Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Robert Soliva‐Fortuny | -- | 2253 | 2023-10-18 16:27:05 | | | |
| 2 | Wendy Huang | Meta information modification | 2253 | 2023-10-19 04:51:43 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Manthei, A.; López-Gámez, G.; Martín-Belloso, O.; Elez-Martínez, P.; Soliva-Fortuny, R. Enhancing the Health-Promoting Effect of Dietary Fiber. Encyclopedia. Available online: https://encyclopedia.pub/entry/50479 (accessed on 07 February 2026).
Manthei A, López-Gámez G, Martín-Belloso O, Elez-Martínez P, Soliva-Fortuny R. Enhancing the Health-Promoting Effect of Dietary Fiber. Encyclopedia. Available at: https://encyclopedia.pub/entry/50479. Accessed February 07, 2026.
Manthei, Alina, Gloria López-Gámez, Olga Martín-Belloso, Pedro Elez-Martínez, Robert Soliva-Fortuny. "Enhancing the Health-Promoting Effect of Dietary Fiber" Encyclopedia, https://encyclopedia.pub/entry/50479 (accessed February 07, 2026).
Manthei, A., López-Gámez, G., Martín-Belloso, O., Elez-Martínez, P., & Soliva-Fortuny, R. (2023, October 18). Enhancing the Health-Promoting Effect of Dietary Fiber. In Encyclopedia. https://encyclopedia.pub/entry/50479
Manthei, Alina, et al. "Enhancing the Health-Promoting Effect of Dietary Fiber." Encyclopedia. Web. 18 October, 2023.
Copy Citation
Fruit and vegetable by-products are rich in dietary fiber (DF), which consists of plant carbohydrate polymers. These polymers encompass oligo- and polysaccharides (e.g., cellulose, pectin, hemicellulose, resistant starch, lignin), often linked with non-carbohydrate compounds, and are not digestible or absorbable in the small intestine. DF is composed of a major insoluble part (IDF), primarily cellulose but also lignin, and a water-soluble fraction (SDF), comprised of some hemicellulosic but mainly pectic substances.
dietary fiber
fruit and vegetable
physicochemical properties
techno-functional properties
health-promoting
antidiabetic potential
hypocholesterolemic effect
fermentability
1. Introduction
The fruit and vegetable industry contributes significantly to global annual food waste on a weight basis (44%) [1][2]. Approximately 25% of the fruits and vegetables in the world are wasted post-harvest, mainly due to product grading to meet quality and acceptability standards, and during processing for juice and pulp extraction [2][3]. Juice production alone accounts for 5.5 million metric tonnes (MMT) of waste per year [3]. This waste contributes to climate change to a large extent when decomposing in landfills, emitting greenhouse gases and occupying critical resources, such as land, water, and energy [4]. Therefore, studies must be conducted to minimize post-harvest waste, as proposed in the Sustainable Development Goals of the United Nations, by developing sustainable technologies to enable the utilization of these by-products. The primary focus of interest lies in their application in food products as fiber enrichment due to their high content of dietary fiber (DF). Although the beneficial health effect of DF is widely known, the average intake of most European countries and others, including the USA, Australia, and New Zealand, does not meet the recommended intake of 25–35 g/d for adults [5]. Hence, the incorporation of fruit and vegetable by-products into food products would not only overcome potential environmental problems but also help to close the gap between actual and recommended DF intake in the population and improve human health.
Binding capacities and the cholesterol- and glucose-lowering effect of DF are the result of a complex interaction of structural and physicochemical properties (i.e., particle size, surface characteristics, and DF composition, including solubility and total phenolic content) and techno-functional properties (i.e., water- and oil-binding, viscosity, and cation exchange capacity) [6]. Hence, certain parameters, such as hydration properties and the presence of different functional groups, can serve as indicators to estimate a high or low effect, as shown in Figure 1.
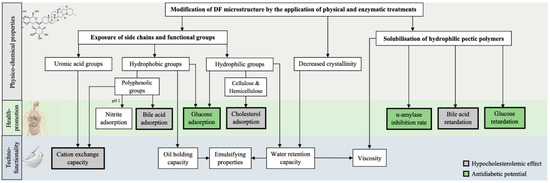
Figure 1. Scheme illustrating the physicochemical modifications of DF induced by the application of novel technologies (i.e., US, HPP, extrusion, microwave, or enzymes) and their impact on improving the health-promoting and techno-functional properties; arrows indicate a positive correlation/improvement of a certain health-related or techno-functional property by the structural alteration.
