Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Fang Ba | -- | 2011 | 2023-10-11 13:29:14 | | | |
| 2 | Lindsay Dong | -3 word(s) | 2008 | 2023-10-13 03:37:48 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Ba, F.; Zhang, Y.; Wang, L.; Liu, W.; Li, J. Applications of Serine Integrases in Synthetic Biology. Encyclopedia. Available online: https://encyclopedia.pub/entry/50128 (accessed on 08 February 2026).
Ba F, Zhang Y, Wang L, Liu W, Li J. Applications of Serine Integrases in Synthetic Biology. Encyclopedia. Available at: https://encyclopedia.pub/entry/50128. Accessed February 08, 2026.
Ba, Fang, Yufei Zhang, Luyao Wang, Wan-Qiu Liu, Jian Li. "Applications of Serine Integrases in Synthetic Biology" Encyclopedia, https://encyclopedia.pub/entry/50128 (accessed February 08, 2026).
Ba, F., Zhang, Y., Wang, L., Liu, W., & Li, J. (2023, October 11). Applications of Serine Integrases in Synthetic Biology. In Encyclopedia. https://encyclopedia.pub/entry/50128
Ba, Fang, et al. "Applications of Serine Integrases in Synthetic Biology." Encyclopedia. Web. 11 October, 2023.
Copy Citation
Serine integrases are emerging as one of the most powerful biological tools for biotechnology. With the fast development of synthetic biology, serine integrases have been used as one of the powerful genetic tools with their unique features of site-specific, orthogonality, irreversibility, high affinity, and high efficiency. Serine integrases are widely used in diverse ways, including genome engineering, biological part and genetic circuit design, and DNA assembly. Moreover, serine integrases also advance multidisciplinary research such as chemical engineering, materials science and engineering, and biomedical engineering.
serine integrase
serine recombinase
synthetic biology
site-specific recombination
1. Introduction
As the genetic information carrier, DNA plays the core role in leading mRNA transcription, directing protein translation, and programming cellular behaviors. The variable change of DNA sequences may reprogram life to confer desired characteristics. Over the past two decades, synthetic biology, which focuses on (re)designing and (re)constructing new biological parts, devices, systems, and organisms, has emerged with intense demands for simple, reliable, and efficient DNA manipulating tools [1]. To meet this demand, synthetic biologists have concentrated on the study of site-specific DNA-modifying enzymes that can catalyze DNA variations with precision, prediction, and high efficiency [2].
Recombinases are DNA-modifying enzymes that recognize specific double strand DNA sequences and catalyze DNA–DNA site-specific recombination. Comparing and aligning the recombinase amino acid sequences indicate two subfamilies of recombinases with distinct catalytic mechanisms: tyrosine recombinases and serine recombinases (also called serine integrases). Tyrosine recombinases cleave single-strand DNA and form covalent 3′-phosphotyrosine bonds [2][3] with the DNA backbone and rejoin DNA strands via a Holliday-Junction-like intermediate state, whereas serine integrases cleave double strand DNA and form covalent 5′-phosphoserine bonds [2][4][5] with a DNA backbone and perform as an “assembly cleavage-rotation-ligation-disassembly” process [2]. In comparison to some tyrosine recombinases with similar DNA recognition sites and reversible reactions (e.g., Cre [6], and FLP [3][7]), serine integrases can recognize and catalyze recombination events between two different and specific DNA sites (approximately 50 bp for each) called attP (attachment site in Phage) and attB (attachment site in Bacteria). Depending on the orientations of attP/attB sites, serine integrases can catalyze DNA sequences as deletion, integration, recombination, and inversion [8]. In general, serine integrase-based DNA recombination is a one-way irreversible reaction; however, this reaction can be reversed when a kind of accessory factor protein (Recombination Directionality Factor, RDF) exists [9].
2. Mechanism of Site-Specific Recombination Mediated by Serine Integrases
Serine integrases are usually discovered from bacteriophages for catalyzing their DNA integration into the recipient genome via site-specific recombination events between the attP–attB attachment site pairs [10]. When integration is finished, two new sites are formed: attL (attachment site on the left) and attR (attachment site on the right). When the host (e.g., bacteria) is converted to a lysogenic state, the prophage will evade the bacterial chromosome by expressing serine integrases with RDFs, which leads to a periodic reverse recombination event [8].
