Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Valerio Manippa | -- | 3090 | 2023-10-11 15:20:25 | | | |
| 2 | Lindsay Dong | Meta information modification | 3090 | 2023-10-16 03:15:19 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Manippa, V.; Palmisano, A.; Ventura, M.; Rivolta, D. The Neural Correlates of Developmental Prosopagnosia. Encyclopedia. Available online: https://encyclopedia.pub/entry/50137 (accessed on 07 February 2026).
Manippa V, Palmisano A, Ventura M, Rivolta D. The Neural Correlates of Developmental Prosopagnosia. Encyclopedia. Available at: https://encyclopedia.pub/entry/50137. Accessed February 07, 2026.
Manippa, Valerio, Annalisa Palmisano, Martina Ventura, Davide Rivolta. "The Neural Correlates of Developmental Prosopagnosia" Encyclopedia, https://encyclopedia.pub/entry/50137 (accessed February 07, 2026).
Manippa, V., Palmisano, A., Ventura, M., & Rivolta, D. (2023, October 11). The Neural Correlates of Developmental Prosopagnosia. In Encyclopedia. https://encyclopedia.pub/entry/50137
Manippa, Valerio, et al. "The Neural Correlates of Developmental Prosopagnosia." Encyclopedia. Web. 11 October, 2023.
Copy Citation
Faces play a crucial role in social interactions. Developmental prosopagnosia (DP) refers to the lifelong difficulty in recognizing faces despite the absence of obvious signs of brain lesions. In recent decades, the neural substrate of this condition has been extensively investigated. While early neuroimaging studies did not reveal significant functional and structural abnormalities in the brains of individuals with developmental prosopagnosia (DPs), the evidence identifies abnormalities at multiple levels within DPs’ face-processing networks.
congenital prosopagnosia
face processing
brain
neuroimaging
1. Introduction
Faces represent the stimuli we rely on the most for social interactions. They provide cues on others’ identity, age, gender, attractiveness, race, approachability, and emotions. Based on the evolutionary relevance of faces, most individuals can recognize others’ identities effortlessly thanks to dedicated face-selective cognitive mechanisms and the respective neural substrates [1]. In fact, unlike most everyday items, human faces are perceived as a whole, rather than assortments of features. According to the “domain-specific hypothesis” faces are processed holistically either due to an innate facial template [2] or because human faces represent the sole uniform stimuli for individual-level discrimination during the sensitive developmental period [3]. Alternatively, according to the “expertise hypothesis”, holistic processing results from automatized attention to whole objects, which is developed with extensive experience in discriminating them [4]. Despite the pivotal role of holistic processing, to date, the more consistent hypothesis (i.e., the “featural/configural hypothesis”) postulates that faces are processed by using both holistic (configural) and featural (i.e., analytic) mechanisms, emphasizing global characteristics (i.e., spatial relations) and specific face components (i.e., eyes, nose, and mouth), respectively [5][6][7]. Holistic and featural analyses of face stimuli can be used alternatively or together based on the task requests, stimulus presentation, and contextual demands [8][9].
The impairment of either or both of these mechanisms plays a role in a specific condition known as prosopagnosia [10], characterized by serious and (often) specific face identification deficits [11][12][13]. Albeit early scientific reports refer to the acquired form of prosopagnosia, where people lost their previously intact face recognition ability after a brain injury, research over the last ~25 years has increasingly focused on the developmental (or congenital) form of prosopagnosia (DP), which refers to the lifelong difficulty in recognizing faces despite the absence of brain damages [7][14][15][16].
Along with studies assessing the cognitive phenotype and interindividual variability of developmental prosopagnosia [14], much research has focused on the neural underpinnings of this condition [17]. This corpus of research is based on evidence from neurotypical individuals, showing the existence of face-sensitive neuro-cognitive mechanisms. Functional magnetic resonance imaging (fMRI) and invasive neuronal recordings in humans (and non-human primates) reveal the existence of a network of face-sensitive regions in the ventral occipito-temporal cortex (VOTC) [18][19][20][21][22]. Face-sensitive regions are mostly (albeit not specifically) right-lateralized and identified in the lateral surface of the inferior occipital cortex (i.e., occipital face area—OFA) [23], the lateral side of the fusiform gyrus (i.e., fusiform face area—FFA) [19], and the posterior part of the superior temporal sulcus (pSTS) [24].
Further neuroimaging evidence has unveiled two main face-sensitive networks in the human brain: (i) the core face network and (ii) the extended face network [21][25]. Following the early-stage analysis of face and structural processing in the OFA [26], information on invariant facial features (i.e., crucial information for face identity) reaches the FFA [21][27], while dynamic (changeable) features such as movements in eye gaze or facial expressions are directed to the pSTS [28][29][30]. Additional face-sensitive regions have also been described in the so-called extended face network. Indeed, the FFA projects to the anterior part of the medial (aMTG) and inferior temporal gyrus (aITG), which process the biographical and semantic information of known faces [31][32] (i.e., as suggested by patients with aTC damage causing the inability to access person-specific information from faces and names [33][34][35]).
As for the temporal dynamics of face-processing, event-related potentials (ERPs) show that faces elicit specific occipito-temporal components [36]. A well-established ERP marker of face-sensitive cortical processing is the N170 [37][38][39], which consists of a large and often right-lateralized electroencephalography (EEG) deflection peaking between 150 and 200 ms over the occipitotemporal cortex in response to faces compared to non-face stimuli [40]. In magnetoencephalography (MEG), a similar component (i.e., the M170) is also observed [41][42][43][44]. Other components involved in face processing include the P1 (indexing a very early stage of face processing [45][46]), the N250 (reflecting the activation of preexisting and acquired face representations [47][48]), and the P600f (indexing later post-perceptual stages of face recognition [38][49]).
Albeit early neuroimaging—mainly single case—studies failed to show functional and morphological abnormalities in individuals with developmental prosopagnosia’s brains [50][51], recent evidence has shown face-network abnormalities at multiple levels [52][53].