2. Relationship between Physicochemical and Techno-Functional Properties of DF
Water retention and oil-holding capacities are important techno-functional characteristics for product development since they confer to DF the ability to stabilize emulsions, replacing fat, flour, or sugar, modifying the texture and sensorial properties of food products, and reducing syneresis (the separation of a liquid from a gel caused by contraction), which leads to improved moisture retention and storage stability [7][8]. Functionality is affected by certain structural properties, such as the soluble content, hydrophilicity, hydrophobicity, and cellulose crystallinity.
Water retention capacity (WRC) accounts for fiber-swelling, gel-forming, and thickening capacities and is based on two mechanisms: (1) Water can be bound to hydrophilic groups of DF, such as hydroxyl, carbonyl, and carboxyl groups, by polar interactions and hydrogen bonding. (2) Adjacent polymer strands of DF can combine in ordered assemblies (junction zones), leading to the formation of a three-dimensional network wherein large amounts of water can be entrapped [9]. SDF is composed of a large number of hydrophilic groups and has a high molecular weight and branched structure, which impart this fraction high hydration capacity and viscosity [6][7][10]. The presence of hydrophobic groups, and thus lipophilic sites, primarily accounts for the oil-holding capacity (OHC). Other structural characteristics that can contribute to an improved OHC are a high charge density, lignin, and protein content of the polymer [11][12][13]. At the same time, high WRC and OHC are linked with improved emulsifying properties of DF, mainly due to the stabilization of the emulsion gel matrix structure [12].
The major DF component is cellulose, consisting of 70% orderly crystalline regions and 30% amorphous regions [14]. Crystalline cellulose is formed by hydrogen bonding and van der Waals forces between adjacent molecules, resulting in a dense and packed structure. On the other hand, amorphous regions contain mainly non-crystalline cellulose, hemicellulose, and lignin [15]. A low crystallinity index, indicating a decreased proportion of dense, crystalline cellulose, correlates with higher porosity; stronger, entangled gel networks; and higher WRC. In contrast, increased cellulose crystallinity is related to enhanced thermal stability and OHC but lower hydration [16].
3. Impact of Physicochemical and Techno-Functional Properties of DF on Its Health-Promoting Effect
The health-promoting effect of DF is highly connected with its ability to reduce blood cholesterol and glucose. This reduction is based on: (1) the physicochemical entrapment of the compounds inside the DF network; (2) the adsorption of these substances by their direct association with functional groups of DF. Both mechanisms, entrapment and adsorption, are responsible for the retardation and thereby the delay of diffusion of the compounds from the lumen to gut epithelial cells [17]. The main factors impacting adsorption and retardation are the DF composition, structure of the component and the environment, including pH, ionic strength, temperature and duration of exposure [18].
As the preferential mechanism, the compounds are captured and entrapped by the viscous polymer network based on the gel-forming properties of DF [19]. The soluble fraction is associated with a higher ability to form gels and increase viscosity linked to its high molecular weight and entangled conformations [7]. Hence, higher solubility, indicated by smaller particle size and higher surface area, provides stronger, more viscous gels and ideal structural conditions to entrap the compounds and reduce their diffusion [20][21]. As mentioned above, when hydrophilic groups are exposed and/or crystallinity is decreased, WRC is enhanced, which facilitates the capturing effect inside the DF network. However, the IDF fraction constitutes a physical obstacle and should be considered as an influencing but secondary factor when evaluating the effect of DF on the delay of diffusion [10].
Direct interactions, primarily non-covalent chemical bonding, such as hydrogen bonds, hydrophobic interactions, van der Waals forces and electrostatic interactions, require the availability and exposure of side chains and functional groups [22]. The major components of DF, namely cellulose, hemicellulose and pectin, provide a high number of hydroxyl and carboxyl groups favouring hydrogen bonding. However, apolar surfaces can be generated depending on the monomer ring conformation, stereochemistry of the glycosidic linkages and the degree of hydration and amount of intra-molecular hydrogen bonding [6]. The presence of non-polar molecules, such as hydrophobic aromatic rings of phenolic compounds and carotenoids, also increase hydrophobicity and probability for interactions. Additionally, DF contains charged polysaccharides, such as carboxyl groups from pectin, which play an important role for mineral absorption [18]. By changing pH and ionic strength, more charges are produced, and components can be additionally retained by electrostatic interactions.
