This information will help identify critical areas for preservation and inform meaningful policies and practices for the conservation of G. monostachia now and into the distant future.
2. Guzmania monostachia in Florida
Guzmania is a member of the Bromeliaceae, a neotropical plant clade consisting of ca. 3140 species from 8 major lineages [1]. The group is placed in the Tillandsioideae, the largest clade within Bromeliaceae, which has an estimated stem age of 15.4 Ma and a proposed Andean origin [2][3]. In comparison to other Guzmania, G. monostachia has the broadest distribution, with populations in northern South America, Central America, the Caribbean, and South Florida [3]. Florida is home to 16 bromeliad species, all of which belong to the Tillandsioideae. Specimen records and historical observations suggest that many of these epiphytes were common throughout the forested freshwater wetlands of South Florida, where they thrived on the humid conditions and trees characterizing these habitats. However, bromeliads in South Florida have experienced rapid population declines recently due to human-related impacts such as habitat loss, poaching, and extensive damage by an invasive bromeliad-eating weevil from Mexico, Metamasius callizona Chevrolat, whose presence in Florida was first documented in 1989. Within its native range, M. callizona is known mainly as an occasional pest in shaded greenhouses, and observations of infestations on naturally occurring bromeliads are sparse [4]. In contrast, infestations on naturally occurring bromeliads in South Florida are common, and damage to individual plants is also more severe [5]. It is unclear why impacts to Floridian bromeliad populations have been so extensive, although a biological agent (e.g., a parasitoid wasp) may control populations of M. callizona within its native range [6].
Despite its inclusion on the Endangered and Threatened Species List for Florida, endangered conservation status, and rapidly declining plant numbers [7][8], basic research on G. monostachia is surprisingly lacking. Florida-based populations represent the northernmost limit of the species’ distribution range, but their evolutionary origin is unknown. Also lacking is information about the genetic identity, diversity, and population structure of the remaining populations and how these characteristics compare with those of other populations in the Americas and Caribbean. Morphological and observation-based evidence, including the close proximity of the gynoecium and androecium within a largely closed corolla, muted bract coloration, and apparent lack of pollinator visitation [9], suggests that Floridian plants may be exclusively autogamous and, consequently, genetically distinct, whereas outcrossing individuals with brighter flower color and hummingbird-mediated pollination have been observed in South America [10][11]. Autogamous breeding systems lead to reduced genetic diversity through inbreeding, increasing a population’s susceptibility to pests, pathogens, or environmental disturbances—for example, those resulting from climate change [12].
2. History of Ecosystems in Modern-Day Florida and Threats to Bromeliad Habitats
Florida is situated atop the shallow Florida Platform [13], a flat geological feature that includes the emergent portion that the researchers currently recognize as the Florida peninsula, and a submerged portion known as the West Florida Escarpment, which extends westward by more than 160 km into the Gulf of Mexico from the modern-day coastline. The Florida Platform is composed primarily of limestone that was deposited by carbonate-producing marine organisms during the Mesozoic (190–66 Ma; [14]). During the Miocene (~23 Ma ago), a portion of the Florida platform was exposed by lower sea levels, allowing silica-based sediments from the eroding Appalachian Mountains to flow southward onto the newly exposed limestone [15]. The fluctuating sea levels that occurred from the Miocene to the present day (~23 Ma ago to present) further affected sediment deposition processes, with submerged and newly exposed areas accumulating more carbonate and sediment, respectively. Roughly one-third to one-half of the Florida Platform is above sea level today, but the extent of the emergent portion has varied through time as a result of changing climate and oceanographic events [13], shaping both its geological form and biota. The superposition of climatic events, global sea level dynamics, and changing soil composition across the Florida Platform contributed to major transitions in environments, affecting species compositions and ranges in space and time, as well as increases and decreases in the areas of suitable habitats.
