Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Dulce Moncho | -- | 3859 | 2023-10-19 11:28:30 | | | |
| 2 | Lindsay Dong | Meta information modification | 3859 | 2023-10-23 02:56:55 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Moncho, D.; Poca, M.A.; Rahnama, K.; Sánchez Roldán, M.�.; Santa-Cruz, D.; Sahuquillo, J. Neurophysiology in Managing Patients with Chiari Malformations. Encyclopedia. Available online: https://encyclopedia.pub/entry/50536 (accessed on 07 February 2026).
Moncho D, Poca MA, Rahnama K, Sánchez Roldán M�, Santa-Cruz D, Sahuquillo J. Neurophysiology in Managing Patients with Chiari Malformations. Encyclopedia. Available at: https://encyclopedia.pub/entry/50536. Accessed February 07, 2026.
Moncho, Dulce, Maria A. Poca, Kimia Rahnama, M. Ángeles Sánchez Roldán, Daniela Santa-Cruz, Juan Sahuquillo. "Neurophysiology in Managing Patients with Chiari Malformations" Encyclopedia, https://encyclopedia.pub/entry/50536 (accessed February 07, 2026).
Moncho, D., Poca, M.A., Rahnama, K., Sánchez Roldán, M.�., Santa-Cruz, D., & Sahuquillo, J. (2023, October 19). Neurophysiology in Managing Patients with Chiari Malformations. In Encyclopedia. https://encyclopedia.pub/entry/50536
Moncho, Dulce, et al. "Neurophysiology in Managing Patients with Chiari Malformations." Encyclopedia. Web. 19 October, 2023.
Copy Citation
Chiari malformation type 1 (CM1) includes various congenital anomalies that share ectopia of the cerebellar tonsils lower than the foramen magnum, in some cases associated with syringomyelia or hydrocephalus. CM1 can cause dysfunction of the brainstem, spinal cord, and cranial nerves. This functional alteration of the nervous system can be detected by various modalities of neurophysiological tests, such as brainstem auditory evoked potentials, somatosensory evoked potentials, motor evoked potentials, electromyography and nerve conduction studies of the cranial nerves and spinal roots, as well as brainstem reflexes.
blink reflex
brainstem auditory evoked potentials
brainstem reflexes
Chiari type 1 malformation
electromyography
evoked potentials
intraoperative neurophysiological monitoring
motor evoked potentials
somatosensory evoked potentials
syringomyelia
1. Introduction
Chiari malformations (CMs) comprise a series of neurodevelopmental disorders characterized by a descent of the cerebellar tonsils through the foramen magnum (FM) [1][2][3][4][5]. CM type 1 (CM1) was first described by the Austrian pathologist Hans Chiari in two papers published in 1891 and 1895 [2][3][4]. Nevertheless, the term “Arnold-Chiari malformation” must still be introduced to search in PubMed or other databases for the literature on this abnormality. However, due to Arnold’s minor role in the original description, “Chiari malformation” is the most extended eponym [6]. In Chiari’s original description, four types of malformations were differentiated (CM1, CM2, CM3, and CM4). CM1 is traditionally defined as a tonsillar descent of 3–5 mm below the FM, quantified in a mid-sagittal section of magnetic resonance imaging (MRI). The choice of cut-off point for tonsillar descent (3 or 5 mm) is somewhat arbitrary and varies depending on the criteria of the different authors [7][8][9][10][11][12][13]. CM type 2 (CM2) is characterized by a descent of the structures of the brainstem or vermis below the FM and is always associated with spinal dysraphisms (spina bifida) in addition to tonsillar ectopia. Patients with CM2 also present a series of brain anomalies associated with spina bifida that are not observed in any other type of CM (gray matter heterotopia, polygyria, and descended tentorium, among others). CM type 3 (craniocervical encephalocele) and CM type 4 (cerebellar hypoplasia) [14] are severe malformations with a low incidence that most authors consider unrelated to CM1 and CM2. The recent International Consensus Conference recommended considering CM3 and CM4 as separate entities [15].
Other variants of CM1 have been described as minor or major forms of CM1. In 1998, Iskandar et al. coined the term “Chiari 0” (CM0) to describe five pediatric patients with syringomyelia, no tonsillar herniation, and a “tight” posterior fossa (PF), which improved after PF decompression. Subsequent studies by this and other groups have confirmed this entity and observed a significant volumetric reduction in the PF and alterations in cerebrospinal fluid (CSF) dynamics around the FM as the common etiopathogenic factor in CM0. Some authors suggested the term “tight cisterna magna” to define the same entity [16][17][18][19].
An additional type, often not included in canonical classifications, is comprised of patients in which any type of CM is associated with different osseous malformations of the craniovertebral junction (CVJ). In a previous paper, scholars proposed the term ‘complex CVJ abnormalities’ when patients present tonsillar herniation and at least two of the following abnormalities: a significant retroflexed odontoid, a basilar impression (BI), platybasia, severe bone abnormalities in the C0–C2 complex, uni- or bilateral occipital condyle hypoplasia, atlantooccipital assimilation, or other abnormalities that condition an anterior compression of the cervical-medullary junction [19][20] (Figure 1). These patients need different clinical management and often require multiple surgical procedures (such as anterior approaches and occipito-cervical fusions) [21].
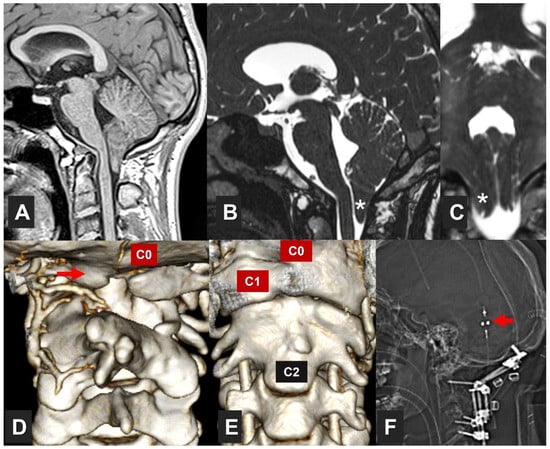
Figure 1. Patient with a complex craniovertebral junction (CVJ) malformation. This 17-year-old male with a VACTERL association (https://rarediseases.info.nih.gov/diseases/5443/vacterl-association) (accessed on 5 October 2023)—a non-random association of congenital disabilities that affects multiple parts of the body—was referred to the institution due to a Chiari malformation type 1 (CM1) detected as the patient presented recurrent episodes of severe occipital headaches. The neurological examination was normal. The patient had CM1 with an asymmetrical tonsillar descent 11 mm below the McRae line. (A) T1*weighted sagittal midline image; (B) T2*weighted sagittal, and (C) T2*weighted coronal slices; (D,E) three-dimensional CT reconstruction of the CVJ. White asterisks in (B,C) indicate the right cerebellar tonsil. The patient presented complete assimilation of the anterior and posterior C1 arches with an associated C2–C3 fusion and partial agenesis of the right C2 lamina. C0: posterior part of the foramen magnum. C1 labels the right lateral mass of the atlas. The red arrow in (D) shows the assimilated posterior C1 arch. Arrow in (F) indicates the valve (Polaris® adjustable valve, Sophysa, Orsay, France) of a ventriculoperitoneal shunt implanted one month before because of hydrocephalus. This patient was treated with halo-vest stabilization for three weeks, followed by a one-step surgical procedure in which a posterior fossa reconstruction was conducted and instrumented occipito-C4 posterior fusion with bone-bank allograft (F).