2. The (In)visible Brain Markers of Developmental Prosopagnosia
2.1. Gray and White Matter Alterations
Contrary to the acquired form, a DP diagnosis requires face-processing difficulties to be present (presumably) since birth, not caused by any sign of a brain lesion, together with normal sensory and intellectual functions [54]. Nevertheless, differences in the cerebral architecture and connectivity have been reported in DP. Using various MRI techniques such as structural MRI, diffusor tensor imaging (DTI), and functional connectivity fMRI, researchers have been able to investigate the differences between DPs and HCs. This has allowed for an analysis of the links between structural and behavioral data.
Most of the studies reported a reduced density or volume in DPs’ temporal lobes compared to HCs, specifically in the pSTS, MTG, and FG (e.g., [21][25]). Such evidence was more consistent within the right hemisphere. As for white matter integrity, lower fractional anisotropy and functional connectivity were found in the core face network, particularly near the r-FFA (e.g., [53][55]). These findings are consistent with studies on the neural basis of face processing [56] and with injuries reported in acquired prosopagnosia [57][58]. On the other hand, the MTG and ITG are not face-selective regions; despite this, they are implicated in identifying and naming famous faces and buildings (i.e., semantic memory) [59].
Albeit DPs’ FFA and OFA gray matter volumes do not seem to be reduced, there is converging evidence about disrupted white matter within DPs’ VOTCs. Particularly, the r-OFA and r-FFA have emerged as central nodes where the connectivity within the core face network is compromised in DPs. Specifically, the r-OFA shows impairment in both the short-range and long-range functional connectivity within the core face network, whereas the r-FFA shows impairment mainly in the long-range functional connectivity and within the extended face network [52][53][60]. Further analyses revealed multiple regions in DPs’ core- and extended-face networks, whose functional connectivity to the r-OFA and r-FFA is decreased; such findings suggest the central role of the interaction between the r-FFA and the other regions of the core face network and the extended face network to successfully recognize faces.
To summarize, despite the absence of a brain injury, gray matter alterations in the temporal lobe and white matter reductions involving the VOTC and aTC seem to characterize the DPs’ brains (see Figure 1). Reduced r-FFA’s white and gray matter volume and short-range functional connectivity would explain DPs’ face perception deficits, whereas disrupted functional connectivity between the r-FFA and r-pSTS and r-aTC seems to explain DPs’ deficits in face learning (i.e., memory) [53][61].
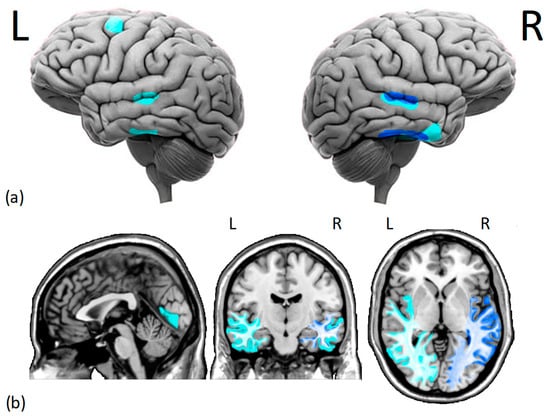
Figure 1. Cortical (a) and subcortical (b) issues in the gray and white matter of DPs brains. Several studies have found (i.e., dark blue) reduced morphometry measurements in the right fusiform face area and right superior temporal sulcus of DPs. Additionally, there is moderate evidence (i.e., light blue) of decreased gray matter density in the middle temporal gyrus, right anterior inferior temporal gyrus, left dorsolateral prefrontal cortex, and lingual gyrus. Moreover, there is a reduction in white matter integrity and functional connectivity within the core face network and middle-anterior temporal cortex, especially in the right hemisphere. L = left hemisphere, R = right hemisphere.
2.2. Face-Induced Brain Activity in Developmental Prosopagnosia
The studies investigating face-induced neural activity in DPs compared to HCs show different methodologies and findings. The low temporal resolution of blood-oxygenation-level-dependent (BOLD) signals from fMRI, the advantage in terms of signal-to-noise ratio, and the technical limitations of performing tasks in the scanner determined a bias towards block designs compared to event-related designs. Indeed, most of the fMRI evidence for DP comes from block designs, particularly those adopting passive viewing and one-back tasks of faces vs. non-face stimuli.
First, Jiahui et al. [62] investigated how selective attention to different aspects of faces affects brain activity in HCs and DPs; their results show that attention towards specific facial features (i.e., selective attention) modulates activity in both ventral areas (OFA and FFA) and dorsal areas (pSTS and inferior frontal gyrus): the modulation profiles in both pathways are similar between HCs and DPs, suggesting that DPs’ difficulties with recognizing faces are not due to attentional alterations, but rather due to face-specific perceptive or memory deficits.
To date, the model that best explains how face-relevant information flows through face-selective areas is based on the presence/absence of faces, which modulates the feed-forward effective connectivity from the primary and secondary visual cortices to the core face network. The connectivity within these networks during face viewing is significantly diminished in DPs relative to HCs, indicating that these connections may contribute to typical face-selective responses as well as accurate facial recognition [63].
Some researchers attempted to dissociate the neural activity related to face memory and perceptual processing; although DPs experience deficits in both face perception and face memory, there is a weak correlation between their performances in these tasks, indicating dissociable neural correlates. Compared to HCs, DPs show separate neural correlates for face memory and face perception within the core face network. Particularly, face memory is associated with activation in the bilateral FFA, while face perception is linked to face selectivity in the r-pSTS [64].
Other studies reported significant alterations beyond the VOTC. For instance, abnormalities in neural activity in response to familiar compared to unfamiliar faces in DPs exist in the left precuneus, anterior, and posterior cingulate cortex [65]. Furthermore, Rivolta et al. reported reduced face sensitivity in the aTC and reduced face–object discrimination in the right parahippocampal gyrus [66]. These regions are part of the extended face network and linked to post-perceptual face-processing stages, such as encoding or the retrieval of semantic and episodic memories about specific individuals [31].