The general structural model and catalytic domains/motifs of the serine integrase subfamily have been identified clearly (Figure 1a) [11][12]. The NTD (N-Terminal catalytic Domain) contains highly conserved residues including serine (catalytic residue), tyrosine, and arginine [13]. NTD performs its function by cleavage and ligation during the recombination process (Figure 1b) [13]. The flexible αE domain plays an important role in the DNA-protein binding process [14]. RD (Recombinase Domain) mediates the attachment between attP/attB sites and the serine integrase monomer [14]. ZD (Zinc ribbon Domain) [15][16] leads to the conformationally distinct of integrase–attP and integrase–attB complexes; CC (Coiled-Coil motif) [14][17], which is embedded in the two ZDs, can assemble the two complexes of “attP-serine integrases dimer” and “attB-serine integrases dimer” to form a DNA-protein homologous tetramer [13][18]. A recently proposed structural model showed the recombination event containing six steps: (1) DNA-protein dimerization [18], (2) assembly of tetramer complex [18][19][20], (3) double strand DNA cleavage within 2 bp overhangs [8], (4) complex 180° rotation [19][21][22], (5) DNA re-ligation [19], and (6) disassembly of tetramer complex [12] (Figure 1b).
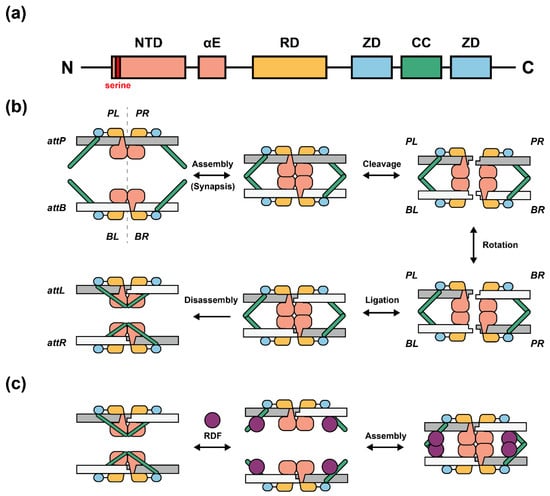
Figure 1. Domains and proposed recombination model of serine integrases. (a) General structural domains and motifs of serine integrases. NTD: N-Terminal catalytic Domain; αE: flexible linker; RD: Recombinase Domain; ZD: Zinc ribbon Domain; CC: Coiled-Coil motif. ZD is embedded between the two ZDs; CC is divided into two antiparallel helical segments. (b) Proposed model of serine integrase-mediated recombination. First, integrase dimers specifically bind to attP or attB site depending on the ZD binding position. Second, dimer–attP and dimer–attB complexes will be automatically assembled as homologous tetramer by stabilization between CCs interaction. Third, integrase monomers cleave attP and attB sites and form 5′-phosphoserine linkages, DNA half-sites, and 3′ dinucleotide overhangs (2 bp). Then, PL-BL or PR-BR dimeric complexes will rotate 180° along the horizontal axis. After that, PL-BR and BL-PR dimeric complexes will be formed by ligation between DNA strands, called attL and attR. Finally, the CCs will be conformationally changed and form a new internal interaction along with the same DNA double strand rather than two heterologous DNA strands, which leads to disassembly and inhibits reversible exchange. (c) Proposed structural model of serine integrase-mediated reverse recombination. RDFs bind to CCs and alter the integrase–attL and integrase–attR internal interactions. The released CCs may interact with each other located on two heterologous DNA strands and reassemble again.
RDF is a small protein encoded by bacteriophages that can alter the recombination direction [23]. Some serine integrases and their paired RDFs have been characterized such as Bxb1 (RDF: gp47) [24], phiC31 (RDF: gp3) [25][26], phiBT1 (RDF: gp3) [27], A118 (RDF: Gp44) [28], and TP901-1 (RDF: orf7) [29]. When RDF exists, a possible model of reverse recombination indicates that RDF may bind to CCs to prohibit the internal dimeric DNA-protein interaction (Figure 1c) [14].
3. Orientation of att Sites
As the origin of serine integrases, bacteriophages facilitate their invasion via site-specific circular DNA integration and later convert into a lysogenic state to excise (delete) their linearized DNA from the host genome by co-existence of integrase and RDF (Figure 2a) [10]. This process inspires researchers to rearrange the orientation of attP/attB sites to utilize them for other synthetic biology and bioengineering applications. For example, when attP/attB sites are located in two different linearized DNA, the two strands will exchange partial fragments specifically to create two recombined DNA strands (Figure 2b) [30]. This strategy can also be developed as multiple linear DNA assembly in one pot [31]. In addition, when recombination occurs between two circular DNA (e.g., plasmids), these two molecules will be assembled into a merged, large circular DNA [32]. When RDF exists, the merged circular DNA can be disassembled and separated into two independent circular DNA molecules (Figure 2c). Furthermore, when attP/attB sites are oppositely located in the same DNA strand, serine integrases (with or without RDFs) enable the inversion of the internal DNA sequence (Figure 2d) [8]. In summary, depending on the orientation of attP/attB sites, serine integrases can rearrange DNA sequences as integration/deletion, recombination, assembly/disassembly, and inversion.
