Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Yuxuan Zhang | -- | 3728 | 2023-10-11 04:30:08 | | | |
| 2 | Rita Xu | -19 word(s) | 3709 | 2023-10-11 04:40:15 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Li, Y.; Zhang, X.; Zhang, X.; Zhang, Y.; Hou, D. Vat Photopolymerization. Encyclopedia. Available online: https://encyclopedia.pub/entry/50093 (accessed on 07 February 2026).
Li Y, Zhang X, Zhang X, Zhang Y, Hou D. Vat Photopolymerization. Encyclopedia. Available at: https://encyclopedia.pub/entry/50093. Accessed February 07, 2026.
Li, Ying, Xueqin Zhang, Xin Zhang, Yuxuan Zhang, Dan Hou. "Vat Photopolymerization" Encyclopedia, https://encyclopedia.pub/entry/50093 (accessed February 07, 2026).
Li, Y., Zhang, X., Zhang, X., Zhang, Y., & Hou, D. (2023, October 11). Vat Photopolymerization. In Encyclopedia. https://encyclopedia.pub/entry/50093
Li, Ying, et al. "Vat Photopolymerization." Encyclopedia. Web. 11 October, 2023.
Copy Citation
Vat photopolymerization (VP), including stereolithography (SLA), digital light processing (DLP), and volumetric printing, employs UV or visible light to solidify cell-laden photoactive bioresin contained within a vat in a point-by-point, layer-by-layer, or volumetric manner.
vat photopolymerization
bioprinting
stereolithography
digital light processing
two photon polymerization
1. Introduction
3D printing, also known as additive manufacturing, is a revolutionary technology that distinguishes itself from traditional subtractive manufacturing. Under the control of a computer, materials with controlled cross-section geometries can be accumulated layer by layer to form 3D objects with virtually any structure. It is particularly suitable for the fabrication of small-batch production or personalized complex structures [1][2]. Recently, 3D printing has been successfully applied in the fields of regenerative medicine and tissue engineering, which is considered a promising method to address the growing demand for living organ transplantation [3]. 3D bioprinting has been used to fabricate artificial substitutes with biological functionalities to repair, enhance, or maintain tissue functions [4]. Cells, biological factors, and bioactive scaffolds are the fundamental building blocks for tissue engineering [5]. Hydrogels are widely utilized as tissue engineering scaffold materials because of their ECM-like network structures, which can be tailored to possess a range of mechanical, chemical, and biological properties that promote cell adhesion, proliferation, and migration [6].
Compared to traditional fabrication techniques such as freeze-drying, electrospinning, and thermally induced phase separation, 3D bioprinting offers numerous advantages in fabricating artificial tissues: 1. high accuracy in replicating multiscale 3D tissue structures [7]; 2. controlled printing of cell-laden complex 3D structures [5]; 3. capable of constructing multi-network structures to facilitate the transportation of nutrients and oxygen for the regeneration of tissues [8].
According to printing principles and materials, 3D bioprinting can roughly be classified as vat photopolymerization (VP) and ink-based bioprinting [9]. VP, including stereolithography (SLA), digital light processing (DLP), and volumetric printing, is a printing technology that employs UV, visible light, or NIR light to solidify cell-laden photoactive bioresin point-by-point, layer-by-layer, or volumetrically within a vat. Ink-based printing includes extrusion bioprinting and inkjet bioprinting. Extrusion bioprinting constructs 3D structures layer by layer via extruding bioink onto a platform [10]. Inkjet bioprinting uses nozzles similar to those in traditional inkjet printers to deposit tiny biological droplets containing cells and biomaterials layer by layer onto a printing platform to build 3D structures [11]. The viscosity of the bioink for ink-based bioprinting can range from 30 cP to 6 × 107 cP, which means there is a wide range of materials to choose from. The ink-based bioprinter, especially the extrusion-based bioprinter, is cost-effective. Thus, ink-based bioprinting is now the most widely applied method for the fabrication of tissue engineering scaffolds [11][12]. However, the printing resolution of extrusion-based bioprinting is limited to 200–1000 μm, which presents a considerable hurdle when aiming to recreate intricate biological tissues [13]. While the printing resolution of inkjet-based bioprinting is higher, typically ranging from 20–50 μm, it still falls short of the requirements for constructing complex biological tissues [14]. Ink-based bioprinting utilizes nozzles or syringes to extrude bioink, which may lead to low cell viability [15]. In contrast, VP offers unique advantages compared to ink-based bioprinting in terms of manufacturing precision, printing quality, reproducibility, and molding efficiency. The printing resolution for VP-based bioprinting can be less than 100 nm, making VP-based bioprinting a highly promising technology for constructing hierarchical structures that closely mimic natural tissue organization. Thus, VP shows great potential in the customized fabrication of tissue scaffolds, dental materials, soft robots, microfluidics, etc. [16].