To sum up, DP is characterized by abnormal face representations that differ qualitatively from HCs. Indeed, DPs may rely on different aspects of facial features for successful face recognition (i.e., parts-based strategies) due to the FFA’s grey and white matter disruptions. Moreover, DPs who exhibit typical face perception performance show differential activation patterns compared to HCs, suggesting that some DPs develop compensation strategies. Overall, DPs exhibit abnormalities in neural face representation in both the FFA and r-OFA, indicating difficulties in both holistic and featural face processing.
3. EEG and MEG
The functional impairment of the face-processing systems in DPs has been largely investigated via ERPs, which allow for examining real-time brain dynamics underlying face processing [36]. The N170—occurring around approximately 170 ms at the right lateral temporal electrode sites following the presentation of facial stimuli—represents a reliable marker of the early activation of facial representations [38][67], when features are perceptually “glued” into an indecomposable holistic whole [49][68].
Some ERP studies provide evidence for abnormal N170 responses in DPs. Since the first evidence from [69], the non-specificity of this component in DP has been reported in multiple studies showing that DPs exhibit same-amplitude N170 in response to faces and objects [50][70][71][72], as well as no typical N170 right laterality [73][74]. Larger-than-normal noise elicited from the N170 might account for the abovementioned non-selectivity of this component in DPs [75].
Evidence for reliable N170 amplitude differences between faces and nonface stimuli in DPs has also been reported [76]. It should be noted that neurophysiological discrepancies among studies might stem from differences in diagnostic criteria and participants’ characteristics (e.g., heterogeneous performances among DPs). This supports the idea that DP reflects a heterogonous impairment (i.e., with face-processing deficits being on a continuum) and that some behavioral deficits might not necessarily correlate with a lack of the face-selective N170 [77].
While face inversion in HCs leads to an enhanced and delayed N170 due to a loss of configural information [78][79], no such effect of upside-down faces is reported in DPs [80]. This could represent a functional deficit in configural face processing (i.e., less efficient use of prototypical spatial–configural information provided by upright faces). Indeed, DPs might prominently rely on feature-based strategies when processing faces [81]. In line with the idea of a reduced face-selectivity of visual areas in DPs, the paradoxical larger N170 for upright stimuli reported in some DPs might stem from the activation of object-sensitive areas in response to faces [80].
ERP evidence in DPs includes components such as the P1 (indexing a very early stage of face processing) [45][46], the N250 (reflecting the activation of preexisting and acquired face representations) [47][48], and the P600f (indexing later post-perceptual stages of face recognition) [38][49]. While evidence from the P1 component shows no inversion effect in DPs [81], N250 responses are reported for non-recognized faces in some DPs [82]. However, they seem to be attenuated, delayed, or qualitatively different in DPs compared to HCs [83][84][85]. These results suggest that stored visual representations of known faces might be available for DPs [86].
Further support for the abnormal electrophysiological responses in DP include the attenuated neural responses to unfamiliar faces in DPs compared to HCs (assessed via Fast Periodic Visual Stimulation EEG) [87], as well as abnormal and delayed EEG responses to faces (i.e., similar to those for processing non-face stimuli) [88]. This highlights the engagement of different cognitive processes during face recognition between DPs and HCs, with the former relying on a pathway more commonly associated with objects [88].
As compared to EEG, MEG provides good spatial resolution to investigate the neural sources of face-elicited responses [89]. Multiple studies have found a strong magnetic response (M170) to face stimuli compared with non-face stimuli over occipitotemporal brain regions in HCs [90]. The neural generators of the M170 are identified within the VOTC (e.g., [91]). Face-selective M170 patterns are reported in DPs, suggesting that impaired face recognition in developmental prosopagnosia is not necessarily characterized by an absence of face-specific responses [92].
Despite DPs’ face perceptions being associated with both typical and atypical brain responses, the activity in face-sensitive areas seems to be altered in DPs compared to HCs, with (i) electrophysiological markers demonstrating the occurrence of overt face processing, (ii) differential activity patterns (e.g., laterality), and (iii) the activation of object-sensitive areas in response to faces, suggesting the adoption of insufficient or inadequate strategies. The main reliance on feature-based processing mechanisms and the lack of configural strategies seem to be consistent across studies.
4. Conclusions
DP is a neurodevelopmental disorder in which brain structural, functional, and electrophysiological alterations are observed. Consistent with the strong heritability of face recognition in the general population, DP has a genetic component (precisely, it may be a monogenic, autosomal dominant disorder) [93][94][95]. However, little is known about its onset, which is thought to be heterogeneous (i.e., of multiple etiologies). Similarly to other selective neurodevelopmental conditions, one hypothesis involves neural migration errors in the occipital and temporal regions during brain development [96][97]. This would also account for DP’s heterogeneity, based on how circumscribed these errors occur. However, given the lack of evidence, DP etiology represents a matter of debate [16].
The available MRI evidence highlights some recurring patterns in DP. Reduced gray matter volume is often observed in DPs’ temporal lobes, specifically in the pSTS, MTG, and FG. White matter alterations have been found in the core face network, particularly near the r-FFA. fMRI studies assessing brain activation in response to faces indicate that DPs exhibit lower activity in the right FFA and right OF, potentially due to disrupted feed-forward connectivity from the visual cortices to the core face network. The predominant right-lateralization of the impairments is in line with the evidence supporting the right hemisphere’s dominance in face processing [98]. Neural activation abnormalities in DP also extend beyond the VOTC to regions involved in post-perceptual face processing and object visual processing. Evidence from EEG/MEG studies reveal atypical N170 responses, with non-specificity and a lack of right laterality.