A common feature of CMs is that the cerebellar tonsillar descent causes compression of the neural structures and hinders CSF passage at the cervico–medullary joint, being able to alter brainstem and upper spinal cord function. This neural structures compression may generate dysfunctions in brainstem pathways, cranial nerve nuclei, or their exit from the brainstem, sleep-regulating regions, and cardiorespiratory centers [22]. The frequent association of syringomyelia with any form of CM can induce additional damage to the spinal cord and the spinal roots that emerge from the gray matter. These functional alterations of the nervous system can be detected by multimodal neurophysiological tests: brainstem auditory evoked potentials (BAEPs), somatosensory evoked potentials (SEPs), motor evoked potentials (MEPs), electromyography (EMG) of the cranial nerves/spinal roots, and brainstem reflexes.
2. Exploring Brainstem and Spinal Cord Functionality
2.1. Brainstem Auditory Evoked Potentials (BAEPs)
BAEPs were first described in humans in 1970 [23]. They allow the recording of a series of five to seven positive electrical signals generated in response to sounds, each of which has a well-defined electrical generator [20][24]; because waves VI and VII are not constant in the healthy population, they are not routinely evaluated. BAEPs explore the functional integrity of a limited portion of the brainstem, both in the rostrocaudal direction—from the VIII cranial nerve entry at the pontomedullary junction to the upper part of the pons-midbrain—as well as in the transversal plane. The ventral part of the brainstem is not explored with BAEPs. It is widely accepted that alterations in waves I to III reflect the involvement of neural structures ipsilateral to the auditive stimulation. In contrast, abnormalities in the amplitude or latency of waves IV and V indicate contralateral structural impairment [24]. BAEPs can be abnormal in several processes: (1) peripheral auditory pathology (conductive or cochlear hearing loss); (2) primary anatomical modifications of the brainstem due to congenital pathologies (CM, Joubert syndrome, Dandy–Walker syndrome, etc.), or secondary (vascular, tumor, demyelinating, or degenerative); (3) anoxia and ischemia; and (4) intracranial hypertension with transtentorial herniation [20]. Due to the close relationship between the cranial nerves and the brainstem, BAEPs are routinely used in IONM and are important in surgical procedures in which the brainstem and cranial nerves are at risk [25].
2.2. Somatosensory Evoked Potentials (SEPs)
SEPs explore the conduction of the electrical impulse through the lemniscal or dorsal column system, collecting the integrated responses obtained at different anatomical levels after applying a repetitive electrical stimulus on a peripheral sensory nerve or the sensory portion of a mixed nerve—usually the median nerve (MN) for the upper limbs and the posterior tibial nerve (PTN) for the lower limbs. In addition, other nerves, such as the cubital, trigeminal, saphenous, or pudendal, can be explored [26][27][28][29]. SEPs explore the somesthetic pathway from the peripheral nerve, preganglionic or postganglionic plexus, roots, spinal cord, brainstem, thalamus, and suprathalamic structures [20]. In anoxic or traumatic coma, they are useful for evaluating cortical and subcortical function. SEPs are now routinely used in the IONM of surgical procedures with a risk of damaging structures that generate and transmit the electrical signal [29]. In addition, ‘dorsal column mapping’ has also been introduced to guide the surgical team in procedures with a risk of spinal cord damage [30][31].
Cutaneous heat stimulations with a laser beam can selectively activate thermoalgesic A-delta and C nociceptors, leading to the generation of laser-evoked potentials (LEPs) in the cortex [32][33]. LEPs have been recognized as reliable neurophysiological tools for investigating neuropathic pain [34]. Pure spinothalamic lesions, such as syringomyelia, brainstem syndromes, or small fiber neuropathy, are characterized by normal SEPs but abnormal LEPs [33][35]. However, spinal injuries involving the cervical or lumbar dorsal horn can also alter or abolish components of classical SEPs, such as N13 or N22 [29][36]. Despite their potential utility, LEPs have not been widely adopted due to technical difficulties, the need for skilled personnel, and associated risks like skin burns and hyperpigmentation. Contact heat evoked potentials (CHEPs) have emerged as a safer alternative to LEPs and are easier to use but require patient cooperation and are unsuitable for IONM [37].
2.3. Motor Evoked Potentials
The term “motor evoked potentials” refers to potentials recorded from muscle or nerve after stimulation of the primary motor areas in the CNS. MEPs are the responses obtained along the spinal cord, peripheral nerve, or muscle by stimulating the central motor paths (spinal cord or cerebral hemispheres) using either transcranial magnetic stimulation (TMS) or electrical stimulation. Electrical stimulation is utilized routinely for IONM, whereas magnetic stimulation is generally used for diagnostic studies in awake patients because of its better tolerance.
TMS is carried out using coils of different magnitudes and shapes. A magnetic field applied via the skull induces a stimulus in the underneath brain with minimal current affecting the skin and subcutaneous tissue. For lower extremity MEPs, the coil is placed in the Cz area; for the upper limb, it is just lateral to this reference. Per peripheral recordings, three MEP characteristics are employed: latency, amplitude, and threshold. MEP amplitude is highly variable but is generally considered abnormal if it is less than 20% of the compound muscle action potential (CMAP) amplitude obtained on peripheral neurography [38]. Central motor conduction time (CMCT) is the most commonly used parameter to identify CNS disorders [39] and is calculated by subtracting the latency of spinal MEPs from the latency of cortical MEPs [40]. TMS MEPs are used to study central demyelinating disease, motoneuron disease, epilepsy, movement disorders, ataxia, myelopathies, neuropathies, and radiculopathies [41].
