The thermal energy storage and conversion process possesses high energy losses in the form of waste heat. The losses associated with energy conversion achieve almost 90% of the worldwide energy supply, and approximately half of these losses are waste heat. Hence, waste heat recovery approaches intend to recuperate that large amount of wasted heat from chimneys, vehicles, and solar energy systems, among others. The novel class of thermal fluids designated by nanofluids has a high potential to be employed in waste heat recovery. It has already been demonstrated that nanofluids enhance energy recovery efficiency by more than 20%. Also, the use of nanofluids can improve the energy capacity of steelworks systems by around three times. In general, nanofluids can improve efficiency and reduce exergy destruction and carbon emissions in devices like heat exchangers.
1. Waste Heat Sources
Heat is one of the common types of energy used in definitive form in heating and cooling applications or under intermediate form derived from the traditional power generation from fuels. The primary source of heat is the combustion of fuels, which release vast quantities of energy
[1]. As an example, the average heat content of natural gas is approximately 8.6 MJ/m
3 at normal temperature and pressure
[2]. A significant part of industrial applications uses the heat contained in vapor, acting as a heat transfer medium, having high, medium, and low pressure grades of vapor
[3]. Nonetheless, the main problem with heat is that it requires a temperature gradient to be transferred from the higher-temperature stream to the lower-temperature stream, leaving waste heat that cannot be further transferred from the heat source to the heat dissipator
[4]. The energy losses in different processes are a thermodynamic fact that is rather difficult to avert, given that no thermal process can attain a complete energy efficiency of 100%, and most of the energy losses are released under the form of heat. According to the work of Forman et al.
[5], the global waste heat comes from exhausts/effluents (52%), energy services (28%), and other losses (20%). By sectors, and according to the same study, waste heat is mainly produced by the electricity sector (61%), transportation (18%), industrial (9%), residential (7%), and commercial (6%). A work elaborated by authors Vance et al.
[6] has shown that in the US industry, around 113.6 TWh of waste heat comes from chimneys, furnaces, steel electric arc furnaces, and the glass industry. Moreover, researchers Cheng et al.
[7] showed that high-temperature solid granular material in, for instance, the cement, coke, and steel industries were associated with approximately 160, 700, and 1400 TWh of waste heat in India, the United States, and China, respectively. A significant part of waste heat amount can be linked to energy losses, which account for 45% of conventional power plants, 40% of pumps and fans, and up to 80% of compressors
[8]. This clearly shows that waste heat sources are widely spread across different processes to the level that it is hard to find a particular industry without waste heat. The feasibility of waste heat recovery depends mainly on the quality and quantity of waste heat and the state (gas, liquid, or solid) of the waste heat stream
[8].
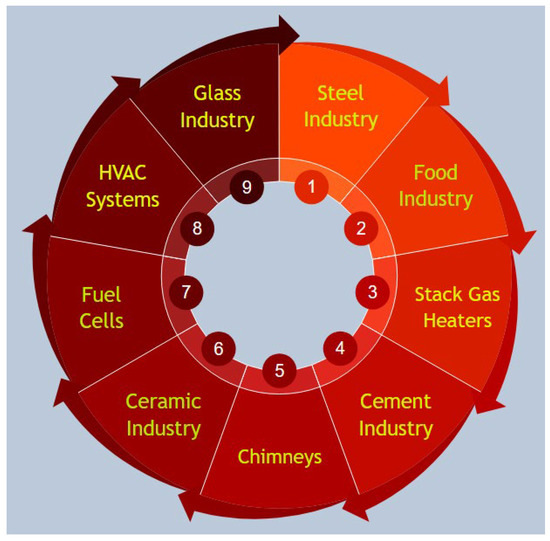
Figure 1 summarizes the fundamental waste heat recovery applications with nanofluids.
Figure 1. Fundamental waste heat recovery applications with nanofluids.
2. Nanofluids
2.1. Preparation Methods
Nanofluids can be produced by dispersing nanoparticles in base fluids. A uniform dispersion that mitigates the clustering and sedimentation of the included nanoparticles is paramount. In this direction, these are usually added to nanofluid surfactants to improve their stability over time. Apart from this, the modification of the surface of the dispersed nanoparticles and application of strong force on their clusters can enhance the stability over time of nanofluids. The one-step and two-step methods are the main preparation methodologies to synthesize nanofluids.