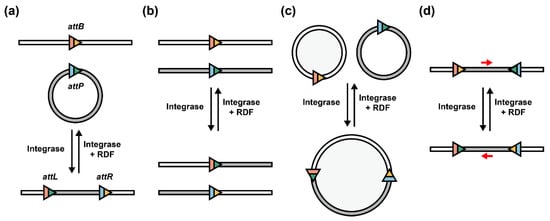
Figure 2. Serine integrases with different att site orientations. Serine integrases catalyze attP and attB recombination to make attL and attR sites. When integrases and RDFs exist simultaneously, the direction will be reversed. (a) Integration mediated by serine integrases, and deletion when RDFs exist. (b) Recombination between two linear DNA strands. (c) Circular DNA assembly and disassembly. (d) Inversion of internal DNA sequence located in att sites. Red arrow: direction of internal DNA sequence.
4. Recent Achievements of Serine Integrases in Synthetic Biology
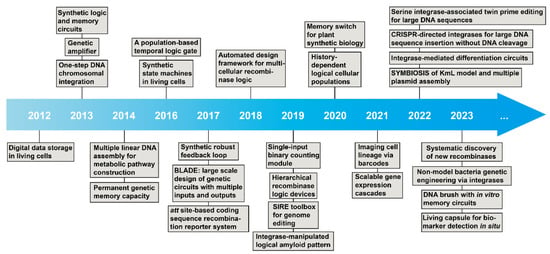
In 2012, Bonnet et al. first created a rewriteable recombinase addressable data (RAD) module, which utilized serine integrases and excisionases to invert DNA sequences (e.g., promoter) as reversible inversion [33]. This innovative design opened the next decade of emerging studies of serine integrases in synthetic biology (Figure 3). Next year, two papers reported significant designs, which were inspired by electronics engineering. Siuti et al. [34] and Bonnet et al. [35] brought Boolean logic circuits to serine integrase-based genetic logic circuit design (e.g., AND, OR, and NOT gates). Meanwhile, Bonnet et al. reported a genetic amplifier via the RNA polymerase flow by the programmable serine integrases [35]. After that, a series of serine integrase-based biological parts and genetic circuit designs were established nearly every year, for instance, genetic memory circuits [34][36][37], population-based logic circuits [38][39], state machines [40], synthetic feedback loops [41], comprehensive layered circuit systems [42][43], coding sequence manipulation [44], binary counting module [45], genetic cascades [46], “keys match locks” model [47], and cellular differentiation circuits [38][39][48].

Figure 3. Timeline of milestones in serine integrase-associated synthetic biology.
Another serine integrase utilization is in vitro linear DNA assembly for defined purposes. In 2014, Colloms et al. first reported a strategy for constructing metabolic pathways via assembling multiple linearized DNA fragments by orthogonal serine integrases [31].
5. Serine Integrase Applications
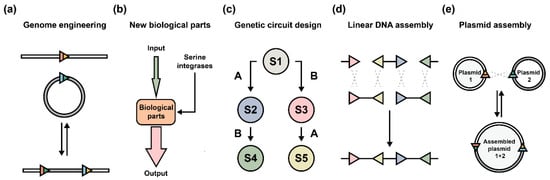
Depending on the purposes, serine integrases can be utilized as DNA manipulation tools both in vitro and in vivo. First, serine integrases are broadly developed as diverse genome engineering tools in different hosts (e.g., bacteria [49], yeast [50], mammalian cells [51][52][53][54], animals [55][56], and plants [57][58][59][60]), and catalyze various reactions (e.g., integration and deletion) (Figure 4a). Second, serine integrases inspire the creative designs of new biological parts (Figure 4b) and the assembly of comprehensive genetic circuits (Figure 4c). Furthermore, orthogonal serine integrase systems enable in vitro assembly of either linear DNA (Figure 4d) [31] or circular DNA (Figure 4e) [47]. Both can produce large biobricks and assemble several independent modules for the designed applications, for example, the biosynthesis of carotenoid [61], erythromycin [62], and the co-expression of chromoproteins [47].