In order to elicit an MEP by transcranial electric stimulation (TcMEP) for IONM, subcutaneous electrodes are positioned on the scalp in C1–C2 or C3–C4 (10–20 EEG International System). In general, a 50% reduction in amplitude from the average baseline value during surgery is considered significant and a warning sign of damage to the corticospinal tract. Similarly, corticobulbar motor evoked potentials (CoMEPs) elicit cranial nerve responses [42].
2.4. Electromyography and Nerve Conduction Studies
Practically all primary neuromuscular disorders cause changes in the electrical activity recorded in muscle fibers. These changes can best be explored employing needle electrodes introduced into the muscle to record free and voluntary electromyography (EMG). EMG is a technique that is basic for the diagnosis of motor unit disorders, involving anterior horn cells, peripheral nerves, neuromuscular junctions, and muscles. EMG complements nerve conduction studies (NCS) in localizing neuromuscular disorders [40][41]. Normal muscle fibers at rest show no spontaneous electrical activity except in the end-plate region. Voluntary muscle activity is produced by the lower motor neurons and their corresponding innervated muscle fibers that form the ‘motor unit potentials ‘(MUPs). The extent and distribution of the EMG patterns allow us to define the type and severity of the disease, its stage, and the anatomical location of the lesion [40].
EMG is also used in IONM via continuous free-run and stimulated EMG. Free-run EMG records spontaneous muscle activity in real time to detect surgically induced mechanical irritation of the peripheral and cranial nerves before irreversible damage may occur. Stimulated EMG consists of electrically stimulating the peripheral motor nerves or roots registering in the corresponding muscles and can be used to localize peripheral or cranial nerves that are difficult to distinguish from tumors, fibers, or fatty tissues [43].
2.5. Brainstem Reflexes
Blink reflex. The blink reflex (BR) is the neural response obtained in the orbicularis oculi muscle after stimulating the trigeminal nerve with electrical, mechanical, or other stimuli. The BR is the most widely used brainstem reflex in clinical practice. The trigeminal BR is mediated by the first division of the trigeminal nerve (afferent branch) and facial nerve (efferent branch). In normal individuals, two responses (R1 and R2) are obtained when recording from the ipsilateral muscles to the stimulated nerve, and a single (R2) response is obtained from the contralateral muscles [44]. R1 is constituted by the oligosynaptic reflex arc, which includes trigeminal afferents, brainstem connections between the sensory part of the trigeminal nucleus, the motor nucleus of the facial nerve, the facial nerve proper, and the orbicularis oculi muscle. The R2 component has polysynaptic connections within the brainstem but has the same afferent/efferent pathways as R1. BR is useful in the study of trigeminal and facial nerve lesions, peripheral neuropathy, posterior fossa lesions, multiple sclerosis, and extrapyramidal diseases [45]. Under general anesthesia in the operating room, only the R1 response can be recorded [46][47][48][49].
Trigeminal–hypoglossal reflex. Brainstem trigeminal–hypoglossal reflexes (THRs), also known as jaw-tongue reflexes, coordinate the tongue’s position in the mouth during chewing, swallowing, vocalization, and breathing. Recently, a novel methodology for obtaining jaw-opening THR of the brainstem under general anesthesia has been described. This technique could be helpful for the intraoperative monitoring of surgeries involving the trigeminal, hypoglossal nerves, and lower brainstem lesions; however, further studies are still required to validate it [50][51][52][53][54].
H reflex in the masseter muscle. The H reflex in the masseter muscle is a monosynaptic trigeminal–trigeminal reflex transmitted through the mesencephalic nucleus of the trigeminal nerve. It reflects conduction through the midbrain and the median pons [55][56][57]. It can be helpful in studying patients with brainstem or trigeminal lesions, and can be obtained under general anesthesia, thus representing a new method for intraoperative monitoring, especially for lesions involving the midbrain and pons.
3. Neurophysiological Limitations in the Developing Central Nervous System
Neurophysiological test references change during the maturational process of the CNS and, therefore, exhibit some peculiarities according to the patient’s age. In full-term newborns, the most reproducible waves in BAEPs are I, III, and V [58]. However, unlike adults, it is normal for wave I to have a greater amplitude than complex IV/V. The myelin maturation process shortens the latency of the successive waves and the central conduction time (CCT or I-V interval). It has also been demonstrated that the auditory pathway responsible for generating the first and last components of BAEP responses matures at a different rate [59].
A similar process occurs in SEPs; the maturation of peripheral segments of the sensory pathway progresses more rapidly than that of central segments. N9 latency—brachial plexus potential—shortens during the first year of life and subsequently increases as the patient’s size increases. The N9–N11 interval—conduction between the brachial plexus and the dorsal column—decreases with age.
Independent of the changes described, the motor stimulation threshold is higher in children than in adults under 18 years of age [60]. CMCT is a dependent value of CNS maturation but has different times. For example, with relaxation (resting motor threshold), children reach adult values approximately at the age of ten; however, with facilitation (active motor threshold), they reach normal values at two years of age [60][61].
When evaluating peripheral nerves in children, the myelination process also affects parameters commonly used for assessing NCS [62]. For instance, conduction velocity increases in proportion to nerve fiber diameters and the distance between the nodes of Ranvier, achieving faster motor and sensitive velocities with growth; distal latencies and amplitudes also change.
The LAR in children is vital for airway protection. In humans, the larynx descends from the neck at 4–6 months. This descent coincides with the transition from obligatory nasal breathing in infants to facultative nasal or oral breathing in adults. It is possible that alterations in this transition are related to sudden infant death syndrome, whose frequency is maximum at the same age at which laryngeal descent is completed (4 to 6 months) [63][64].
4. Follow-Up Neurophysiological Studies in Patients with CM1
4.1. Brainstem Auditory Evoked Potentials
To our knowledge, the first paper was published in 1983 by Stone et al. [65], describing BAEP findings in a 16-year-old boy with CM2 and symptoms suggestive of brainstem involvement and neuroimaging revealing brainstem compression. The authors diagnosed CM2; however, as the reported patient did not have any visible spinal lesion, it believes that CM1.5 was the most likely diagnosis [13]. BAEPs were abnormal bilaterally, with I-V interpeak latency (IPL) prolongations and no visible wave III identified upon stimulation of the right ear. The patient underwent bilateral posterior fossa decompression (PFD) and cervical laminectomy. Six months later, he presented neurological improvement and bilateral normalization of his BAEPs. A few authors have also described case reports with retrocochlear findings in the BAEPs of CM1 patients, in both adults [66][67] and children [68][69].