Florida is the only state in the continental United States (US) that is characterized by subtropical ecosystems, and 17% of its wildlife species do not occur in other states [16]. Thus, Florida harbors unique biodiversity and habitats that are vital to conserve. In particular, the wetlands of Florida have great ecological importance, providing habitat for numerous plant and animal species, sequestering carbon, removing pollutants from flowing water, and mitigating potential flooding to surrounding areas by acting as sinks [17]. Despite their significance, these habitats have sustained considerable damage inflicted by humans. From 1780 to 1980, Florida lost 9.3 million acres of wetlands—the largest loss in the USA. Currently, Florida has only 54% of its original wetland coverage remaining [18], with losses primarily caused by the draining and filling of wetlands for agricultural land and urban development.
In addition to areas lost to human development activities, many remaining wetlands across Florida are significantly altered. The plant community composition of wetlands is heavily dependent on hydrologic regimes [19], and the draining and filling of some regions can drastically affect wetlands elsewhere in the state [20]. Climate change also has the potential to alter hydrologic regimes, perhaps with drastic consequences. For example, under various climate models, mean annual precipitation is expected to decrease in the Everglades, leading to drier conditions and a shift away from plant communities that depend on deeper and longer flooding periods [21].
In Florida, G. monostachia inhabits primarily two types of ecosystems: pop ash/pond apple sloughs on the west coast and tropical hardwood hammocks on the east coast (Figure 1). Pop ash/pond apple sloughs are wetland ecosystems that are unique to South Florida and dominated by Fraxinus caroliniana Mill. (pop ash) and Annona glabra (L.) (pond apple). These ecosystems are characterized by long hydroperiods of deep water and mucky soil [22]. Tropical hardwood hammocks are densely vegetated with evergreen tropical trees, including Bursera simaruba (L.) Sarg. (gumbo limbo), Coccoloba diversifolia Jacq. (pigeon plum), and Quercus virginiana Mill. (southern live oak), and they support a wide variety of epiphytes, including bromeliads and orchids. These hardwood hammocks are relatively small and disjunct, perched on limestone outcrops elevated by at least one meter above the surrounding valleys, and characterized by shallow soils [23]. In Florida, these hammocks are found as far north as Martin County, near Lake Okeechobee, but occur primarily in the south, from northern Miami-Dade County through the Everglades, along the Miami Rock Ridge, and the southern portion of the geological formation known as the Atlantic Coastal Ridge [24].

Figure 1. Guzmania monostachia (a) in a tropical hardwood hammock habitat (b) and pop ash/pond apple slough (c).
Currently, six distinct populations of G. monostachia are known from five areas in Florida, and these populations are largely fragmented and disjunct (Figure 2). The area with the largest abundance of G. monostachia is the Fakahatchee Strand State Preserve, which is located in Collier County in southwest Florida. This preserve encompasses numerous pop ash/pond apple sloughs that host populations of G. monostachia. Prior to the introduction of M. callizona, the total estimated number of G. monostachia in Fakahatchee Strand exceeded 2 million individuals. However, only half that number are estimated to exist within the preserve today. Additionally, the preference of M. callizona for larger plants has led to a drastic shift in population demographics; the proportion of reproductively mature individuals within the population has been reduced from roughly 50% to only 10–20% today [25]. Adjacent to and east of the Fakahatchee Strand is Big Cypress National Preserve. The latter is home to two highly disjunct populations of G. monostachia, which are located at the extreme north and south ends of the preserve, respectively, and with nearly 2900 km2 of land between them. The northern population consists of roughly 200 individuals and is found within a drier pop ash/pond apple slough habitat that also contains cypress, whereas the southern population comprises approximately 1000 individuals and inhabits a more traditional pop ash/pond apple slough. A fourth area that includes G. monostachia is the Everglades National Park, which is home to a single population. The population is located on the east side of the park, within a tropical hardwood hammock that is only approximately 150 m in diameter. In stark contrast to the Fakahatchee Strand and Big Cypress populations, this population is currently in critical condition and includes only three individuals. The remaining populations are located in Fuchs and Meissner Hammock Preserve. These contiguous areas are located on the outskirts of Homestead and are surrounded by agricultural land and suburban housing. The larger Fuchs Hammock, which is historically known as Sykes Hammock, includes only three individuals of G. monostachia, while two individuals are present in the Meissner Hammock.