Figure 4. Application of serine integrases in different ways. (a) The development of serine integrase-based genome engineering strategies includes exogenous DNA integration and genome sequence manipulation (e.g., deletion and inversion). (b) Designs of new biological parts with programmable serine integrases as controllers. (c) Serine integrase-based genetic circuits organize multiple biological parts to achieve more complex functions in living cells. In general, engineered circuits consist of several input signals, multilayered genetic regulators (including logic gates, amplifiers, and memory modules), and diverse output signals. Meanwhile, the host organism can be programmed into predictable states (e.g., S1–S5 means different states: state 1 to state 5). Serine integrases are represented by A and B. (d) Assembly of linear DNA fragments require the matched attP/attB sites localized on fragment ends. Orthogonal serine integrases or orthogonal 2 bp overhangs should be engineered and applied. (e) Assembly of circular DNA (e.g., plasmids) requires orthogonal attP/attB sites located in different DNA molecules. Multiple rounds of assembly can produce predicted and complicated larger plasmids.
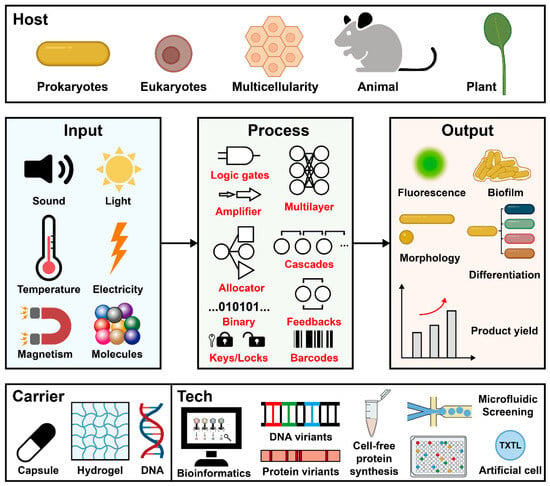
6. Serine Integrases Accelerate the Synthetic Biology Research
In 2000, the innovation of the genetic toggle switch [63] and repressilator [64] represented the beginning of synthetic biology research. After more than 10 years, the first serine integrase-based genetic converter was designed in 2012 [33]. After that, serine integrase accelerated synthetic biology over the next decade with many remarkable milestones (Figure 3). The emerged designs covered multidisciplinary fields. Figure 5 summarizes the published papers and propose a scheme within the “design-build-test-learn” cycle, including three modules as “input-process-output” genetic workflow, and three independent dimensions as host organism, external carrier, and enabling technology.

Figure 5. Schematic overview of serine integrases-based synthetic biology. TXTL: transcription-translation.
Input signals enable cells to sense and respond the external environment for altering their metabolism and behavior (Figure 5, middle left). Up to now, the main inducers for serine integrase expression are chemical molecules [40] such as arabinose, anhydrotetracycline, 2,4-diacetylphloroglucinol (DAPG), and some metal ions like cadmium [65] with strictly regulated inducible circuits to prohibit leakage.
The signal process occurs when the recipient receives the input signals and converts them into genetic information (Figure 5, middle). Unique properties of serine integrase like site-specific, orthogonality, predictable, and high efficiency, empower it as a genetic information processor. The simple Boolean logic gates could be designed as single-layered circuits (e.g, binary counting module [45], and “keys match locks” module [47]), and also could be assembled as multilayered networks [66] such as amplifier [35], allocator [66], genetic cascades [46], feedback loops [41], and modified genetic barcodes [55].
Depending on genetic information processing, the host can export diverse outputs (Figure 5, middle right). The general output signal is fluorescence, including fluorescent proteins and luciferases. Additionally, engineered microorganisms could produce amyloid fibers [67] and natural products [62][68][69] as output signals. Furthermore, the processed signals enable bacteria to reprogram their phenotypes.
As previously reported, serine integrases were active in a wide range of hosts, including not only prokaryotes (E. coli [49], Pseudomonas [70], Rhodococcus [71], and other non-model bacteria [72]), but also eukaryotes (Saccharomyces cerevisiae [50] and mammalian cell lines [51][52][53][54][73][74]), animals (Drosophila [55] and mouse [56]), and plants (tobacco [58][59] and Arabidopsis [57][60]) (Figure 5, top).
References
- Meng, F.; Ellis, T. The second decade of synthetic biology: 2010–2020. Nat. Commun. 2020, 11, 5174.
- Grindley, N.D.F.; Whiteson, K.L.; Rice, P.A. Mechanisms of Site-Specific Recombination. Annu. Rev. Biochem. 2006, 75, 567–605.