Although most of the structural and functional impairments observed in DPs primarily involve the right hemisphere, the involvement of the left hemisphere regions is also common. Indeed, the neural face-processing network is distributed across both hemispheres, although a relative right-hemispheric dominance has been predominantly reported [99]. For example, Thome et al. [100] used fMRI to evaluate the cerebral face perception network in 108 healthy adults. While the average brain activity was higher in right-hemispheric areas than in left-hemispheric regions, this asymmetry was rather mild when compared to other lateralized brain functions such as language and spatial attention. This asymmetry differed greatly across individuals. The differences in lateralization between the core face network regions were not significant, and left-handed people did not display a general leftward shift in lateralization. However, when compared to right-handed men, left-handed men demonstrated a pronounced left-lateralization in the FFA.
These structural and functional alterations lead to face recognition and learning difficulties in DPs. To date, three main hypotheses have been proposed to explain the face-processing impairment in DPs: (i) the inefficient use of cognitive mechanisms devoted to face processing [101]; (ii) the impairment of within-class discrimination mechanisms that are not specific to faces [102][103]; and (iii) the reliance on different neurocognitive mechanisms to HCs, (i.e., with faces processed similarly to non-face stimuli) [104][105][106]. This latter hypothesis, which does not exclude an inefficient use of face-specific cognitive mechanisms, is extensively supported by the cognitive literature on DPs’ reliances on atypical aspects of facial features for successful face recognition [107].
The behavioral deficits in DPs have been strictly linked to the FFA white and grey matter abnormalities, as demonstrated by reduced face-selective activity in both the OFA and FFA. Indeed, although OFA gray matter seems not to be affected, the disrupted functional connectivity between the OFA and FFA could contribute to the normal reconstruction of individual facial features in a holistically integrated configuration, resulting in more feature-based processing [106][108][109].
At the behavioral level, not all DPs display reduced accuracy in face perception tasks, while face learning and memory are always impaired [110][111][112]. The use of inefficient face-processing strategies might interfere with the encoding of face identity as well as semantic and biographical information. EEG and MEG data provide support for this hypothesis.
Some relevant considerations can be drawn for future directions in this field. Future studies should select homogeneous samples of DPs based on an accurate assessment of their behavioral manifestations to account for disease heterogeneity. Different aspects of face processing (i.e., recognition, memory, discrimination) and face features (identity, expression, gaze) should be assessed simultaneously to uncover systematic associations and dissociations between different face deficits, which will unveil the varied behavioral profiles of face recognition deficits.
References
- Tsao, D.Y.; Livingstone, M.S. Mechanisms of Face Perception. Annu. Rev. Neurosci. 2008, 31, 411–437.
- Morton, J.; Johnson, M.H. CONSPEC and CONLERN: A Two-Process Theory of Infant Face Recognition. Psychol. Rev. 1991, 98, 164–181.
- McKone, E.; Kanwisher, N.; Duchaine, B.C. Can Generic Expertise Explain Special Processing for Faces? Trends Cogn. Sci. 2007, 11, 8–15.
- Richler, J.J.; Wong, Y.K.; Gauthier, I. Perceptual Expertise as a Shift from Strategic Interference to Automatic Holistic Processing. Curr. Dir. Psychol. Sci. 2011, 20, 129–134.
- Malatesta, G.; Manippa, V.; Tommasi, L. Crying the Blues: The Configural Processing of Infant Face Emotions and Its Association with Postural Biases. Atten. Percept. Psychophys. 2022, 84, 1403–1410.
- Rossion, B. Picture-Plane Inversion Leads to Qualitative Changes of Face Perception. Acta Psychol. 2008, 128, 274–289.
- Negrini, M.; Brkić, D.; Pizzamiglio, S.; Premoli, I.; Rivolta, D. Neurophysiological Correlates of Featural and Spacing Processing for Face and Non-Face Stimuli. Front. Psychol. 2017, 8, 333.
- Ventura, M.; Palmisano, A.; Innamorato, F.; Tedesco, G.; Manippa, V.; Caffò, A.O.; Rivolta, D. Face Memory and Facial Expression Recognition Are Both Affected by Wearing Disposable Surgical Face Masks. Cogn. Process 2023, 24, 43–57.
- Ventura, M.; Innamorato, F.; Palmisano, A.; Cicinelli, G.; Nobile, E.; Manippa, V.; Keller, R.; Rivolta, D. Investigating the Impact of Disposable Surgical Face-Masks on Face Identity and Emotion Recognition in Adults with Autism Spectrum Disorder. Autism Res. 2023, 16, 1063–1077.
- Bodamer, J. Die Prosop-Agnosie: Die Agnosie Des Physiognomieerkennens. Arch. Für Psychiatr. Nervenkrankh. 1947, 179, 6–53.
- Damasio, A.R.; Damasio, H.; Van Hoesen, G.W. Prosopagnosia: Anatomic Basis and Behavioral Mechanisms. Neurology 1982, 32, 331.
- Rossion, B. Distinguishing the Cause and Consequence of Face Inversion: The Perceptual Field Hypothesis. Acta Psychol. 2009, 132, 300–312.
- Rivolta, D.; Lawson, R.P.; Palermo, R. More than Just a Problem with Faces: Altered Body Perception in a Group of Congenital Prosopagnosics. Q. J. Exp. Psychol. 2017, 70, 276–286.
- Duchaine, B.C.; Nakayama, K. Developmental Prosopagnosia: A Window to Content-Specific Face Processing. Curr. Opin. Neurobiol. 2006, 16, 166–173.
- Monti, C.; Sozzi, M.; Bossi, F.; Corbo, M.; Rivolta, D. Atypical Holistic Processing of Facial Identity and Expression in a Case of Acquired Prosopagnosia. Cogn. Neuropsychol. 2019, 36, 358–382.
- Susilo, T.; Duchaine, B. Advances in Developmental Prosopagnosia Research. Curr. Opin. Neurobiol. 2013, 23, 423–429.