2.3. Thermal Conductivity Influencing Factors
2.3.1. Base Fluid
The thermal conductivity of nanofluids is closely linked to the thermal conductivity of the base fluid. There is a linear relationship between the two thermal conductivities: the nanofluid exhibits higher thermal conductivity in cases where the base fluid has high thermal conductivity.
2.3.2. Type of Nanoparticles
The type or nature of the incorporated nanoparticles in the base fluids significantly impacts the thermal conductivity of the final nanofluid. The addition of high thermally conductive nanoparticles is usually the common choice since they impart a higher thermal conductivity to the nanofluid.
2.3.3. Concentration of the Nanoparticles
The thermal conductivity of nanofluids normally increases with increasing nanoparticle concentration. Most of the existing effective thermal conductivity models include the concentration of the nanoparticles, and a linear and non-linear correlation between the thermal conductivity and concentration of the nanoparticles was experimentally found by the research community.
2.3.4. Size of the Nanoparticles
The thermophysical properties of nanofluids, like, for instance, thermal conductivity, are influenced by the size of the incorporated nanoparticles. The thermal conductivity of nanofluids, in most of the published cases, increases with the decreasing size of nanoparticles. If the nanoparticles are small, the Brownian motion becomes dominant, and the randomly wised motion of the nanoparticles in the base fluid increases, and consequently, the thermal conductivity of the nanofluid also increases. Also, the surface-to-volume ratio increases with the decreasing size of the nanoparticles.
2.3.5. Shape of the Nanoparticles
The shape of the added nanoparticles also impacts the thermal conductivity of nanofluids, as demonstrated by Hamilton-Crosser
[9]. The influence of the shape of the added nanoparticles was studied by researchers Timofeeva et al.
[10]. Authors used alumina nanoparticles dispersed in a mixture of water and ethylene glycol and confirmed that the cylindrical nanoparticles exhibited the highest thermal conductivity. Additionally, it was already found that the brick-shaped nanoparticles had higher thermal conductivity than the nanoplatelets and blade-shaped nanoparticles
[11].
2.3.6. Operating Temperature
The thermal conductivity of nanofluids can be directly related to the operating temperature. In this sense, several researchers have found an ameliorated thermal conductivity of nanofluids with increasing working temperature. The thermal conductivity increases with increasing temperature since the Brownian motion of the nanoparticles becomes stronger.
2.3.7. Addition of Surfactants
The surfactants are usually added to nanofluids to improve their stability over time by diminishing the agglomeration of the nanoparticles. Nonetheless, the surfactant may decrease the thermal conductivity of the nanofluid in the stabilization process.
2.5. Rheological Behavior
The rheological behavior of a fluid can be interpreted by the relationship between the shear stress and the shear rate. The shear stress can be defined as the tangential force per unit area, and the shear rate can be defined as the alteration of the shear strain per unit time. The viscosity of a fluid can be defined as the ratio between the shear stress and shear rate, and it is a measure of the resistance given by the adjacent layers to one another during the fluid flow. The fluid behavior can be categorized as Newtonian and non-Newtonian. The non-Newtonian can be divided in pseudoplastic, Bingham, Bingham plastic, and dilatant. In the cases where a fluid exhibits Newtonian behavior, its viscosity remains constant, whereas its shear stress exhibits a linear relation with the shear rate. In the case where a fluid shows non-Newtonian behavior, the viscosity varies with the shear rate, and the relationship between the shear stress and shear rate exhibits Bingham behavior. Moreover, the rheological behavior of nanofluids directly impacts their pressure drop and brings some useful insights about nanoparticle structuring, which can be very useful in estimating the thermal conductivity of nanofluids. The rheological behavior can be determined with the aid of rheometers. The titanium oxide-water nanofluids usually show shear thinning behavior, and the titanium oxide-ethylene glycol nanofluids present Newtonian behavior even for high shear rates. Additionally, the