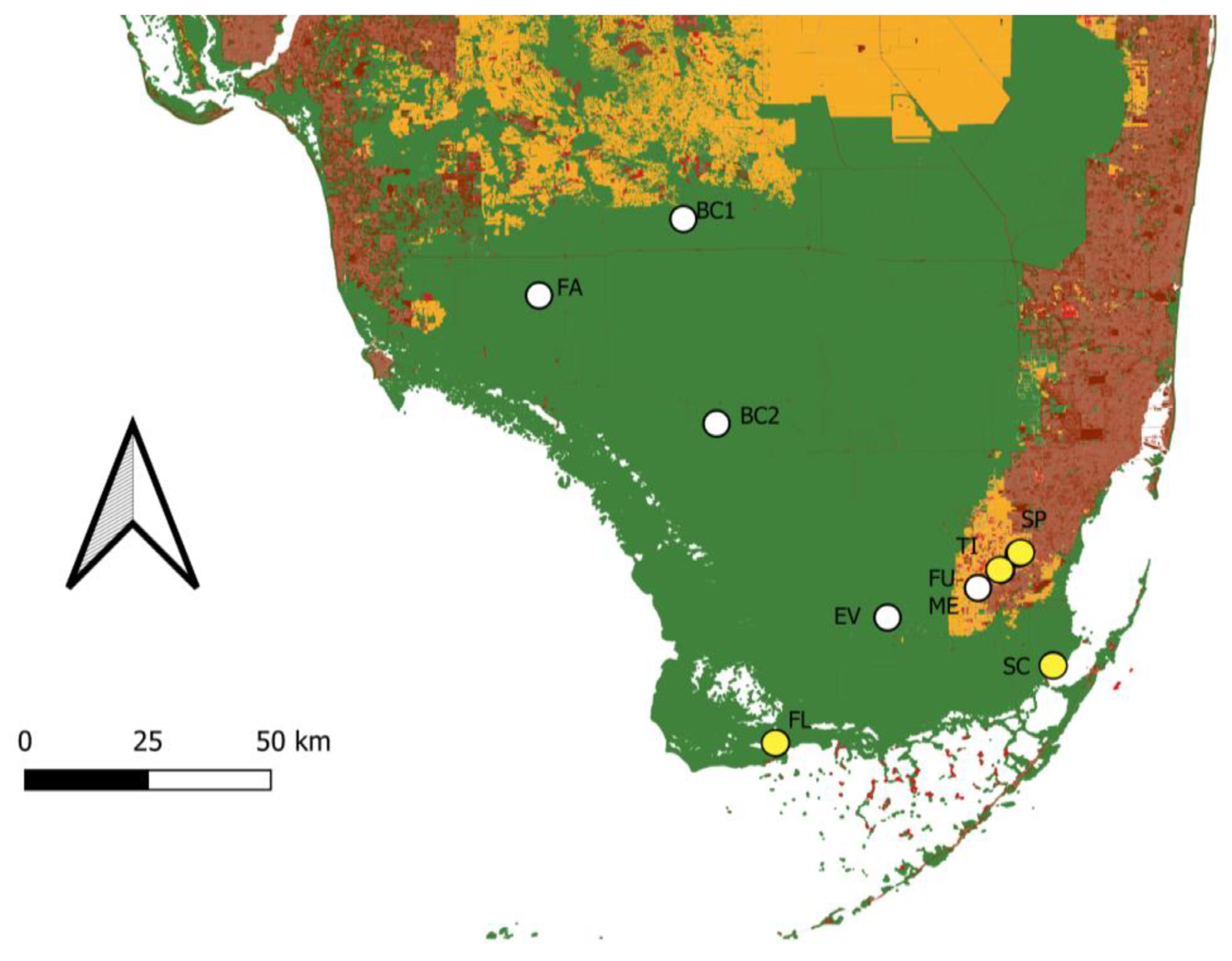
Figure 2. Land cover map of south Florida with general localities of known populations of Guzmania monostachia. White points are populations that are still present. Yellow points are populations that have been extirpated. Areas in green are natural lands. Areas in orange are agricultural lands. Areas in light red are rural areas. Areas in dark red are urbanized areas. Map constructed by using data from the Florida Cooperative Land Cover Map version 3.5. Population identifiers: Fakahatchee Strand (FA), Northern Big Cypress (BC1), Southern Big Cypress (BC2), Everglades (EV), Fuchs Hammock (FU), Meissner Hammock (ME), Flamingo (FL), South of Cutler (SC), Timms Hammock (TI), Silver Palm Hammock (SP).