- Evans, B.R.; Chen, J.-W.; Parsons, R.L.; Bauer, T.K.; Teplow, D.B.; Jayaram, M. Identification of the Active Site Tyrosine of Flp Recombinase. Possible Relevance of its Location to the Mechanism of Recombination. J. Biol. Chem. 1990, 265, 18504–18510.
- Smith, M.C.A.; Till, R.; Brady, K.; Soultanas, P.; Thorpe, H.; Smith, M.C.M. Synapsis and DNA cleavage in phiC31 integrase-mediated site-specific recombination. Nucleic Acids Res. 2004, 32, 2607–2617.
- Thorpe, H.M.; Smith, M.C.M. In vitro site-specific integration of bacteriophage DNA catalyzed by a recombinase of the resolvase/invertase family. Proc. Natl. Acad. Sci. USA 1998, 95, 5505–5510.
- Abremski, K.; Hoess, R. Bacteriophage P1 Site-specific Recombination. Purification and Properties of the Cre Recombinase Protein. J. Biol. Chem. 1984, 259, 1509–1514.
- Andrews, B.J.; Proteau, G.A.; Beatty, L.G.; Sadowski, P.D. The FLP Recombinase of the 2 micron Circle DNA of Yeast: Interaction with Its Target Sequences. Cell 1985, 40, 795–803.
- Merrick, C.A.; Zhao, J.; Rosser, S.J. Serine Integrases: Advancing Synthetic Biology. ACS Synth. Biol. 2018, 7, 299–310.
- Olorunniji, F.J.; McPherson, A.L.; Rosser, S.J.; Smith, M.C.M.; Colloms, S.D.; Stark, W.M. Control of serine integrase recombination directionality by fusion with the directionality factor. Nucleic Acids Res. 2017, 45, 8635–8645.
- Groth, A.C.; Calos, M.P. Phage Integrases: Biology and Applications. J. Mol. Biol. 2004, 335, 667–678.
- Li, H.; Sharp, R.; Rutherford, K.; Gupta, K.; Van Duyne, G.D. Serine Integrase attP Binding and Specificity. J. Mol. Biol. 2018, 430, 4401–4418.
- Rutherford, K.; Van Duyne, G.D. The ins and outs of serine integrase site-specific recombination. Curr. Opin. Struct. Biol. 2014, 24, 125–131.
- Yuan, P.; Gupta, K.; Van Duyne, G.D. Tetrameric Structure of a Serine Integrase Catalytic Domain. Structure 2008, 16, 1275–1286.
- Gupta, K.; Sharp, R.; Yuan, J.B.; Li, H.; Van Duyne, G.D. Coiled-coil interactions mediate serine integrase directionality. Nucleic Acids Res. 2017, 45, 7339–7353.
- Prorocic, M.M.; Wenlong, D.; Olorunniji, F.J.; Akopian, A.; Schloetel, J.-G.; Hannigan, A.; McPherson, A.L.; Stark, W.M. Zinc-finger recombinase activities in vitro. Nucleic Acids Res. 2011, 39, 9316–9328.
- McEwan, A.R.; Raab, A.; Kelly, S.M.; Feldmann, J.; Smith, M.C.M. Zinc is essential for high-affinity DNA binding and recombinase activity of phiC31 integrase. Nucleic Acids Res. 2011, 39, 6137–6147.
- McEwan, A.R.; Rowley, P.A.; Smith, M.C.M. DNA binding and synapsis by the large C-terminal domain of phiC31 integrase. Nucleic Acids Res. 2009, 37, 4764–4773.
- Keenholtz, R.A.; Rowland, S.-J.; Boocock, M.R.; Stark, W.M.; Rice, P.A. Structural basis for catalytic activation of a serine recombinase. Structure 2011, 19, 799–809.
- Chang, Y.; Johnson, R.C. Controlling tetramer formation, subunit rotation and DNA ligation during Hin-catalyzed DNA inversion. Nucleic Acids Res. 2015, 43, 6459–6472.
- Dhar, G.; McLean, M.M.; Heiss, J.K.; Johnson, R.C. The Hin recombinase assembles a tetrameric protein swivel that exchanges DNA strands. Nucleic Acids Res. 2009, 37, 4743–4756.
- Trejo, C.S.; Rock, R.S.; Stark, W.M.; Boocock, M.R.; Rice, P.A. Snapshots of a molecular swivel in action. Nucleic Acids Res. 2018, 46, 5286–5296.