multi-walled carbon nanotubes (MWCNTs) exhibit both Newtonian and non-Newtonian behaviors, depending on the type of base fluid in which they are dispersed. Also, a shear thinning behavior was reported by MWCNTs dispersed in water, resin, and oil. Nanofluids containing MWCNTs in silicone oil and glycerol have Newtonian behavior for all concentrations and operating temperatures. It should be noted that the MWCNTs nanofluids with high volume fractions exhibit non-Newtonian behavior, whereas they show Newtonian behavior when they possess lower concentrations. The MWCNTs nanofluids usually exhibit shear thinning behavior for low shear rates and sometimes show Newtonian behavior at high shear rates. The silica nanofluids exhibit Newtonian behavior regardless of the base fluid. A large part of the alumina aqueous nanofluids exhibit non-Newtonian behavior, with the exceptions of the alumina-ethylene glycol and alumina polyethylene glycol, which behave as Newtonian fluids. The aqueous nanofluids with micro-sized alumina particles exhibit a shear-thinning behavior. Also, the alumina nanofluids show a transition from shear thinning behavior to shear thickening as the shear rate exceeds a certain critical level, which increases with increasing concentration of nanoparticles. The copper oxide nanofluids exhibit almost a Newtonian behavior, but with the addition of xanthan gum, they show a shear-thinning behavior. In sum, it can be concluded that a significant part of nanofluids with low concentrations of nanoparticles behave as Newtonian fluids, and nanofluids with high concentrations exhibit non-Newtonian behavior. Nanofluids present Newtonian behavior at low shear rates and non-Newtonian behavior at high shear rates.
3. Heat-to-Heat Waste Heat Recovery with Nanofluids
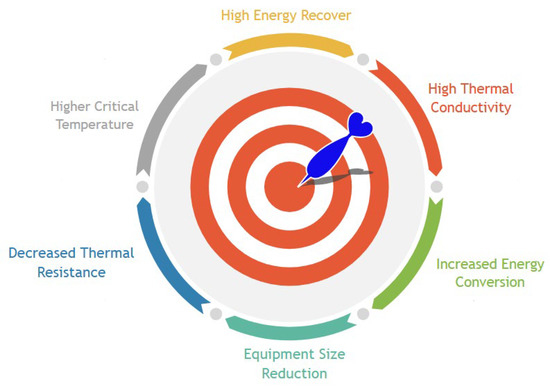
The diverse approaches using nanofluids to recover thermal energy from different waste heat sources will be presented. Hence, there will be described the main heat-to-heat, heat-to-work, and heat-to-power waste heat recovery routes where the usage of nanofluids takes an active role. Figure 2 summarizes nanofluids characteristics that are very suitable for waste heat recovery processes.
Figure 2. Nanofluids target characteristics for waste heat recovery.
3.1. Waste Heat Recovery Using Heat Exchangers
There are several types of heat exchangers, the most common being the double pipe, shell and tube, helical or coiled, and plate heat exchangers, having diverse configurations for heat transfer performance and efficiency enhancements
[12]. The effectiveness of the heat exchangers depends fundamentally on the temperature values and thermophysical features of the hot and cold streams, together with the constitutive material and channel configuration of the heat exchangers
[13]. The improved heat transfer across the heat exchange surface in a heat exchanger from waste heat stream to the nanofluid, and within the nanofluid, shows a more dispersed heat transfer across the nanofluid due to the suspended nanoparticles resulting in an overall plasmonic effect. The heat transfer beams showed a primary heat transfer across the surface from the waste heat stream to the nanofluid. Additionally, due to the presence of the nanoparticles, secondary heat transfer beams within the nanofluid itself are generated, hence improving the heat transfer efficiency beyond that of conventional fluids.
3.2. Waste Heat Recovery Using Heat Pipes
The heat pipes are composed of an evaporator, condenser, and adiabatic sections. The evaporator is located at one end of the heat pipe, where the heat is absorbed, and the working fluid is vaporized. The condenser is located at the other end, where the vapor is condensed, and the heat is released. The adiabatic section is located between the evaporator and condenser, where the two phases flow in opposite directions trough the core and wick sections
[14]. The heat pipe contains the fluid and vapor phase, where the saturated fluid turns into vapor, and then it is transferred to the condenser section, where it goes back to liquid form. The fluid that has been condensed is converted to vapor again through the wick section using capillary wicking. The different types of heat pipes include the tubular heat pipe, thermosyphon heat pipe, pulsating heat pipe, rotating heat pipe, heat pipe heat exchanger, loop heat pipe, and gravity heat pipe. Diverse types of heat pipes have already been studied for waste heat recovery purposes.