In 1999, Hort-Legrand and Emery [70] reported 79 patients with syringomyelia, of which 48 cases were associated with CM1. BAEPs were abnormal in 13 of the 59 patients studied, and the most frequent finding was prolongation of the I-V IPL, more often unilaterally. Another study on syringomyelia and CM1 [71] found that BAEP abnormalities had a better correlation with clinical and radiological findings than SEPs.
4.2. Somatosensory Evoked Potentials
In 1986, Anderson et al. [72] described the SEPs of nine syringomyelia patients, eight with associated CM1. The most frequent findings were unilateral or bilateral decrease or absence of the cervical potential. In all but one patient with CM1 and syringomyelia, an increased or asymmetric CCT was found; in 1988, Forcadas et al. reported similar results [73].
Moncho et al. found that SEPs were altered in 43.5% of CM1 patients. The most common finding in MN SEPs was an increase in N13-N20 IPL, while for PTN SEPs, it was an increase in N22-P37 IPL, sometimes associated with an abnormal cervical response. In a logistic regression model, only age and tonsillar herniation degree showed statistical significance for predicting abnormal SEPs. These results indicated a higher chance of obtaining pathological SEPs in older patients with greater tonsillar descent [19][74]; recently, Guvenc et al. described similar findings [75].
4.3. Motor Evoked Potentials
Few authors have described TMS MEP alterations in patients with CM1, most of them with syringomyelia. The most frequent findings were increased CMTCs [70][76][77][78].
Referring to pre- and post-surgical MEP evaluations, Cristante et al. [79] described the MEP findings in eight CM1 patients. Preoperative TMS MEPs showed that functionally impaired muscles exhibited neurophysiologic abnormalities, and even one patient without any motor deficits had abnormal MEPs. Interestingly, the postoperative functional motor recovery of five of the eight patients―mostly partial―was not reflected by a similar improvement in MEPs.
4.4. NCS, EMG, and Other Peripheral Nerve Studies
In 1992, Gerard et al. described the case of a five-year-old girl―with CM1 and BI―admitted due to insufficiency of the soft palate [80]. EMG activity was recorded bilaterally in the levator palatini and anterior faucial pillars, showing ample biphasic or polyphasic action potentials at rest (probably spontaneous activity). When the child cried, the frequency of these potentials increased poorly, and recruitment was impaired. Clinical examination and EMG findings led to a suspicion of denervation of the IX, X, and XI cranial nerves.
CM1 should be a differential diagnosis in patients with adult dysphagia onset requiring MRI examination, even when presenting with “typical” lower motor neuron signs in bulbar muscles. SEPs, NCS, and EMG can help diagnose these cases [81].
In patients with CM1 and associated syringomyelia, NCS and EMG are useful in detecting anterior horn affectation caused by the syringomyelia. This syndrome characteristically presents with spontaneous activity while performing needle EMG if acute or denervating activity occurs, with large, polyphasic (neurogenic) MUPs, reduced voluntary recruitment, and normal sensory nerve conductions [82][83][84][85][86][87].
4.5. Brainstem Reflexes
We only detected two reports regarding CM1 follow-up with brainstem reflexes, specifically, the blink reflex. Amoiridis et al. [88] reported a case of a 25-year-old man with CM1 and holocord syrinx. They performed the BR that showed Rl absent bilaterally and R2 latency with left-side stimulation prolonged on both sides, even though the patient did not present any anomaly in clinical cranial nerve exploration. Jacome, in 2001, described four CM1 patients presenting with blepharoclonus, one with altered R1, two with altered R2, and one with normal BR. Also, facial EMG showed complex repetitive discharges of the right mentalis muscle in one patient [89].
5. Intraoperative Neurophysiological Monitoring in CM1
5.1. Posterior Fossa Surgery
The role of intraoperative neuromonitoring (IONM) during PF surgery for CM1 is controversial, and there is still no consensus on the usefulness of the technique for the prevention of new neurological deficits nor on the most effective modality to use. Anderson et al. studied changes in intraoperative BAEPs and SEPs in 11 pediatric patients during suboccipital decompression for CM1. BAEP conduction times were compared at four surgical stages: (1) before and (2) after positioning, (3) after craniotomy, and (4) at the end of durotomy. Their data indicated that significant improvement in conduction times occurs after bony decompression and division of the atlantooccipital membrane rather than after dura opening. They also highlighted the risk of altering SEPs and BAEPs during positioning [90].
In 2012, Chen et al. analyzed BAEP and SEP parameters in 13 consecutive pediatric patients who underwent suboccipital decompression to treat symptomatic CM1. They recorded the MN SEP, PTN SEP, and BAEP latencies at four stages: preoperatively, following craniotomy, following durotomy, and following closure [91].
The largest pediatric population series―with 156 patients―was reviewed in 2015 by Kennedy et al., in children with PFD but not dural opening [92]. IONM with SEPs and BAEPs was performed before and after prone positioning and during surgery. They found that 78% of patients exhibited significant BAEP conduction time improvement after bony decompression. SEPs were used to assess the lack of problems with patient positioning during surgery.
5.2. Surgery for the Direct Treatment of Syringomyelia in MC1
The first articles about IONM for syringomyelia in CM1 patients were published by Milhorat et al., with 32 syringomyelia patients (21 with CM1). IONM with MN SEPs demonstrated a significant decrease in N20 latency and a nonsignificant increase in N20 amplitude 30 min after syrinx decompression. They concluded that the improvement in N20 latency was indirect evidence of preexisting long tract compression. However, these conclusions are questionable in patients with CM1, in whom SEP improvements may have been caused, in part, by previous PF surgery [93][94].
5.3. CM1 in Scoliosis Surgery
The relationship between Chiari malformation syrinx and scoliosis has been widely reported [95][96][97][98]. The associated percentage of scoliosis fluctuates from 15 to 50% in patients with Chiari malformation [99][100][101]. There is a potential risk from scoliosis surgery in patients with syringomyelia-associated scoliosis, which might be higher with longer and wider syrinxes.
5.4. Exploratory Research on the Subject
In the first, craniocervical hinge―22 CM1 patients―participated in the study. The authors concluded that the function of each of the XI CN rootlets appears to be specific. Thus, the cranial root contributes, separately from the spinal root to the innervation of the vocal folds, which makes it a specific entity. The spinal root innervates the sternocleidomastoid and trapezius muscles with a craniocaudal motor organization of its cervical rootlets. Giampicolo et al. conducted an exploratory and preliminary study on cerebello-cortical stimulation in 10 patients undergoing PF surgery, one of them with CM1. A third of children undergoing cerebellar resections can present cerebellar mutism. According to recent evidence, it is suggested that this may arise from damage to cerebellar efferents, either uni- or bilaterally, affecting the cortex along the cerebello-dento-thalamo-cortical pathway. There is currently no neurophysiological technique available to intraoperatively monitor this pathway.