The populations identified above represent all known populations existing in Florida today. However, historical records identify at least four additional areas where G. monostachia once occurred. The plants are presumably extirpated from these areas today, highlighting the drastic range contraction that G. monostachia has experienced within the last hundred years [8]. Interestingly, the areas were largely unaffected by weevil activity, which points to urbanization and land conversions as the most probable causes of range contractions in this species [26]. Two of the four extirpated populations existed within what is now known as the Miami Metropolitan Area, a region of urbanized land stretching 161 km along the southeastern coast of Florida in Broward, Miami-Dade, and Palm Beach Counties. This statistical area includes several large cities, such as Miami, Fort Lauderdale, and West Palm Beach [27]. This urbanized region has expanded rapidly in recent decades and is one of the most populous human settlements in the world. From 1950 to 1995, over 2100 km2 of natural land within Broward, Miami-Dade, and Palm Beach counties was converted to urban use [28]. This urban expansion has continued in recent decades, albeit at a slower rate. For example, the Miami Metropolitan Area gained only 860 km2 of urban area between 1992 and 2016 [27]. While several hammocks that historically supported populations of G. monostachia remain as preserved and protected natural lands within the Miami Metropolitan Area today, their hydrology and microclimates have likely changed considerably as a consequence of increasing urbanization in the areas surrounding them. Development activities by humans are likely to have disrupted historical patterns of hydrological flow within these areas, and the hammocks no longer offer suitable habitat conditions for G. monostachia.
3. Conserving Bromeliads in Florida
The conservation of native bromeliads is critical, in part because of the important ecosystem services they provide. Water collected in the leaf bases of tank bromeliads such as G. monostachia, which are known as a “phytotelmata”, sustains life for a variety of organisms, including some invertebrates that exist solely on these plants. Thus, it may be appropriate to view individual bromeliads as ecosystems within ecosystems [29], and the loss of these plants could have cascading negative effects on the biodiversity within a given area. Additionally, Florida populations of G. monostachia represent the northernmost edge of an extensive distribution range, which is the most extensive of any Guzmania, and may harbor genetic diversity that is potentially important to the long-term survival of the species. Numerous studies show that populations occupying the periphery of species ranges are often genetically distinct from those closer to the center [e.g., [30][31]]. As global temperatures rise and species ranges shift toward the poles, these leading-edge gene pools could prove critical for the evolutionary or adaptive potential of the species [32]. Thus, the genetic diversity of G. monostachia in Florida is critical to investigate, ultimately to be able to generate meaningful conservation strategies.
Ecological niche models (ENM) make use of known species occurrence data, as well as data about the environments in which they occur, to reconstruct past and future distribution ranges [33]. By using data from paleoclimatic and future climate projections, ENMs can help generate species distribution models (SDMs) that hypothesize historical and future geographic species ranges. ENMs have become an important tool for conservation, enabling researchers to make predictions about the impacts of climate change on the geographic ranges of imperiled species [34][35]. Predictions about the future range and distribution of G. monostachia in Florida can inform effective conservation strategies, priorities, and actions that ensure the persistence of G. monostachia in Florida by pinpointing populations at risk of extirpation, areas that should be preserved as potential future habitats for the species, and other important elements in their associated plant communities. Paleohistorical projections of the distribution of G. monostachia can provide both insights into the historical interactions between climate changes and distribution ranges and context for interpretations of current range and distribution patterns. Studies show that climate and distribution range interactions are generally conserved over paleohistorical time, with periods of warmer climate shifting distribution ranges toward the pole, and periods of cooler climate shifting distribution ranges toward the equator [36].
References
- Barfuss, M.H.J.; Till, W.; Leme, E.M.C.; Pinzón, J.P.; Manzanares, J.M.; Halbritter, H.; Samuel, R.; Brown, G.K. Taxonomic revision of Bromeliaceae subfam. Tillandsioideae based on a multi-locus DNA sequence phylogeny and morphology. Phytotaxa 2016, 279, 1.