3.1. Antidiabetic Potential
Determination of glucose adsorption and retardation capacities (GAC/GRC) serve as parameters to evaluate the in vitro antidiabetic potential of DF. Direct interactions between DF and glucose molecules can be assigned to polar and non-polar groups [23] whereas dipole-dipole interactions might be the primary non-covalent bonding type due to the high polarity of glucose. Several studies have reported positive correlations between porosity, surface area and the soluble content on GAC and GRC [24][25]. For instance, Huang et al. [25] compared the delay of diffusion and glucose adsorption of fiber-rich orange pomace, cellulose and psyllium containing a high soluble DF content. The highest GRC and GAC were found for psyllium, which was attributed to the higher number of soluble fibers. Therefore, the increase of the soluble content within a fiber system containing insoluble and soluble fraction might be favourable particularly to form a more viscous network structure and entrap glucose molecules.
The reduction of blood glucose level and the risk of suffering type 2 diabetes are also influenced by the adsorption and inhibition of α-amylase (AAIR) by DF components. DF impedes the accessibility to the enzyme substrate and, consequently, the inhibition of starch degradation to glucose [26]. The main mechanisms behind this inhibition are entrapping the enzyme and starch inside of the fiber matrix, and the adsorption of α-amylase and starch by DF components which leads to a reduced contact rate and hydrolysis. An enhanced SDF content and viscosity facilitate embedding the compounds and reducing their accessibility [12][14]. In contrast, insoluble substances, mainly cellulose, are involved in adsorption [27][28], which is affected by its crystallinity index.
3.2. Hypocholesterolemic Effect
Cholesterol reduction is based on two mechanisms: the adsorption and increment in the excretion of cholesterol and, secondly, the adsorption and enhanced excretion of bile acids, preventing their reabsorption by the liver and promoting the synthesis of new bile salts from cholesterol [29]. Suitable parameters to predict the hypocholesterolemic effect in vitro are adsorption and retardation capacities of bile acids (BAC, BRC), the adsorption capacity of cholesterol (CAC) and the cation exchange capacity (CEC). Bile acids have a steroid nucleus and an aliphatic side chain, thus contain a hydrophobic and hydrophilic surface enabling them to form micelles above a certain concentration (CMC) and interact with an oil-water interface. Similarly, cholesterol shows amphiphilic nature since it is comprised of an apolar ring system, which is associated with a hydrocarbon chain and a hydroxyl group [30].
The main mechanism causing the enhanced excretion of cholesterol is due to the physical entrapment of bile acids and cholesterol and their micelles, promoted by high SDF content and increased viscosity [6][29][31]. Additionally, SDF is discussed to support the physical barrier properties of the unstirred water layer, which covers the luminal side of the enterocytes, and therefore impair the uptake of bile salt-cholesterol micelles [32]. Soluble carboxymethyl cellulose (CMC) is discussed as the main soluble component of DF which can entrap cholesterol crystals by forming cholesterol-CMC-composites [33]. However, a study with heat damaged oat fiber with decreased viscosity did not show the expected decrease of BAC and indicated that not solely viscosity and solubility impact cholesterol lowering but direct binding of the components [31].
The mechanism of the adsorption of bile salts and cholesterol by DF is still unclear [29][31]. Research is mainly focused on studying the interaction between bile acids and SDF since it is expected to be the main process of cholesterol reduction besides entrapment. Bile acids were better absorbed when DF had a hydrophobic surface suggesting that hydrophobic interaction is the predominant non-covalent binding type [6]. This might explain the correlation between the ability to bind bile acids and to retain fat linked by the hydrophobic nature of DF [13]. However, the relationship between OHC and BAC is also determined by SDF since certain soluble components, including pectin, arabinoxylan and arabinogalactan, have high affinity for lipid materials [34]. Hydrophobic surface of DF or rather potential binding sites can be enlarged by the presence of free hydrophobic groups from polyphenols [6]. Different studies confirmed that the presence of polyphenolic compounds, such as phenolic acids and flavonoids, and lignin, contribute to BAC through hydrophobic interactions [35][36]. Other factors, such as ionic strength, might as well have an impact since it promotes electrostatic interactions, but studies are lacking [17][18]. Contrasting, cholesterol was suggested to be preferably bound to insoluble hemicellulosic and cellulosic components. For instance, coffee parchment samples high in IDF, composed by hemicellulosic and cellulosic profile, exhibited high CAC [37]. However, direct interactions of IDF with cholesterol have a lower contribution to the hypocholesterolemic effect than bile acid binding by hydrophobic attractions [31].