- Olorunniji, F.J.; Buck, D.E.; Colloms, S.D.; McEwan, A.R.; Smith, M.C.M.; Stark, W.M.; Rosser, S.J. Gated rotation mechanism of site-specific recombination by phiC31 integrase. Proc. Natl. Acad. Sci. USA 2012, 109, 19661–19666.
- Ghosh, P.; Bibb, L.A.; Hatfull, G.F. Two-step site selection for serine-integrase-mediated excision: DNA-directed integrase conformation and central dinucleotide proofreading. Proc. Natl. Acad. Sci. USA 2008, 105, 3238–3243.
- Ghosh, P.; Wasil, L.R.; Hatfull, G.F. Control of Phage Bxb1 Excision by a Novel Recombination Directionality Factor. PLoS Biol. 2006, 4, e186.
- Fan, H.-F.; Hsieh, T.-S.; Ma, C.-H.; Jayaram, M. Single-molecule analysis of phiC31 integrase-mediated site-specific recombination by tethered particle motion. Nucleic Acids Res. 2016, 44, 10804–10823.
- Khaleel, T.; Younger, E.; McEwan, A.R.; Varghese, A.S.; Smith, M.C.M. A phage protein that binds phiC31 integrase to switch its directionality. Mol. Microbiol. 2011, 80, 1450–1463.
- Zhang, L.; Zhu, B.; Dai, R.; Zhao, G.; Ding, X. Control of Directionality in Streptomyces Phage phiBT1 Integrase-Mediated Site-Specific Recombination. PLoS ONE 2013, 8, e80434.
- Mandali, S.; Gupta, K.; Dawson, A.R.; Van Duyne, G.D.; Johnson, R.C. Control of Recombination Directionality by the Listeria Phage A118 Protein Gp44 and the Coiled-Coil Motif of Its Serine Integrase. J. Bacteriol. 2017, 199, e00019-17.
- Breuner, A.; Brondsted, L.; Hammer, K. Novel Organization of Genes Involved in Prophage Excision Identified in the Temperate Lactococcal Bacteriophage TP901-1. J. Bacteriol. 1999, 181, 7291–7297.
- Marken, J.P.; Murray, R.M. Addressable and adaptable intercellular communication via DNA messaging. Nat. Commun. 2023, 14, 2358.
- Colloms, S.D.; Merrick, C.A.; Olorunniji, F.J.; Stark, W.M.; Smith, M.C.M.; Osbourn, A.; Keasling, J.D.; Rosser, S.J. Rapid metabolic pathway assembly and modification using serine integrase site-specific recombination. Nucleic Acids Res. 2014, 42, e23.
- Neil, K.; Allard, N.; Jordan, D.; Rodrigue, S. Assembly of large mobilizable genetic cargo by double recombinase operated insertion of DNA (DROID). Plasmid 2019, 104, 102419.
- Bonnet, J.; Subsoontorn, P.; Endy, D. Rewritable digital data storage in live cells via engineered control of recombination directionality. Proc. Natl. Acad. Sci. USA 2012, 109, 8884–8889.
- Siuti, P.; Yazbek, J.; Lu, T.K. Synthetic circuits integrating logic and memory in living cells. Nat. Biotechnol. 2013, 31, 448–452.
- Bonnet, J.; Yin, P.; Ortiz, M.E.; Subsoontorn, P.; Endy, D. Amplifying Genetic Logic Gates. Science 2013, 340, 599–603.
- Yang, L.; Nielsen, A.A.K.; Fernandez-Rodriguez, J.; McClune, C.J.; Laub, M.T.; Lu, T.K.; Voigt, C.A. Permanent genetic memory with >1-byte capacity. Nat. Methods 2014, 11, 1261–1266.
- Siuti, P.; Yazbek, J.; Lu, T.K. Engineering genetic circuits that compute and remember. Nat. Protoc. 2014, 9, 1292–1300.
- Zúñiga, A.; Guiziou, S.; Mayonove, P.; Meriem, Z.B.; Camacho, M.; Moreau, V.; Ciandrini, L.; Hersen, P.; Bonnet, J. Rational programming of history-dependent logic in cellular populations. Nat. Commun. 2020, 11, 4758.
- Hsiao, V.; Hori, Y.; Rothemund, P.W.; Murray, R.M. A population-based temporal logic gate for timing and recording chemical events. Mol. Syst. Biol. 2016, 12, 869.
- Roquet, N.; Soleimany, A.P.; Ferris, A.C.; Aaronson, S.; Lu, T.K. Synthetic recombinase-based state machines in living cells. Science 2016, 353, aad8559.