3.2.1. Pulsating Heat Pipes
There are three types of forces acting on the nanoparticles dispersed in a nanofluid: gravitational, electrical repulsion, and attraction forces. The electrical repulsion force (zeta potential charge) makes the nanofluid stable, whereas the other forces induce instability to the nanofluid because of the formation of larger clusters, resulting in the nanoparticles’ deposition. The application of a magnetic field could aid the stabilization of the nanoparticles with magnetic properties since it can enhance the heat transfer performance and reduce the need for the ultrasonication preparation phase and surfactant incorporation.
3.2.2. Gravity Heat Pipes
The gravity heat pipes normally exhibit superior thermal performance due to the cyclic phase transformation of the operating fluid. As an important thermal management device for waste heat recovery, the heat transfer capability of the gravity heat pipes improves, the performance and efficiency of waste heat recovery improve, and more wasted heat can be stored more rapidly. In this direction, researchers Qian et al.
[15] explored nano-diamond, which has the highest thermal conductivity dispersed in water, to improve the thermal performance of a gravity heat pipe. Additionally, the influences of filling ratio, mass fraction, and heat flux on thermal performance require further studies. Researchers reported that the heat flux had the most significant impact on the thermal performance, followed by the filling ratio and mass fraction. The thermal performance was the best when the optimal parameters of a filling ratio of 20% and a mass fraction of 1% wt. were chosen at a heat flux of 20 × 10
4 W/m
2. The diamond nanofluid heat transfer behavior is studied by observing the flow patterns. The orthogonal experiment and range analysis investigate the influence of the filling ratio, concentration of the diamond nanoparticles, and heat flux on heat transfer capability. The research team also confirmed that the heat transfer capability decreased with increasing filling ratio due to the poor generation of bubbles and vapor flow rate.
3.2.3. Heat Pipes Heat Exchangers
The heat pipe heat exchangers seem like ordinary finned coils, but each successive tube is independent and not connected to the other tubes. Each tube possesses an internal capillary wick material and is evacuated, filled with a compatible fluid according to the range of operating temperatures, and sealed. With the tubes installed horizontally, one half of the heat exchanger will act as an evaporator, and the other half will play the role of a condenser. The high-temperature air stream passes through the evaporator half of the unit, and the low-temperature air stream passes through the condenser half. The high-temperature air stream passes over one half of all the tubes. Since the working fluid is heated and vaporized in the evaporator half, the vapor pressure difference directs the vapor to the condenser end section of the heat tube. In the condenser section, the fluid releases the latent energy of vaporization as it condenses, thus heating the low-temperature air stream. The fluid will return to the evaporator end through the internal wick.
3.3. Pool Boiling Waste Heat Recovery
Heat extraction from wastewater or incinerators is an important and challenging task in the industry’s thermal management systems. Heat recovery boilers are often used in the industry for heat recovery, and their thermal performance is mainly dependent on the Critical Heat Flux (CHF) of the working fluid. Researchers are using nanofluids as a better alternative to conventional base fluids. Also, the pool boiling process is of relevance for the energy sustainability of the wastewater heat recovery and incinerator heat recovery processes.
3.4. Waste Heat Recovery Using Phase-Change Materials
The phase-change materials absorb and release energy in the form of latent heat, which is normally dominant when compared to the sensible heat, and low-temperature gradients are the driving force for heat transfer improvement
[16]. The phase-change materials can be categorized according to the kind of latent heat being transported into liquid–gas, solid–solid, solid–liquid, and solid–gas. The solid–gas and liquid–gas have higher latent heat and, consequently, heat capacity
[17]. Nonetheless, the fundamental concern linked with these two types of phase-change materials is the increased gas volume that evolved during the phase transition, making them unsuitable for large-scale ends. Apart from this, the solid–solid phase change material has a higher phase transition temperature but lower latent heat in comparison with the solid–liquid phase change material that presents a wide phase transition temperature range. The phase-change materials can be divided according to the type of the constitutive material into organic, inorganic, and eutectic mixtures of organic–organic, inorganic–inorganic, and organic–inorganic phase-change materials. The phase-change materials, in general, and the solid–liquid, in specific, are widely utilized in energy storage purposes and have been successfully applied for waste heat recovery
[18].