References
- Cleland. Contribution to the Study of Spina Bifida, Encephalocele, and Anencephalus. J. Anat. Physiol. 1883, 17, 257–292.
- Chiari, H. Über Veränderungen Des Kleinhirns Infolge von Hydrocephalie Des Grosshirns. Dtsch. Med. Wochenschr. 1891, 17, 1172–1175.
- Chiari, H. Über veränderungen des Kleinhirns, des Pons un der Medulla Oblongata in folge von congenitaler Hydrocephalie des Grosshirns. Denkschr. Akad. Wiss 1896, 63, 71–116.
- Chiari, H. Concerning Alterations in the Cerebellum Resulting from Cerebral Hydrocephalus. 1891. Pediatr. Neurosci. 1987, 13, 3–8.
- Cruveilhier, J. L’anatomie Pathologique Du Corps Humain; Descriptions Avec Figures Lithographiées et Coloriées; Diverses Altérations Morbides Dont Le Corps Humain et Susceptible; Bailliere: Paris, France, 1829.
- Mortazavi, M.M.; Tubbs, R.S.; Brockerhoff, M.A.; Loukas, M.; Oakes, W.J. The First Description of Chiari I Malformation with Intuitive Correlation between Tonsillar Ectopia and Syringomyelia. J. Neurosurg. Pediatr. 2011, 7, 257–260.
- Barkovich, A.J.; Wippold, F.J.; Sherman, J.L.; Citrin, C.M. Significance of Cerebellar Tonsillar Position on MR. AJNR Am. J. Neuroradiol. 1986, 7, 795–799.
- Bogdanov, E.I.; Heiss, J.D.; Mendelevich, E.G.; Mikhaylov, I.M.; Haass, A. Clinical and Neuroimaging Features of “Idiopathic” Syringomyelia. Neurology 2004, 62, 791–794.
- Botelho, R.V.; Bittencourt, L.R.A.; Rotta, J.M.; Tufik, S. A Prospective Controlled Study of Sleep Respiratory Events in Patients with Craniovertebral Junction Malformation. J. Neurosurg. 2003, 99, 1004–1009.
- Meadows, J.; Kraut, M.; Guarnieri, M.; Haroun, R.I.; Carson, B.S. Asymptomatic Chiari Type I Malformations Identified on Magnetic Resonance Imaging. J. Neurosurg. 2000, 92, 920–926.
- Milhorat, T.H.; Nishikawa, M.; Kula, R.W.; Dlugacz, Y.D. Mechanisms of Cerebellar Tonsil Herniation in Patients with Chiari Malformations as Guide to Clinical Management. Acta Neurochir. 2010, 152, 1117–1127.
- Sekula, R.F.; Jannetta, P.J.; Casey, K.F.; Marchan, E.M.; Sekula, L.K.; McCrady, C.S. Dimensions of the Posterior Fossa in Patients Symptomatic for Chiari I Malformation but without Cerebellar Tonsillar Descent. Cerebrospinal Fluid Res. 2005, 2, 11.
- Tubbs, R.S.; Iskandar, B.J.; Bartolucci, A.A.; Oakes, W.J. A Critical Analysis of the Chiari 1.5 Malformation. J. Neurosurg. 2004, 101, 179–183.
- Tubbs, R.S.; Demerdash, A.; Vahedi, P.; Griessenauer, C.J.; Oakes, W.J. Chiari IV Malformation: Correcting an over One Century Long Historical Error. Child’s Nerv. Syst. 2016, 32, 1175–1179.
- Ciaramitaro, P.; Massimi, L.; Bertuccio, A.; Solari, A.; Farinotti, M.; Peretta, P.; Saletti, V.; Chiapparini, L.; Barbanera, A.; Garbossa, D.; et al. Diagnosis and Treatment of Chiari Malformation and Syringomyelia in Adults: International Consensus Document. Neurol. Sci 2022, 43, 1327–1342.
- Isik, N.; Elmaci, I.; Kaksi, M.; Gokben, B.; Isik, N.; Celik, M. A New Entity: Chiari Zero Malformation and Its Surgical Method. Turk. Neurosurg. 2011, 21, 264–268.
- Kyoshima, K.; Kuroyanagi, T.; Oya, F.; Kamijo, Y.; El-Noamany, H.; Kobayashi, S. Syringomyelia without Hindbrain Herniation: Tight Cisterna Magna. Report of Four Cases and a Review of the Literature. J. Neurosurg. 2002, 96, 239–249.
- Tubbs, R.S.; Elton, S.; Grabb, P.; Dockery, S.E.; Bartolucci, A.A.; Oakes, W.J. Analysis of the Posterior Fossa in Children with the Chiari 0 Malformation. Neurosurgery 2001, 48, 1050–1054.
- Moncho, D.; Poca, M.A.; Minoves, T.; Ferré, A.; Cañas, V.; Sahuquillo, J. Are Evoked Potentials Clinically Useful in the Study of Patients with Chiari Malformation Type 1? J. Neurosurg. 2017, 126, 606–619.
- Moncho, D.; Poca, M.A.; Minoves, T.; Ferré, A.; Rahnama, K.; Sahuquillo, J. Brainstem auditory evoked potentials and somatosensory evoked potentials in Chiari malformation. Rev. Neurol. 2013, 56, 623–634.
- Sahuquillo, J.; Moncho, D.; Ferré, A.; López-Bermeo, D.; Sahuquillo-Muxi, A.; Poca, M.A. A Critical Update of the Classification of Chiari and Chiari-like Malformations. J. Clin. Med. 2023, 12, 4626.
- Ferré Masó, A.; Poca, M.A.; de la Calzada, M.D.; Solana, E.; Romero Tomás, O.; Sahuquillo, J. Sleep Disturbance: A Forgotten Syndrome in Patients with Chiari I Malformation. Neurologia 2014, 29, 294–304.
- Jewett, D.L.; Romano, M.N.; Williston, J.S. Human Auditory Evoked Potentials: Possible Brain Stem Components Detected on the Scalp. Science 1970, 167, 1517–1518.
- Stockard, J.J.; Rossiter, V.S. Clinical and Pathologic Correlates of Brain Stem Auditory Response Abnormalities. Neurology 1977, 27, 316–325.
- Møller, A.R. Monitoring Auditory Evoked Potentials. In Intraoperative Neurophysiological Monitoring; Humana Press: Totowa, NJ, USA, 2006; pp. 85–124. ISBN 978-1-59745-018-8.