- Folliard, T.; Steel, H.; Prescott, T.P.; Wadhams, G.; Rothschild, L.J.; Papachristodoulou, A. A Synthetic Recombinase-Based Feedback Loop Results in Robust Expression. ACS Synth. Biol. 2017, 6, 1663–1671.
- Guiziou, S.; Mayonove, P.; Bonnet, J. Hierarchical composition of reliable recombinase logic devices. Nat. Commun. 2019, 10, 456.
- Weinberg, B.H.; Pham, N.T.H.; Caraballo, L.D.; Lozanoski, T.; Engel, A.; Bhatia, S.; Wong, W.W. Large-scale design of robust genetic circuits with multiple inputs and outputs for mammalian cells. Nat. Biotechnol. 2017, 35, 453–462.
- Bland, M.J.; Ducos-Galand, M.; Val, M.-E.; Mazel, D. An att site-based recombination reporter system for genome engineering and synthetic DNA assembly. BMC Biotechnol. 2017, 17, 62.
- Zhao, J.; Pokhilko, A.; Ebenhöh, O.; Rosser, S.J.; Colloms, S.D. A single-input binary counting module based on serine integrase site-specific recombination. Nucleic Acids Res. 2019, 47, 4896–4909.
- Kim, T.; Weinberg, B.; Wong, W.; Lu, T.K. Scalable recombinase-based gene expression cascades. Nat. Commun. 2021, 12, 2711.
- Ba, F.; Liu, Y.; Liu, W.-Q.; Tian, X.; Li, J. SYMBIOSIS: Synthetic manipulable biobricks via orthogonal serine integrase systems. Nucleic Acids Res. 2022, 50, 2973–2985.
- Williams, R.L.; Murray, R.M. Integrase-mediated differentiation circuits improve evolutionary stability of burdensome and toxic functions in E. coli. Nat. Commun. 2022, 13, 6822.
- Riley, L.A.; Payne, I.C.; Tumen-Velasquez, M.; Guss, A.M. Simple and Rapid Site-Specific Integration of Multiple Heterologous DNAs into the Escherichia coli Chromosome. J. Bacteriol. 2023, 205, e0033822.
- Xu, Z.; Brown, W.R.A. Comparison and optimization of ten phage encoded serine integrases for genome engineering in Saccharomyces cerevisiae. BMC Biotechnol. 2016, 16, 13.
- Gomide, M.S.; Sales, T.T.; Barros, L.R.C.; Limia, C.G.; de Oliveira, M.A.; Florentino, L.H.; Barros, L.M.G.; Robledo, M.L.; José, G.P.C.; Almeida, M.S.M.; et al. Genetic switches designed for eukaryotic cells and controlled by serine integrases. Commun. Biol. 2020, 3, 255.
- Pristovšek, N.; Nallapareddy, S.; Grav, L.M.; Hefzi, H.; Lewis, N.E.; Rugbjerg, P.; Hansen, H.G.; Lee, G.M.; Andersen, M.R.; Kildegaard, H.F. Systematic Evaluation of Site-Specific Recombinant Gene Expression for Programmable Mammalian Cell Engineering. ACS Synth. Biol. 2019, 8, 758–774.
- Gaidukov, L.; Wroblewska, L.; Teague, B.; Nelson, T.; Zhang, X.; Liu, Y.; Jagtap, K.; Mamo, S.; Tseng, W.A.; Lowe, A.; et al. A multi-landing pad DNA integration platform for mammalian cell engineering. Nucleic Acids Res. 2018, 46, 4072–4086.
- Stoll, S.M.; Ginsburg, D.S.; Calos, M.P. Phage TP901-1 Site-Specific Integrase Functions in Human Cells. J. Bacteriol. 2002, 184, 3657–3663.
- Chow, K.-H.K.; Budde, M.W.; Granados, A.A.; Cabrera, M.; Yoon, S.; Cho, S.; Huang, T.-H.; Koulena, N.; Frieda, K.L.; Cai, L.; et al. Imaging cell lineage with a synthetic digital recording system. Science 2021, 372, eabb3099.
- Low, B.E.; Hosur, V.; Lesbirel, S.; Wiles, M.V. Efficient targeted transgenesis of large donor DNA into multiple mouse genetic backgrounds using bacteriophage Bxb1 integrase. Sci. Rep. 2022, 12, 5424.
- Guiziou, S.; Maranas, C.J.; Chu, J.C.; Nemhauser, J.L. An integrase toolbox to record gene-expression during plant development. Nat. Commun. 2023, 14, 1844.