Cation exchange capacity (CEC) is considered as a techno-functional property since it measures the ability of DF to retain cations on the surface or rather the amount of cation that can be exchanged by another. DF with high CEC can contribute to the destabilization and disintegration of micelles when forming fiber-micelle complexes which act as barriers and reduce the diffusion and adsorption of lipids and cholesterol [38]. Hydroxyl and carboxyl groups of polyphenols, lignin and from uronic acids of the pectin and hemicellulose fraction (i.e., glucuronoxylan) have exchange ability [18][39]. Hence, the contents of polyphenols, lignin and uronic acid of DF should be taken into account as contributing factors to impact CEC and the reduction of cholesterol.
3.3. Fermentability
Fermentability of DF is mainly measured by the in vitro production of gas and SCFA as main metabolites, measurement of pH and bacterial composition during batch or continuous fermentation. The uptake and metabolization of DF by gut microbiota species are strongly connected with DF structure whereas soluble DF is more easily fermentable than insoluble DF [7]. This is mainly due to the lower degradability of insoluble DF for bacterial polysaccharide hydrolases based on their lower accessible, more dense and cross-linked cell wall structure [40] and the enhanced metabolization of shortened DF fractions of low DP, such as cello- xylo and pectin oligosaccharides [41]. Additionally, the utilization of carbohydrates by microbiota is strain-dependent since the bacterial genome encodes enzymes that hydrolyze carbohydrate linkages, such as glycosyl hydrolases (GH), and other protein degrading enzymes responsible for carbohydrate-binding and transport [42]. Studies are still scarce investigating the relationship between different DF structures and fermentation profiles. Some studies suggest that not only the DF composition, such as monosaccharides, but also the chain structure, such as linkages and oligosaccharide units, play a key role in the utilization of DF and production of metabolites [42]. In addition, the presence of polyphenolic components, which are highly present in fruit by-products, has been linked to the inhibited growth of some pathogenic species, such as H. pylori, but also the growth of several beneficial bacteria strains. As a result, DF sources containing a high TPC promote a positive balance in the gut microbiota composition and thereby show enhanced fermentability [43].
References
- FAO. Global Initiative on Food Loss and Waste Reduction; FAO: Rome, Italy, 2014.
- FAO. The State of Food and Agriculture 2019. Moving Forward on Food Loss and Waste Reduction; FAO: Rome, Italy, 2019.
- Sagar, N.A.; Pareek, S.; Sharma, S.; Yahia, E.M.; Lobo, M.G. Fruit and Vegetable Waste: Bioactive Compounds, Their Extraction, and Possible Utilization. Compr. Rev. Food Sci. Food Saf. 2018, 17, 512–531.
- Vilariño, M.V.; Franco, C.; Quarrington, C. Food Loss and Waste Reduction as an Integral Part of a Circular Economy. Front. Environ. Sci. 2017, 5, 21.
- Stephen, A.M.; Champ, M.M.J.; Cloran, S.J.; Fleith, M.; van Lieshout, L.; Mejborn, H.; Burley, V.J. Dietary Fibre in Europe: Current State of Knowledge on Definitions, Sources, Recommendations, Intakes and Relationships to Health. Nutr. Res. Rev. 2017, 30, 149–190.
- Blackwood, A.D.; Salter, J.; Dettmar, P.W.; Chaplin, M.F. Dietary Fibre, Physicochemical Properties and Their Relationship to Health. J. R. Soc. Promot. Health 2000, 120, 242–247.
- Elleuch, M.; Bedigian, D.; Roiseux, O.; Besbes, S.; Blecker, C.; Attia, H. Dietary Fibre and Fibre-Rich by-Products of Food Processing: Characterisation, Technological Functionality and Commercial Applications: A Review. Food Chem. 2011, 124, 411–421.
- Yang, Y.Y.; Ma, S.; Wang, X.X.; Zheng, X.L. Modification and Application of Dietary Fiber in Foods. J. Chem. 2017, 2017, 9340427.
- Chaplin, M.F. Fibre and Water Binding. Proc. Nutr. Soc. 2003, 62, 223–227.
- López, G.; Ros, G.; Rincón, F.; Periago, M.J.; Martínez, M.C.; Ortuño, J. Relationship between Physical and Hydration Properties of Soluble and Insoluble Fiber of Artichoke. J. Agric. Food Chem. 1996, 44, 2773–2778.
- Moczkowska, M.; Karp, S.; Niu, Y.; Kurek, M.A. Enzymatic, Enzymatic-Ultrasonic and Alkaline Extraction of Soluble Dietary Fibre from Flaxseed—A Physicochemical Approach. Food Hydrocoll. 2019, 90, 105–112.