- Grüter, T.; Grüter, M.; Carbon, C.-C. Neural and Genetic Foundations of Face Recognition and Prosopagnosia. J. Neuropsychol. 2008, 2, 79–97.
- Perrett, D.I.; Rolls, E.T.; Caan, W. Visual Neurones Responsive to Faces in the Monkey Temporal Cortex. Exp. Brain Res. 1982, 47, 329–342.
- Kanwisher, N.; McDermott, J.; Chun, M.M. The Fusiform Face Area: A Module in Human Extrastriate Cortex Specialized for Face Perception. J. Neurosci. 1997, 17, 4302–4311.
- Haxby, J.V.; Gobbini, M.I. Distributed Neural Systems for Face Perception. In The Oxford Handbook of Face Perception; Oxford University Press: Oxford, UK, 2011.
- Haxby, J.V.; Hoffman, E.A.; Gobbini, M.I. The Distributed Human Neural System for Face Perception. Trends Cogn. Sci. 2000, 4, 223–233.
- Meyers, E.M.; Borzello, M.; Freiwald, W.A.; Tsao, D. Intelligent Information Loss: The Coding of Facial Identity, Head Pose, and Non-Face Information in the Macaque Face Patch System. J. Neurosci. 2015, 35, 7069–7081.
- Pitcher, D.; Walsh, V.; Duchaine, B. The Role of the Occipital Face Area in the Cortical Face Perception Network. Exp. Brain Res. 2011, 209, 481–493.
- Bernstein, M.; Yovel, G. Two Neural Pathways of Face Processing: A Critical Evaluation of Current Models. Neurosci. Biobehav. Rev. 2015, 55, 536–546.
- Haxby, J.V.; Hoffman, E.A.; Gobbini, M.I. Human Neural Systems for Face Recognition and Social Communication. Biol. Psychiatry 2002, 51, 59–67.
- Pitcher, D.; Walsh, V.; Yovel, G.; Duchaine, B. TMS Evidence for the Involvement of the Right Occipital Face Area in Early Face Processing. Curr. Biol. 2007, 17, 1568–1573.
- Andrews, T.J.; Ewbank, M.P. Distinct Representations for Facial Identity and Changeable Aspects of Faces in the Human Temporal Lobe. Neuroimage 2004, 23, 905–913.
- Said, C.P.; Moore, C.D.; Engell, A.D.; Todorov, A.; Haxby, J.V. Distributed Representations of Dynamic Facial Expressions in the Superior Temporal Sulcus. J. Vis. 2010, 10, 11.
- Yovel, G.; O’Toole, A.J. Recognizing People in Motion. Trends Cogn. Sci. 2016, 20, 383–395.
- Mazard, A.; Schiltz, C.; Rossion, B. Recovery from Adaptation to Facial Identity Is Larger for Upright than Inverted Faces in the Human Occipito-Temporal Cortex. Neuropsychologia 2006, 44, 912–922.
- Gobbini, M.I.; Haxby, J.V. Neural Systems for Recognition of Familiar Faces. Neuropsychologia 2007, 45, 32–41.
- Leveroni, C.L.; Seidenberg, M.; Mayer, A.R.; Mead, L.A.; Binder, J.R.; Rao, S.M. Neural Systems Underlying the Recognition of Familiar and Newly Learned Faces. J. Neurosci. 2000, 20, 878–886.
- Borghesani, V.; Narvid, J.; Battistella, G.; Shwe, W.; Watson, C.; Binney, R.J.; Sturm, V.; Miller, Z.; Mandelli, M.L.; Miller, B. “Looks Familiar, but I Do Not Know Who She Is”: The Role of the Anterior Right Temporal Lobe in Famous Face Recognition. Cortex 2019, 115, 72–85.
- Gainotti, G. Face Familiarity Feelings, the Right Temporal Lobe and the Possible Underlying Neural Mechanisms. Brain Res. Rev. 2007, 56, 214–235.
- Gainotti, G. Different Patterns of Famous People Recognition Disorders in Patients with Right and Left Anterior Temporal Lesions: A Systematic Review. Neuropsychologia 2007, 45, 1591–1607.
- Di Russo, F.; Berchicci, M.; Bianco, V.; Perri, R.L.; Pitzalis, S.; Quinzi, F.; Spinelli, D. Normative Event-Related Potentials from Sensory and Cognitive Tasks Reveal Occipital and Frontal Activities Prior and Following Visual Events. Neuroimage 2019, 196, 173–187.
- Bentin, S.; Allison, T.; Puce, A.; Perez, E.; McCarthy, G. Electrophysiological Studies of Face Perception in Humans. J. Cogn. Neurosci. 1996, 8, 551–565.
- Gosling, A.; Eimer, M. An Event-Related Brain Potential Study of Explicit Face Recognition. Neuropsychologia 2011, 49, 2736–2745.
- Barbieri, M.; Negrini, M.; Nitsche, M.A.; Rivolta, D. Anodal-tDCS over the Human Right Occipital Cortex Enhances the Perception and Memory of Both Faces and Objects. Neuropsychologia 2016, 81, 238–244.
- Towler, J.; Eimer, M. Electrophysiological Studies of Face Processing in Developmental Prosopagnosia: Neuropsychological and Neurodevelopmental Perspectives. Cogn. Neuropsychol. 2012, 29, 503–529.
- Gao, X.; Cassidy, A.; Schwarzschild, M.A.; Rimm, E.B.; Ascherio, A. Habitual Intake of Dietary Flavonoids and Risk of Parkinson Disease. Neurology 2012, 78, 1138–1145.
- Watanabe, S.; Miki, K.; Kakigi, R. Mechanisms of Face Perception in Humans: A Magneto-and Electro-Encephalographic Study. Neuropathology 2005, 25, 8–20.
- Borra, D.; Bossi, F.; Rivolta, D.; Magosso, E. Deep Learning Applied to EEG Source-Data Reveals Both Ventral and Dorsal Visual Stream Involvement in Holistic Processing of Social Stimuli. Sci. Rep. 2023, 13, 7365.