- Bernabé-Orts, J.M.; Quijano-Rubio, A.; Vazquez-Vilar, M.; Mancheño-Bonillo, J.; Moles-Casas, V.; Selma, S.; Gianoglio, S.; Granell, A.; Orzaez, D. A memory switch for plant synthetic biology based on the phage phiC31 integration system. Nucleic Acids Res. 2020, 48, 3379–3394.
- Shao, M.; Kumar, S.; Thomson, J.G. Precise excision of plastid DNA by the large serine recombinase Bxb1. Plant Biotechnol. J. 2014, 12, 322–329.
- Thomson, J.G.; Chan, R.; Smith, J.; Thilmony, R.; Yau, Y.-Y.; Wang, Y.; Ow, D.W. The Bxb1 recombination system demonstrates heritable transmission of site-specific excision in Arabidopsis. BMC Biotechnol. 2012, 12, 9.
- Abioye, J.; Lawson-Williams, M.; Lecanda, A.; Calhoon, B.; McQue, A.L.; Colloms, S.D.; Stark, W.M.; Olorunniji, F.J. High fidelity one-pot DNA assembly using orthogonal serine integrases. Biotechnol. J. 2023, 18, 2200411.
- Gao, H.; Taylor, G.; Evans, S.K.; Fogg, P.C.M.; Smith, M.C.M. Application of serine integrases for secondary metabolite pathway assembly in Streptomyces. Synth. Syst. Biotechnol. 2020, 5, 111–119.
- Gardner, T.S.; Cantor, C.R.; Collins, J.J. Construction of a genetic toggle switch in Escherichia coli. Nature 2000, 403, 339–342.
- Elowitz, M.B.; Leibler, S. A synthetic oscillatory network of transcriptional regulators. Nature 2000, 403, 335–338.
- Akboğa, D.; Saltepe, B.; Bozkurt, E.U.; Şeker, U.Ö.Ş. A Recombinase-Based Genetic Circuit for Heavy Metal Monitoring. Biosensors 2022, 12, 122.
- Müller, M.; Ausländer, S.; Spinnler, A.; Ausländer, D.; Sikorski, J.; Folcher, M.; Fussenegger, M. Designed cell consortia as fragrance-programmable analog-to-digital converters. Nat. Chem. Biol. 2017, 13, 309–316.
- Kalyoncu, E.; Ahan, R.E.; Ozcelik, C.E.; Seker, U.O.S. Genetic Logic Gates Enable Patterning of Amyloid Nanofibers. Adv. Mater. 2019, 31, 1902888.
- Effendi, S.S.W.; Ng, I.-S. Reprogramming T7RNA Polymerase in Escherichia coli Nissle 1917 under Specific Lac Operon for Efficient p-Coumaric Acid Production. ACS Synth. Biol. 2022, 11, 3471–3481.
- Li, L.; Zheng, G.; Chen, J.; Ge, M.; Jiang, W.; Lu, Y. Multiplexed site-specific genome engineering for overproducing bioactive secondary metabolites in actinomycetes. Metab. Eng. 2017, 40, 80–92.
- Khan, N.; Yeung, E.; Farris, Y.; Fansler, S.J.; Bernstein, H.C. A broad-host-range event detector: Expanding and quantifying performance between Escherichia coli and Pseudomonas species. Synth. Biol. 2020, 5, ysaa002.
- Round, J.W.; Robeck, L.D.; Eltis, L.D. An Integrative Toolbox for Synthetic Biology in Rhodococcus. ACS Synth. Biol. 2021, 10, 2383–2395.
- Elmore, J.R.; Dexter, G.N.; Baldino, H.; Huenemann, J.D.; Francis, R.; Peabody, G.L.; Martinez-Baird, J.; Riley, L.A.; Simmons, T.; Coleman-Derr, D.; et al. High-throughput genetic engineering of nonmodel and undomesticated bacteria via iterative site-specific genome integration. Sci. Adv. 2023, 9, eade1285.
- Chao, G.; Travis, C.; Church, G. Measurement of large serine integrase enzymatic characteristics in HEK293 cells reveals variability and influence on downstream reporter expression. FEBS J. 2021, 288, 6410–6427.
- Xu, Z.; Thomas, L.; Davies, B.; Chalmers, R.; Smith, M.; Brown, W. Accuracy and efficiency define Bxb1 integrase as the best of fifteen candidate serine recombinases for the integration of DNA into the human genome. BMC Biotechnol. 2013, 13, 87.
More
Information
Subjects:
Biotechnology & Applied Microbiology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.0K
Revisions:
2 times
(View History)
Update Date:
13 Oct 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