- Zheng, Y.; Li, Y. Physicochemical and Functional Properties of Coconut (Cocos nucifera L) Cake Dietary Fibres: Effects of Cellulase Hydrolysis, Acid Treatment and Particle Size Distribution. Food Chem. 2018, 257, 135–142.
- Navarro-González, I.; García-Valverde, V.; García-Alonso, J.; Periago, M.J. Chemical Profile, Functional and Antioxidant Properties of Tomato Peel Fiber. Food Res. Int. 2011, 44, 1528–1535.
- Ma, M.M.; Mu, T.H. Effects of Extraction Methods and Particle Size Distribution on the Structural, Physicochemical, and Functional Properties of Dietary Fiber from Deoiled Cumin. Food Chem. 2016, 194, 237–246.
- He, Y.; Li, W.; Zhang, X.; Li, T.; Ren, D.; Lu, J. Physicochemical, Functional, and Microstructural Properties of Modified Insoluble Dietary Fiber Extracted from Rose Pomace. J. Food Sci. Technol. 2020, 57, 1421–1429.
- Qi, J.; Yokoyama, W.; Masamba, K.G.; Majeed, H.; Zhong, F.; Li, Y. Structural and Physico-Chemical Properties of Insoluble Rice Bran Fiber: Effect of Acid-Base Induced Modifications. RSC Adv. 2015, 5, 79915–79923.
- Mudgil, D. The Interaction Between Insoluble and Soluble Fiber. In Dietary Fiber for the Prevention of Cardiovascular Disease; Elsevier: Amsterdam, The Netherlands, 2017; pp. 35–59. ISBN 9780128051306.
- Guillon, F.; Champ, M. Structural and Physical Properties of Dietary Fibres, and Consequences of Processing on Human Physiology. Food Res. Int. 2000, 33, 233–245.
- Zheng, Y.; Li, Y.; Xu, J.; Gao, G.; Niu, F. Adsorption Activity of Coconut (Cocos nucifera L.) Cake Dietary Fibers: Effect of Acidic Treatment, Cellulase Hydrolysis, Particle Size and PH. RSC Adv. 2018, 8, 2844–2850.
- Peerajit, P.; Chiewchan, N.; Devahastin, S. Effects of Pretreatment Methods on Health-Related Functional Properties of High Dietary Fibre Powder from Lime Residues. Food Chem. 2012, 132, 1891–1898.
- Chen, J.; Gao, D.; Yang, L.; Gao, Y. Effect of Microfluidization Process on the Functional Properties of Insoluble Dietary Fiber. Food Res. Int. 2013, 54, 1821–1827.
- Jakobek, L.; Mati, P. Non-Covalent Dietary Fiber—Polyphenol Interactions and Their Influence on Polyphenol Bioaccessibility. Trends Food Sci. Technol. 2019, 83, 235–247.
- Yu, G.; Bei, J.; Zhao, J.; Li, Q.; Cheng, C. Modification of Carrot (Daucus carota Linn. Var. Sativa Hoffm.) Pomace Insoluble Dietary Fiber with Complex Enzyme Method, Ultrafine Comminution, and High Hydrostatic Pressure. Food Chem. 2018, 257, 333–340.
- Zhang, F.; Yi, W.; Cao, J.; He, K.; Liu, Y.; Bai, X. Microstructure Characteristics of Tea Seed Dietary Fibre and Its Effect on Cholesterol, Glucose and Nitrite Ion Adsorption Capacities in vitro: A Comparison Study among Different Modifications. Int. J. Food Sci. Technol. 2020, 55, 1781–1791.
- Huang, Y.-L.; Ma, Y.-S.; Tsai, Y.-H.; Chang, S.K.C. In Vitro Hypoglycemic, Cholesterol-Lowering and Fermentation Capacities of Fiber-Rich Orange Pomace as Affected by Extrusion. Int. J. Biol. Macromol. 2019, 124, 796–801.
- Gourgue, C.M.P.; Champ, M.M.J.; Delort-Laval, J.; Lozano, Y. Dietary Fiber from Mango Byproducts: Characterization and Hypoglycemic Effects Determined by in vitro Methods. J. Agric. Food Chem. 1992, 40, 1864–1868.
- Chau, C.F.; Chen, C.H.; Lee, M.H. Comparison of the Characteristics, Functional Properties, and in vitro Hypoglycemic Effects of Various Carrot Insoluble Fiber-Rich Fractions. LWT Food Sci. Technol. 2004, 37, 155–160.