The phase-change materials can be in bulk or encapsulated form. In the bulk form, the heat transfer behavior is influenced by the morphology of the container
[19]. The phase change material encapsulation is explored to enhance the heat transfer area, decreasing the corrosivity degree of some phase-change materials by avoiding direct contact, controlling the volume alteration during the phase transition, and reducing the backdraws from subcooling
[20]. The encapsulation can be done in the form of a core-shell or shape-stabilized configuration in which the phase change material is kept inside a porous material with a capillary effect. One of the fundamental problems associated with phase-change materials is their low temperature and poor thermal conductivity, which needs prolonged periods and greater surface area. As an example, the paraffin-based phase-change materials, which are one of the most used phase-change materials, have excellent availability, cost-effectiveness, superior thermal and chemical stabilities, and suitable melting points between 18 °C and 30 °C and are adequate to be used in a broad range of applications, have only a poor thermal conductivity of 0.2 W/m K
[21].
3.5. Main Areas of Actuation of the Heat-to-Heat Waste Heat Recovery
3.5.1. HVAC Systems
Heating, ventilation, and air-conditioning, commonly designated by HVAC systems, usually account for up to 60% of the energy demand in residential buildings. Hence, many heat recovery methods are increasingly adopted to decrease the heating and cooling demands of the HVAC systems through pre-heating or pre-cooling. In energy recovery ventilation, the technological solutions for waste energy recovering are studied. The exploration of air-to-air energy recovery devices and systems in HVAC systems continues to increase. The more demanding outdoor air requirements needed to accomplish the ASHRAE standards regarding the building ventilation for the indoor air acceptable quality entailed appreciably the cooling and heating charges. The augmentation of the outdoor air loads will increase the operating and equipment costs. Such facts had a great interest in energy recovery systems and their economic implications. The new and retrofit energy recovery methodologies can be divided into process-to-process, process-to-comfort, and comfort-to-comfort approaches. In the process-to-process route, the sensible heat is taken from the exhaust stream, and it is transported to the supply one. In a significant part of the process-to-comfort approaches, the recovery of energy involves the capture and transfer of sensible heat only. Waste heat is transferred to makeup or outdoor air streams. This is effective during winter months, but it requires modulation during spring and autumn to prevent overheating of the residential buildings.
3.5.2. Cement Industry Waste Heat Recovery
Cement production is one of the most intensive, energy-consuming, and largest carbon-emitting industrial sectors due to the very high-temperature values required to obtain the cement clinker. The cost of energy consumption in the cement industry signifies 20% to 40% of the total production cost. According to the International Energy Agency, energy consumption can still be as high as approximately 0.5 GJ/ton of cement (Canada, 2018). Waste heat sources from the cement plants comprise the exhaust gases from the pre-heater and the ejection of hot air from the cooler of the clinker. In terms of cogeneration power, these waste heat sources, which have various temperature levels, can be employed independently or in combination. The rotary kilns are worldwide explored by various industries to manufacture a wide range of products such as lime, cement, magnesia, alumina, vermiculite, and iron ore pellets. There are many published studies on waste heat recovery processes in the cement sector that incorporate waste heat recovery from flue gases and rotary kiln surfaces. Whereas the former has attracted much interest from the industry, and waste heat recovery plants for flue gases are installed in cement plants, the latter is still not followed because of some practical concerns regarding the kiln operation. The cement plant kiln releases an appreciable heat quantity to the surroundings through its surface. Therefore, the heat can be extracted by developing and implementing a suitable waste heat recovery system over the kiln shell.