- Jean-Michel, G. Les Potentiels Évoqués, 2nd ed.; Masson: Paris, France, 1993; Volume 1, ISBN 2-225-84107-1.
- Desmedt, J.E. Physiology and Physiopathology of Somatic Sensations Studied in Man by the Method of Evoked Potentials. J. Physiol. 1987, 82, 64–136.
- Cracco, R.Q.; Cracco, J.B. Somatosensory Evoked Potential in Man: Far Field Potentials. Electroencephalogr. Clin. Neurophysiol. 1976, 41, 460–466.
- Cruccu, G.; Aminoff, M.J.; Curio, G.; Guerit, J.M.; Kakigi, R.; Mauguiere, F.; Rossini, P.M.; Treede, R.-D.; Garcia-Larrea, L. Recommendations for the Clinical Use of Somatosensory-Evoked Potentials. Clin. Neurophysiol. 2008, 119, 1705–1719.
- Nair, D.; Kumaraswamy, V.M.; Braver, D.; Kilbride, R.D.; Borges, L.F.; Simon, M.V. Dorsal Column Mapping via Phase Reversal Method: The Refined Technique and Clinical Applications. Neurosurgery 2014, 74, 437–446.
- Simon, M.V.; Chiappa, K.H.; Borges, L.F. Phase Reversal of Somatosensory Evoked Potentials Triggered by Gracilis Tract Stimulation: Case Report of a New Technique for Neurophysiologic Dorsal Column Mapping. Neurosurgery 2012, 70, E783–E788.
- Cruccu, G.; García-Larrea, L. Clinical Utility of Pain—Laser Evoked Potentials. Suppl. Clin. Neurophysiol. 2004, 57, 101–110.
- Garcia-Larrea, L. Pain Evoked Potentials. In Handbook of Neurology; Elsevier: Amsterdam, The Netherlands, 2006; Volume 81, pp. 439–462.
- Cruccu, G.; Anand, P.; Attal, N.; Garcia-Larrea, L.; Haanpää, M.; Jørum, E.; Serra, J.; Jensen, T.S. EFNS Guidelines on Neuropathic Pain Assessment. Eur. J. Neurol. 2004, 11, 153–162.
- Kakigi, R.; Inui, K.; Tamura, Y. Electrophysiological Studies on Human Pain Perception. Clin. Neurophysiol. 2005, 116, 743–763.
- Restuccia, D.; Mauguière, F. The Contribution of Median Nerve SEPs in the Functional Assessment of the Cervical Spinal Cord in Syringomyelia. A Study of 24 Patients. Brain 1991, 114B Pt 1, 361–379.
- Dotson, R.M.; Sandroni, P. Contact heat evoked potentials. In Clinical Neurophsyiology; Contemporary Neurology Series; OUP: New York, NY, USA, 2009; pp. 688–692. ISBN 978-0-19-538511-3.
- Hallett, M.; Chokroverty, S. (Eds.) Magnetic Stimulation in Clinical Neurophysiology, 2nd ed.; Elsevier Butterworth-Heinemann: Philadelphia, PA, USA, 2005; ISBN 978-0-7506-7373-0.
- Claus, D. Central Motor Conduction: Method and Normal Results. Muscle Nerve 1990, 13, 1125–1132.
- Strommen, J.A. Motor Evoked Potentials. In Clinical Neurophsyiology; Contemporary Neurology Series; OUP: New York, NY, USA, 2009; pp. 385–397. ISBN 978-0-19-538511-3.
- Kimura, J. Motor Evoked Potentials. In Electrodiagnosis in Diseases of Nerve and Muscle: Principles and Practice; Oxford University Press: New York, NY, USA, 2013; pp. 526–551. ISBN 978-0-19-935316-3.
- Deletis, V.; Fernández-Conejero, I. Intraoperative Monitoring and Mapping of the Functional Integrity of the Brainstem. J. Clin. Neurol. 2016, 12, 262–273.
- Simon, M.V. Intraoperative Clinical Neurophysiology: A Comprehensive Guide to Monitoring and Mapping; Demos Medical: New York, NY, USA, 2010; ISBN 978-1-933864-46-4.
- Kugelberg, E. Facial reflexes. Brain 1952, 75, 385–396.
- Smith, B.E. Cranial Reflexes and Related Techniques. In Clinical Neurophsyiology; OUP: New York, NY, USA, 2009; pp. 529–542. ISBN 978-0-19-538511-3.
- Deletis, V.; Urriza, J.; Ulkatan, S.; Fernandez-Conejero, I.; Lesser, J.; Misita, D. The Feasibility of Recording Blink Reflexes under General Anesthesia. Muscle Nerve 2009, 39, 642–646.
- Aydinlar, E.I.; Kocak, M.; Soykam, H.O.; Mat, B.; Dikmen, P.Y.; Sezerman, O.U.; Elmaci, İ.; Pamir, M.N. Intraoperative Neuromonitoring of Blink Reflex During Posterior Fossa Surgeries and Its Correlation with Clinical Outcome. J. Clin. Neurophysiol. 2022, 39, 299–306.
- Fernández-Conejero, I.; Ulkatan, S.; Deletis, V. Chapter 10—Monitoring Cerebellopontine Angle and Skull Base Surgeries. In Handbook of Clinical Neurology; Intraoperative Neuromonitoring; Nuwer, M.R., MacDonald, D.B., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; Volume 186, pp. 163–176.
- Karakis, I.; Simon, M.V. Neurophysiologic Mapping and Monitoring of Cranial Nerves and Brainstem. In Intraoperative Neurophysiology; Simon, M.V., Ed.; Springer Publishing Company: New York, NY, USA, 2018; pp. 345–388. ISBN 978-1-62070-117-1.
- Mirallave Pescador, A.; Téllez, M.J.; Sánchez Roldán, M.d.L.Á.; Samusyte, G.; Lawson, E.C.; Coelho, P.; Lejarde, A.; Rathore, A.; Le, D.; Ulkatan, S. Methodology for Eliciting the Brainstem Trigeminal-Hypoglossal Reflex in Humans under General Anesthesia. Clin. Neurophysiol. 2022, 137, 1–10.
- Ishiwata, Y.; Ono, T.; Kuroda, T.; Nakamura, Y. Jaw-Tongue Reflex: Afferents, Central Pathways, and Synaptic Potentials in Hypoglossal Motoneurons in the Cat. J. Dent. Res. 2000, 79, 1626–1634.
- Lowe, A.A. The Neural Regulation of Tongue Movements. Prog. Neurobiol. 1980, 15, 295–344.