- Givnish, T.; Millam, K.; Berry, P.; Sytsma, K. Phylogeny, Adaptive Radiation, and Historical Biogeography of Bromeliaceae Inferred from ndhF Sequence Data. Aliso 2007, 23, 3–26.
- Givnish, T.J.; Barfuss, M.H.J.; Van Ee, B.; Riina, R.; Schulte, K.; Horres, R.; Gonsiska, P.A.; Jabaily, R.S.; Crayn, D.M.; Smith, J.A.C.; et al. Phylogeny, adaptive radiation, and historical biogeography in Bromeliaceae: Insights from an eight-locus plastid phylogeny. Am. J. Bot. 2011, 98, 872–895.
- Frank, J.H.; Thomas, M.C. Metamasius callizona (CHEVROLAT) (COLEOPTERA: CURCULIONIDAE), AN IMMIGRANT PEST, DESTROYS BROMELIADS IN FLORIDA. Can. Entomol. 1994, 126, 673–682.
- Frank, J.H.; Cave, R.D. Metamasius callizona is destroying Florida’s native bromeliads. In Proceedings of the Second International Symposium on Biological Control of Arthropods, Davos, Switzerland, 2–16 September 2005.
- Frank, J.H. Exploration in Guatemala and Belize for more parasitoids to use against Metamasius callizona in Florida. J. Bromel. Soc. 2011, 61, 112–125.
- Sunquist-Blunden, C.; Montero-McAllister, N. Florida’s Endangered and Threatened Species; Florida Fish and Wildlife Conservation Commission: Tallahassee, FL, USA, 2022.
- Gann, G.D.; Stocking, G.C. Collaborators Floristic Inventory of South Florida Database Online. Available online: https://regionalconservation.org/ircs/database/database.asp (accessed on 22 September 2022).
- Krupar, S.L. (Department of Biology, University of Florida, Gainesville, FL, USA) Personal communication, 2022.
- Benzing, D.H. Bromeliaceae: Profile of An Adaptive Radiation; Cambridge University Press: Cambridge, UK, 2000; ISBN 0521430313.
- Cascante-Marín, A.; de Jong, M.; Borg, E.D.; Oostermeijer, J.G.B.; Wolf, J.H.D.; den Nijs, J.C.M. Reproductive strategies and colonizing ability of two sympatric epiphytic bromeliads in a tropical premontane area. Int. J. Plant Sci. 2006, 167, 1187–1195.
- Jump, A.S.; Marchant, R.; Peñuelas, J. Environmental change and the option value of genetic diversity. Trends Plant Sci. 2009, 14, 51–58.
- Hine, A.C. Geologic History of Florida, 2nd ed.; University Press of Florida: Gainesville, FL, USA, 2013.
- Gardulski, A.F.; Gowen, M.H.; Milsark, A.; Weiterman, S.D.; Wise, S.W.; Mullins, H.T. Evolution of a deep-water carbonate platform: Upper Cretaceous to Pleistocene sedimentary environments on the west Florida margin. Mar. Geol. 1991, 101, 163–179.
- Missimer, T.M.; Maliva, R.G. Late Miocene fluvial sediment transport from the southern Appalachian Mountains to southern Florida: An example of an old mountain belt sediment production surge. Sedimentology 2017, 64, 1846–1870.
- Dahl, T.E. Florida’s Wetlands: An Update on Status and Trends 1985 to 1996; U.S. Department of the Interior, Fish and Wildlife Service: Washington, DC, USA, 2005.
- McLaughlin, D.L.; Cohen, M.J. Realizing ecosystem services: Wetland hydrologic function along a gradient of ecosystem condition. Ecol. Appl. 2013, 23, 1619–1631.
- Dahl, T.E. Wetlands Losses in the United States 1780’s to 1980’s; U.S. Department of the Interior, Fish and Wildlife Service: Washington, DC, USA, 1990.