- Rivolta, D.; Castellanos, N.P.; Stawowsky, C.; Helbling, S.; Wibral, M.; Grützner, C.; Koethe, D.; Birkner, K.; Kranaster, L.; Enning, F. Source-Reconstruction of Event-Related Fields Reveals Hyperfunction and Hypofunction of Cortical Circuits in Antipsychotic-Naive, First-Episode Schizophrenia Patients during Mooney Face Processing. J. Neurosci. 2014, 34, 5909–5917.
- Taylor, M.J. Non-Spatial Attentional Effects on P1. Clin. Neurophysiol. 2002, 113, 1903–1908.
- Itier, R.J.; Taylor, M.J. Face Recognition Memory and Configural Processing: A Developmental ERP Study Using Upright, Inverted, and Contrast-Reversed Faces. J. Cogn. Neurosci. 2004, 16, 487–502.
- Kaufmann, J.M.; Schweinberger, S.R.; Burton, A.M. N250 ERP Correlates of the Acquisition of Face Representations across Different Images. J. Cogn. Neurosci. 2009, 21, 625–641.
- Tanaka, J.W.; Curran, T.; Porterfield, A.L.; Collins, D. Activation of Preexisting and Acquired Face Representations: The N250 Event-Related Potential as an Index of Face Familiarity. J. Cogn. Neurosci. 2006, 18, 1488–1497.
- Eimer, M. Event-Related Brain Potentials Distinguish Processing Stages Involved in Face Perception and Recognition. Clin. Neurophysiol. 2000, 111, 694–705.
- Hasson, U.; Avidan, G.; Deouell, L.Y.; Bentin, S.; Malach, R. Face-Selective Activation in a Congenital Prosopagnosic Subject. J. Cogn. Neurosci. 2003, 15, 419–431.
- Avidan, G.; Hasson, U.; Malach, R.; Behrmann, M. Detailed Exploration of Face-Related Processing in Congenital Prosopagnosia: 2. Functional Neuroimaging Findings. J. Cogn. Neurosci. 2005, 17, 1150–1167.
- Thomas, C.; Avidan, G.; Humphreys, K.; Jung, K.; Gao, F.; Behrmann, M. Reduced Structural Connectivity in Ventral Visual Cortex in Congenital Prosopagnosia. Nat. Neurosci. 2009, 12, 29–31.
- Zhao, Y.; Zhen, Z.; Liu, X.; Song, Y.; Liu, J. The Neural Network for Face Recognition: Insights from an fMRI Study on Developmental Prosopagnosia. Neuroimage 2018, 169, 151–161.
- Behrmann, M.; Avidan, G. Congenital Prosopagnosia: Face-Blind from Birth. Trends Cogn. Sci. 2005, 9, 180–187.
- Song, S.; Garrido, L.; Nagy, Z.; Mohammadi, S.; Steel, A.; Driver, J.; Dolan, R.J.; Duchaine, B.; Furl, N. Local but Not Long-Range Microstructural Differences of the Ventral Temporal Cortex in Developmental Prosopagnosia. Neuropsychologia 2015, 78, 195–206.
- Fox, C.J.; Iaria, G.; Barton, J.J. Defining the Face Processing Network: Optimization of the Functional Localizer in fMRI. Hum. Brain Mapp. 2009, 30, 1637–1651.
- Barton, J.J. Structure and Function in Acquired Prosopagnosia: Lessons from a Series of 10 Patients with Brain Damage. J. Neuropsychol. 2008, 2, 197–225.
- Sorger, B.; Goebel, R.; Schiltz, C.; Rossion, B. Understanding the Functional Neuroanatomy of Acquired Prosopagnosia. Neuroimage 2007, 35, 836–852.
- Martin, A.; Chao, L.L. Semantic Memory and the Brain: Structure and Processes. Curr. Opin. Neurobiol. 2001, 11, 194–201.
- Zhao, Y.; Li, J.; Liu, X.; Song, Y.; Wang, R.; Yang, Z.; Liu, J. Altered Spontaneous Neural Activity in the Occipital Face Area Reflects Behavioral Deficits in Developmental Prosopagnosia. Neuropsychologia 2016, 89, 344–355.
- Gomez, J.; Pestilli, F.; Witthoft, N.; Golarai, G.; Liberman, A.; Poltoratski, S.; Yoon, J.; Grill-Spector, K. Functionally Defined White Matter Reveals Segregated Pathways in Human Ventral Temporal Cortex Associated with Category-Specific Processing. Neuron 2015, 85, 216–227.
- Jiahui, G.; Yang, H.; Duchaine, B. Attentional Modulation Differentially Affects Ventral and Dorsal Face Areas in Both Normal Participants and Developmental Prosopagnosics. Cogn. Neuropsychol. 2020, 37, 482–493.
- Lohse, M.; Garrido, L.; Driver, J.; Dolan, R.J.; Duchaine, B.C.; Furl, N. Effective Connectivity from Early Visual Cortex to Posterior Occipitotemporal Face Areas Supports Face Selectivity and Predicts Developmental Prosopagnosia. J. Neurosci. 2016, 36, 3821–3828.
- Liu, X.; Li, X.; Song, Y.; Liu, J. Separate and Shared Neural Basis of Face Memory and Face Perception in Developmental Prosopagnosia. Front. Behav. Neurosci. 2021, 15, 668174.
- Avidan, G.; Behrmann, M. Functional MRI Reveals Compromised Neural Integrity of the Face Processing Network in Congenital Prosopagnosia. Curr. Biol. 2009, 19, 1146–1150.
- Rivolta, D.; Woolgar, A.; Palermo, R.; Butko, M.; Schmalzl, L.; Williams, M.A. Multi-Voxel Pattern Analysis (MVPA) Reveals Abnormal fMRI Activity in Both the “Core” and “Extended” Face Network in Congenital Prosopagnosia. Front. Hum. Neurosci. 2014, 8, 925.