- Morimoto, T.; Kawamura, Y. Properties of Tongue and Jaw Movements Elicited by Stimulation of the Orbital Gyrus in the Cat. Arch. Oral Biol. 1973, 18, 361–372.
- Luo, P.; Zhang, J.; Yang, R.; Pendlebury, W. Neuronal Circuitry and Synaptic Organization of Trigeminal Proprioceptive Afferents Mediating Tongue Movement and Jaw-Tongue Coordination via Hypoglossal Premotor Neurons. Eur. J. Neurosci. 2006, 23, 3269–3283.
- Godaux, E.; Desmedt, J.E. Human Masseter Muscle: H- and Tendon Reflexes. Their Paradoxical Potentiation by Muscle Vibration. Arch. Neurol. 1975, 32, 229–234.
- Kimura, J. H, T, and Masseter Reflexes and the Silent Period. In Electrodiagnosis in Diseases of Nerve and Muscle: Principles and Practice; Oxford University Press: New York, NY, USA, 2013; pp. 216–218. ISBN 978-0-19-935316-3.
- Szentagothai, J. Anatomical Considerations on Monosynaptic Reflex Arcs. J. Neurophysiol. 1948, 11, 445–454.
- Tarantino, V.; Stura, M.; Vallarino, R. Development of Auditory Evoked Potentials of the Brainstem in Relation to Age. Pediatr. Med. Chir. 1988, 10, 73–76.
- Reroń, E.; Sekuła, J. Maturation of the Acoustic Path-Way in Brain Stem Responses (ABR) in Neonates. Otolaryngol. Pol. 1994, 48, 363–369.
- Hameed, M.Q.; Dhamne, S.C.; Gersner, R.; Kaye, H.L.; Oberman, L.M.; Pascual-Leone, A.; Rotenberg, A. Transcranial Magnetic and Direct Current Stimulation in Children. Curr. Neurol. Neurosci. Rep. 2017, 17, 11.
- Garvey, M.A. Transcranial Magnetic Stimulation in Children. In Magnetic Stimulation in Clinical Neurophysiology; Hallett, M., Chokroverty, S., Eds.; Elsevier Butterworth-Heinemann: Philadelphia, PA, USA, 2005; pp. 429–433. ISBN 978-0-7506-7373-0.
- Kuntz, N.L. Chapter 6—Clinical Neurophysiology of the Motor Unit in Infants and Children. In Clinical Neurophysiology of Infancy, Childhood, and Adolescence; Holmes, G.L., Jones, H.R., Moshé, S.L., Eds.; Butterworth-Heinemann: Philadelphia, PA, USA, 2006; pp. 130–145. ISBN 978-0-7506-7251-1.
- Sasaki, C.T.; Levine, P.A.; Laitman, J.T.; Crelin, E.S. Postnatal Descent of the Epiglottis in Man. A Preliminary Report. Arch. Otolaryngol. 1977, 103, 169–171.
- Wang, X.; Guo, R.; Zhao, W.; Pilowsky, P.M. Medullary Mediation of the Laryngeal Adductor Reflex: A Possible Role in Sudden Infant Death Syndrome. Respir. Physiol. Neurobiol. 2016, 226, 121–127.
- Stone, J.L.; Bouffard, A.; Morris, R.; Hovsepian, W.; Meyers, H.L. Clinical and Electrophysiologic Recovery in Arnold-Chiari Malformation. Surg. Neurol. 1983, 20, 313–317.
- Brookler, K.H. Vestibular Findings in a 62-Year-Old Woman with Dizziness and a Type I Chiari Malformation. Ear Nose Throat J. 2005, 84, 630–631.
- Hendrix, R.A.; Bacon, C.K.; Sclafani, A.P. Chiari-I Malformation Associated with Asymmetric Sensorineural Hearing Loss. J. Otolaryngol. 1992, 21, 102–107.
- Johnson, G.D.; Harbaugh, R.E.; Lenz, S.B. Surgical Decompression of Chiari I Malformation for Isolated Progressive Sensorineural Hearing Loss. Am. J. Otol. 1994, 15, 634–638.
- Ahmmed, A.U.; Mackenzie, I.; Das, V.K.; Chatterjee, S.; Lye, R.H. Audio-Vestibular Manifestations of Chiari Malformation and Outcome of Surgical Decompression: A Case Report. J. Laryngol. Otol. 1996, 110, 1060–1064.
- Hort-Legrand, C.; Emery, E. Motor and sensory evoked potentials in syringomyelia. Neurochirurgie 1999, 45, 95–104.
- Isik, N.; Elmaci, I.; Isik, N.; Cerci, S.; Basaran, R.; Gura, M.; Kalelioglu, M. Long-Term Results and Complications of the Syringopleural Shunting for Treatment of Syringomyelia: A Clinical Study. Br. J. Neurosurg. 2013, 27, 91–99.
- Anderson, N.E.; Frith, R.W.; Synek, V.M. Somatosensory Evoked Potentials in Syringomyelia. J. Neurol. Neurosurg. Psychiatr. 1986, 49, 1407–1410.
- Forcadas, I.; Hurtado, P.; Madoz, P.; Zarranz, J.J. Somatosensory Evoked Potentials in Syringomyelia and the Arnold-Chiari Anomaly. Clinical and Imaging Correlations. Neurologia 1988, 3, 172–175.
- Moncho, D.; Poca, M.A.; Minoves, T.; Ferré, A.; Rahnama, K.; Sahuquillo, J. Brainstem Auditory and Somatosensory Evoked Potentials in Relation to Clinical and Neuroimaging Findings in Chiari Type 1 Malformation. J. Clin. Neurophysiol. 2015, 32, 130–138.
- Guvenc, G.; Kızmazoglu, C.; Aydın, H.E.; Coskun, E. Somatosensory Evoked Potentials and Cerebrospinal Fluid Flow in Chiari Malformation. Ann. Clin. Anal. Med. 2019, 10, 348–353.
- Stancanelli, C.; Mazzeo, A.; Gentile, L.; Vita, G. Unilateral Hyperhidrosis as Persistently Isolated Feature of Syringomyelia and Arnold Chiari Type 1. Neurol. Sci. 2018, 39, 1607–1608.
- Nogués, M.A.; Pardal, A.M.; Merello, M.; Miguel, M.A. SEPs and CNS Magnetic Stimulation in Syringomyelia. Muscle Nerve 1992, 15, 993–1001.
- Cocito, D.; Peci, E.; Garbossa, D.; Ciaramitaro, P. Neurophysiological Correlates in Patients with Syringomyelia and Chiari Malformation: The Cortico-Diaphragmatic Involvement. J. Clin. Med. 2022, 11, 5080.