- Todd, M.J.; Muneepeerakul, R.; Pumo, D.; Azaele, S.; Miralles-Wilhelm, F.; Rinaldo, A.; Rodriguez-Iturbe, I. Hydrological drivers of wetland vegetation community distribution within Everglades National Park, Florida. Adv. Water Resour. 2010, 33, 1279–1289.
- Kushlan, J.A. External threats and internal management: The hydrologic regulation of the Everglades, Florida, USA. Environ. Manag. 1987, 11, 109–119.
- Todd, M.J.; Muneepeerakul, R.; Miralles-Wilhelm, F.; Rinaldo, A.; Rodriguez-Iturbe, I. Possible climate change impacts on the hydrological and vegetative character of Everglades National Park, Florida. Ecohydrology 2012, 5, 326–336.
- Comer, P.; Faber-Langendoen, D.; Evans, R.; Gawler, S.; Josse, C.; Kittel, G.; Menard, S.; Pyne, M.; Reid, M.; Schulz, K.; et al. Ecological Systems of the United States: A Working Classification of U.S. Terrestrial Systems; NatureServe: Arlington, VA, USA, 2003.
- Meeder, J.F.; Harlem, P.W. Origin and development of true karst valleys in response to late Holocene sea-level change, the Transverse Glades of southeast Florida, USA. Depos. Rec. 2019, 5, 558–577.
- Brooks, W.R.; Lockwood, J.L.; Jordan, R.C. Tropical paradox: A multi-scale analysis of the invasion paradox within Miami Rock Ridge tropical hardwood hammocks. Biol. Invasions 2013, 15, 921–930.
- Owen, M. (Park Biologist, Fakahatchee Strand Preserve State Park, Copeland, FL, USA) Personal communication, 2021.
- Frank, J.H. Bromeliad-Eating Weevils. Selbyana 1999, 20, 40–48.
- Rifat, S.A.A.; Liu, W. Quantifying spatiotemporal patterns and major explanatory factors of urban expansion in miami metropolitan area during 1992–2016. Remote Sens. 2019, 11, 2493.
- Walker, R.; Solecki, W. Theorizing Land-Cover and Land-Use Change: The Case of the Florida Everglades and Its Degradation. Ann. Assoc. Am. Geogr. 2004, 94, 311–328.
- Cooper, T.M.; Frank, J.H.; Cave, R.D. Loss of phytotelmata due to an invasive bromeliad-eating weevil and its potential effects on faunal diversity and biogeochemical cycles. Acta Oecol. 2014, 54, 51–56.
- Van Rossum, F.; Vekemans, X.; Gratia, E.; Meerts, P. A comparative study of allozyme variation of peripheral and central populations of Silene nutans L. (Caryophyllaceae) from Western Europe: Implications for conservation. Plant Syst. Evol. 2003, 242, 49–61.
- Hamilton, J.A.; Eckert, C.G. Population genetic consequences of geographic disjunction: A prairie plant isolated on Great Lakes alvars. Mol. Ecol. 2007, 16, 1649–1660.
- Gibson, S.Y.; Van Der Marel, R.C.; Starzomski, B.M. Climate Change and Conservation of Leading-Edge Peripheral Populations. Conserv. Biol. 2009, 23, 1369–1373.
- Peterson, A.T. Predicting species’ geographic distributions based on ecological niche modeling. Condor 2001, 103, 599–605.
- Kolanowska, M.; Rewicz, A.; Baranow, P. Ecological niche modeling of the pantropical orchid Polystachya concreta (Orchidaceae) and its response to climate change. Sci. Rep. 2020, 10, 14801.
- Naranjo, A.A.; Melton, A.E.; Soltis, D.E.; Soltis, P.S. Endemism, projected climate change, and identifying species of critical concern in the Scrub Mint clade (Lamiaceae). Conserv. Sci. Pract. 2022, 4, e621.
- Martínez-Meyer, E.; Peterson, A.T. Conservatism of ecological niche characteristics in North American plant species over the Pleistocene-to-Recent transition. J. Biogeogr. 2006, 33, 1779–1789.
More
Information
Subjects:
Biodiversity Conservation
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
580
Revisions:
2 times
(View History)
Update Date:
20 Oct 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