In 3D bioprinting, bioink refers to a composite mixture (typically in the form of a hydrogel) composed of one or more types of biomaterials and cells [17]. For VP bioprinting, the term “bioresin” is employed to denote the initial solution that contains cells and from which the 3D objects are polymerized [18]. Over the past decades, substantial strides and achievements have been made in developing bioresin from photoactive biomaterials with the essential biocompatibility and biodegradability to facilitate the creation of functional tissue replicas. A bioresin is composed of photoactive precursors, photoinitiators (PIs), encapsulated cells, and other additives such as growth factors. It must possess exceptional physicochemical and biological properties to ensure both printability and biocompatibility. Biomaterials used in tissue engineering for bioprinting should fulfill several requirements:
-
Printability: The rheology and viscosity properties of bioresin should be compatible with VP to ensure the successful fabrication of intricate and accurate 3D structures with high integrity and high printing precision [19].
-
Biocompatibility: Bioresin before and after photopolymerization should facilitate cell adhesion, proliferation, and differentiation. During the printing process, it is essential that the biomaterials used can protect living cells and other bioactive components from pressure, mechanical forces, and photocrosslinking, as these factors can impact cell fate. The degradation products of the build constructs should also have a minimal impact on cell growth and differentiation [20].
-
Versatility: The inherent characteristics of bioresin, such as adaptability to different printing methods, photocrosslinking mechanisms, and types of incorporated bioactive components, should be adjustable within certain ranges to meet the complex requirements of tissue engineering.
2. Vat Photopolymerization (VP)
VP employs light to irradiate and solidify the bioresin within a vat, point by point, layer by layer, or volumetrically (Figure 1a–g). SLA was the earliest VP method and was invented by Charles Hull as an approach to fabricating solid 3D constructs by crosslinking photoactive material layers wisely [21]. Since then, DLP, CLIP, TPP, and CAL have been developed successively. These techniques have received extensive attention due to their exceptional printing precision, rapid production rate, and adaptability in the development of resin materials. By precisely controlling photoactive materials, growth factors, and cells in terms of space and time, VP-based bioprinting can be used to fabricate complex human tissues in vitro, offering great potential for organ and tissue regeneration. Over the past decades, VP has emerged as a dominant tool for creating tissue scaffolds. Currently, extensive research has been conducted on VP for tissue regeneration, encompassing areas like bone [22], skin [16], liver [23], heart [23], blood vessels [8], and so on.
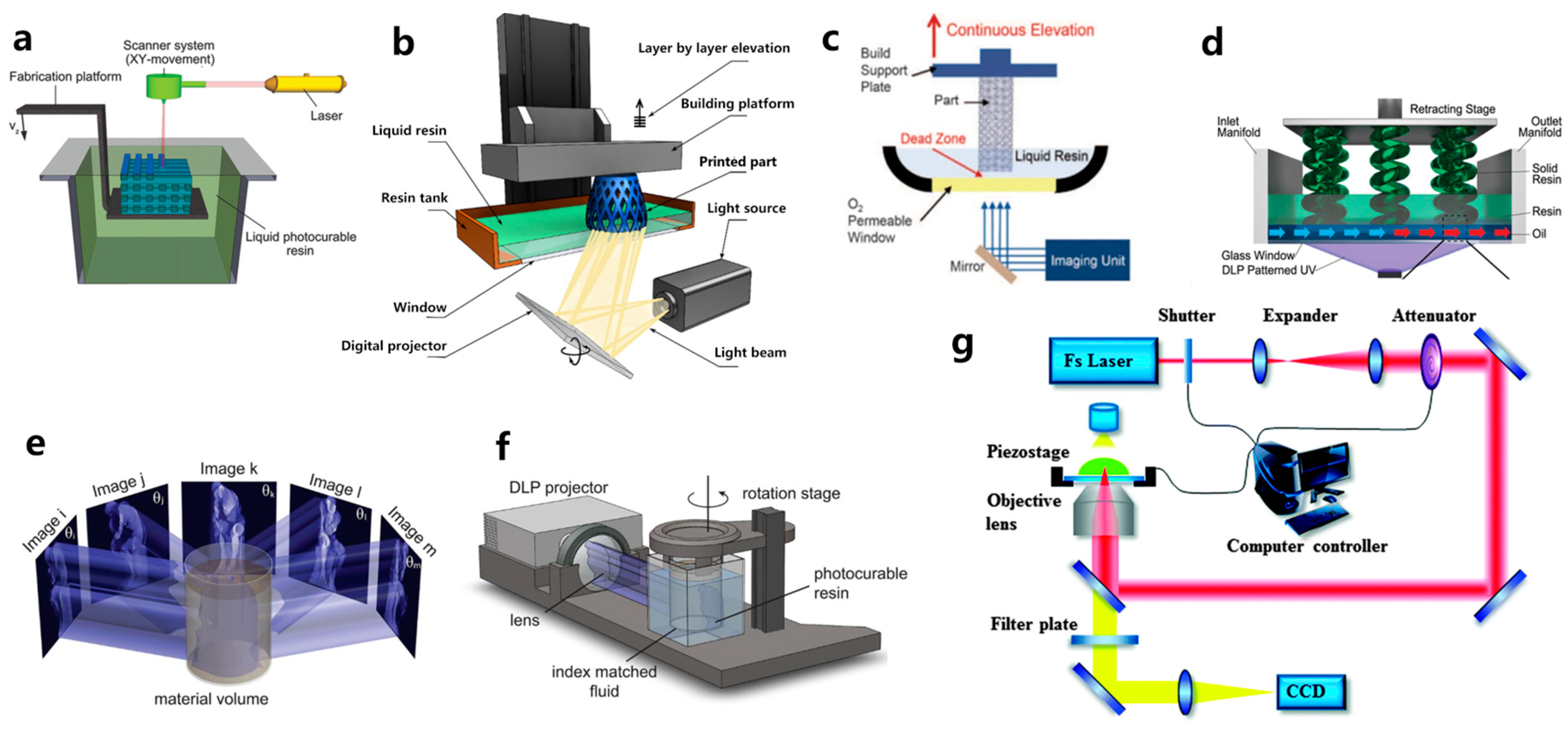
Figure 1. Schematic representation of: (a) SLA; (b) DLP; (c) CLIP; (d) HARP; (e) and (f) CAL; and (g) TPP printing.