- Cristante, L.; Westphal, M.; Herrmann, H.D. Cranio-Cervical Decompression for Chiari I Malformation. A Retrospective Evaluation of Functional Outcome with Particular Attention to the Motor Deficits. Acta Neurochir. 1994, 130, 94–100.
- Gerard, C.L.; Dugas, M.; Narcy, P.; Hertz-Pannier, J. Chiari Malformation Type I in a Child with Velopharyngeal Insufficiency. Dev. Med. Child Neurol. 1992, 34, 174–176.
- Paulig, M.; Prosiegel, M. Misdiagnosis of Amyotrophic Lateral Sclerosis in a Patient with Dysphagia Due to Chiari I Malformation. J. Neurol. Neurosurg. Psychiatry 2002, 72, 270.
- Bagnato, S.; Rizzo, V.; Quartarone, A.; Majorana, G.; Vita, G.; Girlanda, P. Segmental Myoclonus in a Patient Affected by Syringomyelia. Neurol. Sci. 2001, 22, 27–29.
- Scelsa, S. Syringomyelia Presenting as Ulnar Neuropathy at the Elbow. Clin. Neurophysiol. 2000, 111, 1527–1530.
- Muhn, N.; Baker, S.K.; Hollenberg, R.D.; Meaney, B.F.; Tarnopolsky, M.A. Syringomyelia Presenting as Rapidly Progressive Foot Drop. J. Clin. Neuromuscul. Dis. 2002, 3, 133–134.
- Jayamanne, C.; Fernando, L.; Mettananda, S. Chiari Malformation Type 1 Presenting as Unilateral Progressive Foot Drop: A Case Report and Review of Literature. BMC Pediatr. 2018, 18, 34.
- Saifudheen, K.; Jose, J.; Gafoor, V.A. Holocord Syringomyelia Presenting as Rapidly Progressive Foot Drop. J. Neurosci. Rural Pract. 2011, 2, 195–196.
- McMillan, H.J.; Sell, E.; Nzau, M.; Ventureyra, E.C.G. Chiari 1 Malformation and Holocord Syringomyelia Presenting as Abrupt Onset Foot Drop. Child’s Nerv. Syst. 2011, 27, 183–186.
- Amoiridis, G.; Meves, S.; Schols, L.; Przuntek, H. Reversible Urinary Retention as the Main Symptom in the First Manifestation of a Syringomyelia. J. Neurol. Neurosurg. Psychiatry 1996, 61, 407–408.
- Jacome, D.E. Blepharoclonus and Arnold-Chiari Malformation. Acta Neurol. Scand. 2001, 104, 113–117.
- Anderson, R.C.; Dowling, K.C.; Feldstein, N.A.; Emerson, R.G. Chiari I Malformation: Potential Role for Intraoperative Electrophysiologic Monitoring. J. Clin. Neurophysiol. 2003, 20, 65–72.
- Chen, J.A.; Coutin-Churchman, P.E.; Nuwer, M.R.; Lazareff, J.A. Suboccipital Craniotomy for Chiari I Results in Evoked Potential Conduction Changes. Surg. Neurol. Int. 2012, 3, 165.
- Kennedy, B.; Kelly, K.; Phan, M.; Bruce, S.; McDowell, M.; Anderson, R.; Feldstein, N. Outcomes after Suboccipital Decompression without Dural Opening in Children with Chiari Malformation Type I. J. Neurosurg. Pediatr. 2015, 16, 150–158.
- Milhorat, T.H.; Kotzen, R.M.; Capocelli, A.L.; Bolognese, P.; Bendo, A.A.; Cottrell, J.E. Intraoperative Improvement of Somatosensory Evoked Potentials and Local Spinal Cord Blood Flow in Patients with Syringomyelia. J. Neurosurg. Anesthesiol. 1996, 8, 208–215.
- Milhorat, T.H.; Capocelli, A.L.; Kotzen, R.M.; Bolognese, P.; Heger, I.M.; Cottrell, J.E. Intramedullary Pressure in Syringomyelia: Clinical and Pathophysiological Correlates of Syrinx Distension. Neurosurgery 1997, 41, 1102–1110.
- Cheng, J.C.; Guo, X.; Sher, A.H.; Chan, Y.L.; Metreweli, C. Correlation between Curve Severity, Somatosensory Evoked Potentials, and Magnetic Resonance Imaging in Adolescent Idiopathic Scoliosis. Spine 1999, 24, 1679–1684.
- Hausmann, O.N.; Böni, T.; Pfirrmann, C.W.A.; Curt, A.; Min, K. Preoperative Radiological and Electrophysiological Evaluation in 100 Adolescent Idiopathic Scoliosis Patients. Eur. Spine J. 2003, 12, 501–506.
- Zhu, Z.; Qiu, Y.; Wang, B.; Yu, Y.; Qian, B.; Zhu, F. Abnormal Spreading and Subunit Expression of Junctional Acetylcholine Receptors of Paraspinal Muscles in Scoliosis Associated with Syringomyelia. Spine 2007, 32, 2449–2454.
- Eule, J.M.; Erickson, M.A.; O’Brien, M.F.; Handler, M. Chiari I Malformation Associated with Syringomyelia and Scoliosis: A Twenty-Year Review of Surgical and Nonsurgical Treatment in a Pediatric Population. Spine 2002, 27, 1451–1455.
- Zhu, Z.; Yan, H.; Han, X.; Jin, M.; Xie, D.; Sha, S.; Liu, Z.; Qian, B.; Zhu, F.; Qiu, Y. Radiological Features of Scoliosis in Chiari I Malformation Without Syringomyelia. Spine 2016, 41, E276–E281.
- Tubbs, R.S.; Beckman, J.; Naftel, R.P.; Chern, J.J.; Wellons, J.C.; Rozzelle, C.J.; Blount, J.P.; Oakes, W.J. Institutional Experience with 500 Cases of Surgically Treated Pediatric Chiari Malformation Type I. J. Neurosurg. Pediatr. 2011, 7, 248–256.
- Sha, S.; Zhu, Z.; Lam, T.P.; Sun, X.; Qian, B.; Jiang, J.; Cheng, J.C.Y.; Qiu, Y. Brace Treatment versus Observation Alone for Scoliosis Associated with Chiari I Malformation Following Posterior Fossa Decompression: A Cohort Study of 54 Patients. Eur. Spine J. 2014, 23, 1224–1231.
More
Information
Subjects:
Neurosciences
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
843
Revisions:
2 times
(View History)
Update Date:
23 Oct 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