3.5.3. Fuel Cells Waste Heat Recovery
One of the most promising nanofluid uses in the fuel cells technological area is the waste heat recovery approach. The current overview sub-section is dedicated to the use of nanofluids in the different waste heat recovery techniques adequate for fuel cell devices, including Stirling engines, absorption chillers, organic Rankine cycles, and thermoelectric generators. One of the most common ways to recuperate waste heat in fuel cell devices is to drive absorption chillers in the fuel cell for combined cooling power systems producing, at the same time, power, and cooling. This waste heat recovery route was suggested for solid oxide fuel cells
[22] and proton-exchange membrane fuel cells
[23]. The absorption chillers have received increased research interest since these types of chillers are activated by heat instead of electricity and consequently are considered an environmentally benevolent technological solution and a very suitable alternative to the conventional vapor compression chillers
[24]. The ammonia–water and lithium bromide–water are the most common operating mixtures used as absorbent-refrigerant pairs in the absorption chillers. Nanofluids having improved heat transfer and mass transport characteristics can be employed as absorbent material in the absorption chillers to enhance the absorption rate of the refrigerant vapor
[24].
3.5.4. Chimneys Waste Heat Recovery
The very large worldwide number of chimneys used in several industries, restaurants, and homes, among many others, strongly contribute to global warming and climate change because of their release of large amounts of waste heat. Attempting to reduce such negative effects, authors Eldesoukey et al.
[25] analyzed the thermal performance of a thermoelectric generator cooled by a micro-channeled heat spreader using nanofluids for waste heat recovery from a vertical chimney. Researchers employed three-dimensional mathematical models for the thermoelectric generator, microchannels, nanofluids, and heat spreader, which were solved by the
Ansys Fluent™ 2021 R1 software. The effects of Joule, Seebeck, and Thomson were considered in the thermoelectric generator model. The obtained results showed that the thermoelectric power increased with increasing heat spreader and microchannel sizes: a 4-fold increase in the microchannels and heat spreader sizes increased the thermoelectric generator output power by 10%. Under these conditions, it achieved a maximum cooling efficiency of nearly 89% and the net output power peak. Also, the micro-channeled heat spreader increased the system net power by around 125% in reference to the normal channel and reduced the needed cooling fluid flow rate.
3.5.5. Stack Gas Heater Waste Heat Recovery
The impact of using alumina aqueous nanofluids on the thermal performance of an annular enclosure was investigated by authors Dalvand et al.
[26], which can be used for waste heat recovery from a stack of a gas heater. In the first heating steps, the temperature of the inner cylindrical increases, whereas the liquid bulk is approximately at the preceding uniform temperature. Consequently, the wall heat flux was considerably increased at the beginning. After that, it was verified that the wall heat flux deteriorated because of the enhancing Rayleigh number R
a and the correspondent creation of flows in the annulus, which led to a temperature increase in the liquid. The use of nanofluids possesses the benefit of enhancing the main key influencing factors, such as the Nusselt number. Nanofluids with increased concentrations of nanoparticles require less time to react to any alterations in the thermal environment. A greater HTC and improved uniformity of the temperature values in the enclosure were attained by choosing larger nanoparticle concentration nanofluids. The experimental results indicated that the HTC and Nusselt number of nanofluids were comparatively enhanced with time, given that the hotter base fluid results in higher thermal conductivity. In view of the results, the research team concluded that in the initial heating phases, there were observed higher temperature increases in the liquid. The water itself and nanofluids exhibited asymptotic evolutions, achieving nanofluids at a higher temperature because of the increased thermal conductivity due to the Brownian motion of the nanoparticles. At low Rayleigh numbers, conduction is the only heat transfer mechanism of fluid flow. As the Rayleigh number increased, it is established a laminar flow regime at the boundary layer, which is restricted by the boundary layers close to the cylinders. The resulting flow enables the liquid to ascend along the hot wall and descend along the cold wall. The temperature uniformity will be obtained if the volumetric concentration of the nanoparticles enlarges to some extent. The more concentrated nanofluids having higher concentration required a shorter period to reach a specific temperature. Hence, the more concentrated nanofluid, the shorter the response time to any alterations in the thermal environment.