- Rossion, B.; Jacques, C. Does Physical Interstimulus Variance Account for Early Electrophysiological Face Sensitive Responses in the Human Brain? Ten Lessons on the N170. Neuroimage 2008, 39, 1959–1979.
- Sagiv, N.; Bentin, S. Structural Encoding of Human and Schematic Faces: Holistic and Part-Based Processes. J. Cogn. Neurosci. 2001, 13, 937–951.
- Bentin, S.; Deouell, L.Y.; Soroker, N. Selective Visual Streaming in Face Recognition: Evidence from Developmental Prosopagnosia. Neuroreport 1999, 10, 823–827.
- Gilaie-Dotan, S.; Perry, A.; Bonneh, Y.; Malach, R.; Bentin, S. Seeing with Profoundly Deactivated Mid-Level Visual Areas: Non-Hierarchical Functioning in the Human Visual Cortex. Cereb. Cortex 2009, 19, 1687–1703.
- Bentin, S.; DeGutis, J.M.; D’Esposito, M.; Robertson, L.C. Too Many Trees to See the Forest: Performance, Event-Related Potential, and Functional Magnetic Resonance Imaging Manifestations of Integrative Congenital Prosopagnosia. J. Cogn. Neurosci. 2007, 19, 132–146.
- Kress, T.; Daum, I. Event-Related Potentials Reflect Impaired Face Recognition in Patients with Congenital Prosopagnosia. Neurosci. Lett. 2003, 352, 133–136.
- Collins, E.; Dundas, E.; Gabay, Y.; Plaut, D.C.; Behrmann, M. Hemispheric Organization in Disorders of Development. Vis. Cogn. 2017, 25, 416–429.
- Olivares, E.I.; Urraca, A.S.; Lage-Castellanos, A.; Iglesias, J. Different and Common Brain Signals of Altered Neurocognitive Mechanisms for Unfamiliar Face Processing in Acquired and Developmental Prosopagnosia. Cortex 2021, 134, 92–113.
- Németh, K.; Zimmer, M.; Schweinberger, S.R.; Vakli, P.; Kovács, G. The Background of Reduced Face Specificity of N170 in Congenital Prosopagnosia. PLoS ONE 2014, 9, e101393.
- Minnebusch, D.A.; Suchan, B.; Ramon, M.; Daum, I. Event-Related Potentials Reflect Heterogeneity of Developmental Prosopagnosia. Eur. J. Neurosci. 2007, 25, 2234–2247.
- Le Grand, R.; Cooper, P.A.; Mondloch, C.J.; Lewis, T.L.; Sagiv, N.; de Gelder, B.; Maurer, D. What Aspects of Face Processing Are Impaired in Developmental Prosopagnosia? Brain Cogn. 2006, 61, 139–158.
- Rossion, B.; Delvenne, J.-F.; Debatisse, D.; Goffaux, V.; Bruyer, R.; Crommelinck, M.; Guérit, J.-M. Spatio-Temporal Localization of the Face Inversion Effect: An Event-Related Potentials Study. Biol. Psychol. 1999, 50, 173–189.
- Rossion, B.; Gauthier, I.; Tarr, M.J.; Despland, P.; Bruyer, R.; Linotte, S.; Crommelinck, M. The N170 Occipito-Temporal Component Is Delayed and Enhanced to Inverted Faces but Not to Inverted Objects: An Electrophysiological Account of Face-Specific Processes in the Human Brain. Neuroreport 2000, 11, 69–72.
- Towler, J.; Gosling, A.; Duchaine, B.; Eimer, M. The Face-Sensitive N170 Component in Developmental Prosopagnosia. Neuropsychologia 2012, 50, 3588–3599.
- Righart, R.; de Gelder, B. Impaired Face and Body Perception in Developmental Prosopagnosia. Proc. Natl. Acad. Sci. USA 2007, 104, 17234–17238.
- Eimer, M.; Gosling, A.; Duchaine, B. Electrophysiological Markers of Covert Face Recognition in Developmental Prosopagnosia. Brain 2012, 135, 542–554.
- Fisher, K.; Towler, J.; Eimer, M. Face Identity Matching Is Selectively Impaired in Developmental Prosopagnosia. Cortex 2017, 89, 11–27.
- Towler, J.; Fisher, K.; Eimer, M. Holistic Face Perception Is Impaired in Developmental Prosopagnosia. Cortex 2018, 108, 112–126.
- Parketny, J.; Towler, J.; Eimer, M. The Activation of Visual Face Memory and Explicit Face Recognition Are Delayed in Developmental Prosopagnosia. Neuropsychologia 2015, 75, 538–547.
- Rivolta, D.; Palermo, R.; Schmalzl, L.; Coltheart, M. Covert Face Recognition in Congenital Prosopagnosia: A Group Study. Cortex 2012, 48, 344–352.
- Fisher, K.; Towler, J.; Rossion, B.; Eimer, M. Neural Responses in a Fast Periodic Visual Stimulation Paradigm Reveal Domain-General Visual Discrimination Deficits in Developmental Prosopagnosia. Cortex 2020, 133, 76–102.
- Burns, E.J.; Tree, J.J.; Weidemann, C.T. Recognition Memory in Developmental Prosopagnosia: Electrophysiological Evidence for Abnormal Routes to Face Recognition. Front. Hum. Neurosci. 2014, 8, 622.
- Rivolta, D.; Palermo, R.; Schmalzl, L.; Williams, M.A. An Early Category-Specific Neural Response for the Perception of Both Places and Faces. Cogn. Neurosci. 2012, 3, 45–51.
- Liu, J.; Higuchi, M.; Marantz, A.; Kanwisher, N. The Selectivity of the Occipitotemporal M170 for Faces. Neuroreport 2000, 11, 337–341.