- Dhital, S.; Gidley, M.J.; Warren, F.J. Inhibition of α-Amylase Activity by Cellulose: Kinetic Analysis and Nutritional Implications. Carbohydr. Polym. 2015, 123, 305–312.
- Tamargo, A.; Martin, D.; Navarro del Hierro, J.; Moreno-Arribas, M.V.; Muñoz, L.A. Intake of Soluble Fibre from Chia Seed Reduces Bioaccessibility of Lipids, Cholesterol and Glucose in the Dynamic Gastrointestinal Model Simgi®. Food Res. Int. 2020, 137, 109364.
- Ma, H. Cholesterol and Human Health. Nat. Sci. 2004, 2, 17–21.
- Zacherl, C.; Eisner, P.; Engel, K.H. In Vitro Model to Correlate Viscosity and Bile Acid-Binding Capacity of Digested Water-Soluble and Insoluble Dietary Fibres. Food Chem. 2011, 126, 423–428.
- Gunness, P.; Gidley, M.J. Mechanisms Underlying the Cholesterol-Lowering Properties of Soluble Dietary Fibre Polysaccharides. Food Funct. 2010, 1, 149–155.
- Uskoković, V. Composites Comprising Cholesterol and Carboxymethyl Cellulose. Colloids Surf. B Biointerfaces 2008, 61, 250–261.
- Devi, P.B.; Vijayabharathi, R.; Sathyabama, S.; Malleshi, N.G.; Priyadarisini, V.B. Health Benefits of Finger Millet (Eleusine coracana L.) Polyphenols and Dietary Fiber: A Review. J. Food Sci. Technol. 2014, 51, 1021–1040.
- Naumann, S.; Schweiggert-Weisz, U.; Eisner, P. Characterisation of the Molecular Interactions between Primary Bile Acids and Fractionated Lupin Cotyledons (Lupinus angustifolius L.). Food Chem. 2020, 323, 126780.
- Chamnansilpa, N.; Aksornchu, P.; Adisakwattana, S.; Thilavech, T.; Mäkynen, K.; Dahlan, W.; Ngamukote, S. Anthocyanin-Rich Fraction from Thai Berries Interferes with the Key Steps of Lipid Digestion and Cholesterol Absorption. Heliyon 2020, 6, e05408.
- Benitez, V.; Rebollo-hernanz, M.; Hernanz, S.; Chantres, S. Coffee Parchment as a New Dietary Fiber Ingredient: Functional and Physiological Characterization. Food Res. Int. 2019, 122, 105–113.
- Furda, I. Interaction of Dietary Fiber with Lipids—Mechanistic Theories and Their Limitations. In New Developments in Dietary Fiber. Advances in Experimental Medicine and Biology; Furda, I., Brine, C.J., Eds.; Springer: Berlin/Heidelberg, Germany, 1990; Volume 270, pp. 67–82.
- Thibault, J.-F.; Lahaye, M.; Guillon, F. Physico-Chemical Properties of Food Plant Cell Walls. In Dietary Fibre—A Component of Food, Nutritional Function in Health and Disease; Schweizer, T.F., Edwards, C.A., Eds.; Springer: London, UK, 1992; pp. 21–39.
- Lamothe, L.M.; Cantu-Jungles, T.M.; Chen, T.; Green, S.; Naqib, A.; Srichuwong, S.; Hamaker, B.R. Boosting the Value of Insoluble Dietary Fiber to Increase Gut Fermentability through Food Processing. Food Funct. 2021, 12, 10658–10666.
- Míguez, B.; Gómez, B.; Gullón, P.; Gullón, B.; Alonso, J.L. Pectic Oligosaccharides and Other Emerging Prebiotics. In Probiotics and Prebiotics in Human Nutrition and Health; InTech: Vienna, Austria, 2016.
- Hamaker, B.R.; Tuncil, Y.E. A Perspective on the Complexity of Dietary Fiber Structures and Their Potential Effect on the Gut Microbiota. J. Mol. Biol. 2014, 426, 3838–3850.
- Guerra-Valle, M.; Orellana-Palma, P.; Petzold, G. Plant-Based Polyphenols: Anti-Helicobacter pylori Effect and Improvement of Gut Microbiota. Antioxidants 2022, 11, 109.
More
Information
Subjects:
Others
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
889
Revisions:
2 times
(View History)
Update Date:
19 Oct 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