4. Heat-to-Work Waste Heat Recovery with Nanofluids
The heat-to-work waste heat recovery approach can be carried out using diverse heat engines or thermodynamic cycles like the steam and organic Rankine cycle (ORC), Kalina cycle, Stirling engine, and Brayton cycle
[27]. The organic Rankine cycle is the most employed for waste heat recovery for work and power, considering the broad range of fluids to be utilized and adequacy for low-grade waste heat
[28]. Nonetheless, working fluids to be used in the heat engines should exhibit some special characteristics due to the cyclic character of the phase transition. Also, the thermal fluids operating in the Rankine cycle can be vapor steam, organic compounds, or mixtures of organic compounds, whereas, for instance, the Kalyna cycle normally uses an ammonia-water mixture
[29]. Moreover, the application of nanofluids in heat engines is limited and has only been initiated very recently. Nanofluids have been applied as heat transfer fluids in the evaporator and condenser sections to enhance waste heat transport to the operating fluid in essentially the ORC
[30]. One initial example is the work developed by Saadatfar et al.
[31], in which it was proposed the use of nanofluids in the ORC for decreasing the dimensions of the heat exchanger, evaporator, and condenser of the solar organic Rankine cycle integrated power, heating, and cooling tri-generation. Researchers added silver nanoparticles to the pentane base fluid, increasing the thermal conductivity of 0.136 W·m·
−1K
−1 of the pentane to nearly 16 W·m·
−1K
−1 at 0.5% wt. of silver nanoparticles.
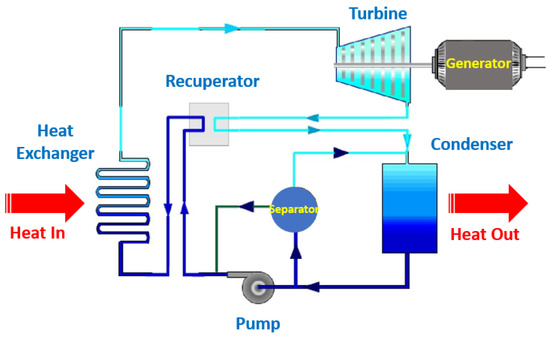
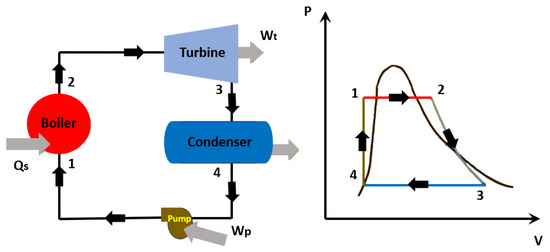
Figure 3 schematically represents the Kalina cycle, and Figure 4 illustrates the Rankine cycle.
Figure 3. Schematic representation of the Kalina cycle.
Figure 4. Schematic representation of the Rankine cycle: 1–2: isobaric expansion; 2–3:isentropic expansion; 3–4: isobaric compression; 4–1: constant volume heating.
5. Heat-to-Power Waste Heat Recovery with Nanofluids
5.1. Solar Thermal Energy Waste Heat Recovery
Several studies have been dedicated to recovering heat energy efficiently by using nanofluids and thermoelectric modules. These studies showed that the hot side of the thermoelectric generators was attached to the backside of the photovoltaic panels to absorb the dissipated heat and convert it to electricity. Also, the cold side was cooled by nanofluids and phase-change materials. A first example is the experimental work carried out by authors Soltani et al.
[32] in which it was evaluated a system having waste heat recovery from photovoltaic cells using the thermoelectric module and iron oxide or silica aqueous nanofluids. These nanofluids were flowing in the cold side of the thermoelectric module made of bismuth telluride, which can bear the working temperature. Also, the backside of the photovoltaic cells is connected to the hot side of the thermoelectric module. The obtained experimental results confirmed that the silica nanofluid cooling was better than the one provided by the iron oxide nanofluid, given that the former enhanced the power output by nearly 54% as compared with the 52% enhancement obtained when using the iron oxide nanofluid. Moreover, researchers Rajaee et al.