- Deffke, I.; Sander, T.; Heidenreich, J.; Sommer, W.; Curio, G.; Trahms, L.; Lueschow, A. MEG/EEG Sources of the 170-Ms Response to Faces Are Co-Localized in the Fusiform Gyrus. Neuroimage 2007, 35, 1495–1501.
- Harris, A.M.; Duchaine, B.C.; Nakayama, K. Normal and Abnormal Face Selectivity of the M170 Response in Developmental Prosopagnosics. Neuropsychologia 2005, 43, 2125–2136.
- Duchaine, B.; Germine, L.; Nakayama, K. Family Resemblance: Ten Family Members with Prosopagnosia and within-Class Object Agnosia. Cogn. Neuropsychol. 2007, 24, 419–430.
- Kennerknecht, I.; Ho, N.Y.; Wong, V.C.N. Prevalence of Hereditary Prosopagnosia (HPA) in Hong Kong Chinese Population. Am. J. Med. Genet. A 2008, 146A, 2863–2870.
- Kennerknecht, I.; Grueter, T.; Welling, B.; Wentzek, S.; Horst, J.; Edwards, S.; Grueter, M. First Report of Prevalence of Non-Syndromic Hereditary Prosopagnosia (HPA). Am. J. Med. Genet. A 2006, 140, 1617–1622.
- Duchaine, B.C.; Yovel, G.; Butterworth, E.J.; Nakayama, K. Prosopagnosia as an Impairment to Face-Specific Mechanisms: Elimination of the Alternative Hypotheses in a Developmental Case. Cogn. Neuropsychol. 2006, 23, 714–747.
- Ramus, F. Neurobiology of Dyslexia: A Reinterpretation of the Data. Trends Neurosci. 2004, 27, 720–726.
- Rossion, B.; Caldara, R.; Seghier, M.; Schuller, A.-M.; Lazeyras, F.; Mayer, E. A Network of Occipito-Temporal Face-Sensitive Areas besides the Right Middle Fusiform Gyrus Is Necessary for Normal Face Processing. Brain 2003, 126, 2381–2395.
- Rossion, B.; Lochy, A. Is Human Face Recognition Lateralized to the Right Hemisphere Due to Neural Competition with Left-Lateralized Visual Word Recognition? A Critical Review. Brain Struct. Funct. 2022, 227, 599–629.
- Thome, I.; García Alanis, J.C.; Volk, J.; Vogelbacher, C.; Steinsträter, O.; Jansen, A. Let’s Face It: The Lateralization of the Face Perception Network as Measured with fMRI Is Not Clearly Right Dominant. NeuroImage 2022, 263, 119587.
- Russell, R.; Duchaine, B.; Nakayama, K. Super-Recognizers: People with Extraordinary Face Recognition Ability. Psychon. Bull. Rev. 2009, 16, 252–257.
- Burns, E.J.; Arnold, T.; Bukach, C.M. P-Curving the Fusiform Face Area: Meta-Analyses Support the Expertise Hypothesis. Neurosci. Biobehav. Rev. 2019, 104, 209–221.
- McGugin, R.W.; Gauthier, I. Perceptual Expertise with Objects Predicts Another Hallmark of Face Perception. J. Vis. 2009, 10, 15.
- Bobak, A.K.; Bennetts, R.J.; Parris, B.A.; Jansari, A.; Bate, S. An In-Depth Cognitive Examination of Individuals with Superior Face Recognition Skills. Cortex 2016, 82, 48–62.
- Bobak, A.K.; Parris, B.A.; Gregory, N.J.; Bennetts, R.J.; Bate, S. Eye-Movement Strategies in Developmental Prosopagnosia and “Super” Face Recognition. Q. J. Exp. Psychol. 2017, 70, 201–217.
- DeGutis, J.; Cohan, S.; Mercado, R.J.; Wilmer, J.; Nakayama, K. Holistic Processing of the Mouth but Not the Eyes in Developmental Prosopagnosia. Cogn. Neuropsychol. 2012, 29, 419–446.
- Tian, X.; Wang, R.; Zhao, Y.; Zhen, Z.; Song, Y.; Liu, J. Multi-Item Discriminability Pattern to Faces in Developmental Prosopagnosia Reveals Distinct Mechanisms of Face Processing. Cereb. Cortex 2020, 30, 2986–2996.
- Zhang, J.; Liu, J.; Xu, Y. Neural Decoding Reveals Impaired Face Configural Processing in the Right Fusiform Face Area of Individuals with Developmental Prosopagnosia. J. Neurosci. 2015, 35, 1539–1548.
- DeGutis, J.; Chatterjee, G.; Mercado, R.J.; Nakayama, K. Face Gender Recognition in Developmental Prosopagnosia: Evidence for Holistic Processing and Use of Configural Information. Vis. Cogn. 2012, 20, 1242–1253.
- Dalrymple, K.A.; Garrido, L.; Duchaine, B. Dissociation between Face Perception and Face Memory in Adults, but Not Children, with Developmental Prosopagnosia. Dev. Cogn. Neurosci. 2014, 10, 10–20.
- Ulrich, P.I.; Wilkinson, D.T.; Ferguson, H.J.; Smith, L.J.; Bindemann, M.; Johnston, R.A.; Schmalzl, L. Perceptual and Memorial Contributions to Developmental Prosopagnosia. Q. J. Exp. Psychol. 2017, 70, 298–315.
- McKone, E.; Hall, A.; Pidcock, M.; Palermo, R.; Wilkinson, R.B.; Rivolta, D.; Yovel, G.; Davis, J.M.; O’Connor, K.B. Face Ethnicity and Measurement Reliability Affect Face Recognition Performance in Developmental Prosopagnosia: Evidence from the Cambridge Face Memory Test–Australian. Cogn. Neuropsychol. 2011, 28, 109–146.
More
Information
Subjects:
Neurosciences
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
717
Revisions:
2 times
(View History)
Update Date:
16 Oct 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