[33] studied the combined usage of a cobalt oxide aqueous nanofluid and a nano-enhanced phase change material composed of paraffin and alumina nanoparticles on the performance of a hybrid photovoltaic/thermoelectric generator system. Researchers concluded that operating with the cobalt oxide aqueous nanofluid increased the electrical power generation by nearly 11% in reference to that attained with water. The system, with the introduction of the phase change material, further augmented the electrical efficiency by 4.5%. The authors also emphasized the need for large-scale photovoltaic module experiments to better evaluate the feasibility of the developed cooling system. Moreover, there were performed numerical analysis on waste heat recovery in solar thermal energy systems. Some examples of such analysis were the ones carried out by authors Sami and Marin
[34]. The authors investigated the influence of the concentration of nanoparticles dispersed in nanofluids on waste heat recovery efficiency from a photovoltaic cell coupled with a thermoelectric generator working with silica, alumina, copper oxide, and iron oxide nanofluids. The adopted model considered nanofluids as homogeneous, isotropic, incompressible, and Newtonian, having stable inlet temperature and velocity. Researchers arrived at the conclusion that a greater concentration of nanoparticles enhanced the heat released by the photovoltaic cell and, consequently, enhanced power generation by the thermoelectric generator. The obtained results were consistent with the ones found in previous works on photovoltaic/thermoelectric generators in thermoelectric generator-rated systems.
Approximately 70% of the chemical energy in gasoline is lost in the form of heat in the engine of vehicles during the combustion process. Hence, it is reasonable to think that waste heat recovery in the automotive technological area should be a key concern. In this direction, authors Smith and Thornton
[35] elaborated a feasibility study for waste heat recovery in vehicles by a thermoelectric generator. Authors reported that waste heat recovery efficiency in vehicles can be between 2% and 3%. Nonetheless, with innovative thermoelectric generators, the same efficiency would get up to 10% to 15%. The research team evaluated a mid-sized automobile, a mid-sized sports vehicle, a Class 8 truck, and a Class 4 truck. It was found that the trucks produced larger amounts of waste heat, so the trucks are the most suitable for the use of a thermoelectric generator to recover these amounts of energy. Moreover, authors Li et al.
[36] carried out experimental work and numeric simulation for a vehicle waste heat recovery system with a thermoelectric generator cooled through the flowing of a nanofluid composed of copper nanoparticles dispersed in a water and ethylene glycol mixture. The fundamental output performance influencing factors were studied, including the concentration of nanoparticles, exhaust gas inlet temperature, and the thermoelectric generator’s total area. Authors reported that the used nanofluid could decrease the average temperature of the cold side of the thermoelectric generator and augment the temperature gradient between its hot and cold sides. The nanofluid increased the power output by nearly 13% and the efficiency of the thermoelectric generator by around 14%.
6. Conclusions
-
There are currently three different approaches in the waste heat recovery area, namely the heat-to-heat, heat-to-work, and heat-to-power. The heat-to-heat is a straightforward and effective methodology using heat exchangers, heat pipes, thermosyphons, waste heat boilers, and phase-change materials. Nonetheless, the recovered energy is still in the form of heat, which can be considered an efficiency-limited form of energy since it is constrained by the temperature difference;
-
The heat-to-power is a waste heat recovery approach that has great potential since it converts heat into electricity, having broad applicability. It can be performed indirectly by extending the heat-to-work through the connection to an electrical generator. Alternatively, it can also be done via thermoelectric generators. Waste heat recovery through thermoelectric generators could be a rated part for several applications because of its installation easiness and facile operation. The utilization of nanofluids could make the thermoelectric generators yield higher power output;
-
Nanofluids have been demonstrated to be an enhanced technological solution for waste heat recovery processes. Their superior thermophysical properties induced by the incorporation of nanoparticles into base fluids, together with the enhanced thermal conductivity and reduced thermal resistance, have been demonstrated to ameliorate the heat transfer performance and recovery rate;
-
Nanofluids used in the heat-to-work waste heat recovery approaches are still limited and fundamentally utilized indirectly to increase heat transport from the primary waste heat source, like in the organic Rankine cycle. The direct utilization of nanofluids as operating fluids in the organic Rankine cycle has been investigated theoretically by numeric simulations with no experimental validation;
-
Nanofluids have been applied so far for waste heat recovery in heat-to-power approaches only to increase the heat transport capability of the primary waste heat source and as refrigerants for the thermoelectric generators’ cold and hot sides;
-
Most studies concerning waste heat recovery in the cement industry have only been conducted on waste gases from the pre-heater and cooler of the clinker. It requires to be further examined intensively to decrease the losses derived from the radiation and convection effects in the cooler. Such losses should be recuperated to improve the efficiency of the clinker cooler.