2.1. Stereolithography, SLA
SLA, invented by Charles W. Hull in 1986, is the first commercially available 3D printing technique [24]. For SLA-based bioprinting, it utilizes laser-induced photopolymerization to selectively solidify bioresins point by point to fabricate 3D structures. As shown in Figure 1a, a focused laser scans over the bioresin surface point by point to solidify the bioresin [11]. When a single layer is finished, the printing platform moves downward by a layer in the Z-axis direction, and the laser again scans the surface of bioresin to photopolymerize another layer of bioresin. The photopolymerization process is repeated again and again until the 3D constructs are successfully built [25][26]. One of the primary advantages of SLA is its ability to achieve high spatial resolution, thanks to the focused laser beam’s small spot size. Hence, SLA is capable of fabricating intricate 3D structures with high resolution, making it extensively utilized in the fields of tissue engineering. Chan et al. [27] stained NIH/3T3 fibroblast cells with green and red fluorescent dyes, respectively. They then employed a mixture of poly(ethylene glycol) diacrylate (PEGDA) and growth factor RGDS amino acids, which were encapsulated with the stained NIH/3T3 cells for SLA 3D bioprinting. After printing, multilayered wheel-like structures with a layer thickness of 100 μm were fabricated. The printed 3D hydrogel exhibited excellent integrity, high resolution, and high cell viability. After 14 days of in vitro cultivation, noticeable cell migration occurred within the hydrogel containing RGDS growth factors. It indicated the excellent biocompatibility of the SLA-printed hydrogels and the facilitative role of the RGDS amino acid sequence in promoting the movement and migration of NIH/3T3 cells.
Despite its remarkable advantages, SLA also has its limitations. SLA employs lasers as a light source, which makes the 3D printer quite expensive. Furthermore, the bioresin not only needs to be photoactive but also needs to be transparent so that lasers can penetrate the ink and irradiate the photopolymerization of photoactive bioresins. Therefore, the development of cost-effective SLA printing devices and appropriate SLA bioresin materials is the future trend.
2.2. Digital Light Processing, DLP
Evolved from SLA, the DLP technique represents the second generation of photopolymerization-based 3D printing. As illustrated in Figure 1b, it differs from SLA by utilizing a patterned exposure technique, enabling the photopolymerization of an entire layer at once [28]. Consequently, even for extremely complex and large structures, the printing time per layer remains constant, which leads to reduced power consumption and higher printing efficiency. This layer-wise solidification approach implies that the more items printed simultaneously, the higher the efficiency gains. Such printing quality and printing speed make DLP technology especially attractive for large-scale industrial production. Compared to other printing methods, DLP offers superior precision, smoother surface quality, enhanced repeatability, improved structural integrity, and better mechanical properties. Moreover, it can also minimize shear forces, which affect the fate of the encapsulated cells [29]. Grigoryan et al. [8] utilized DLP printing to fabricate intricate and functional vascular architectures. PEGDA was used as a monomer, and food dyes were used as PIs. The obtained vascular architectures are capable of exchanging oxygen. This work gained extensive attention when first published, as constructing a complex biomimetic structure that simulates both an intricate vascular system and an airway network poses significant challenges. However, the shortage of photosensitive biomaterials has also emerged as a primary limiting factor in the development of DLP technology.
Compared to extrusion printing, DLP technology offers significantly enhanced printing resolution. Nevertheless, the layer-by-layer curing process can result in a noticeable staircase effect between adjacent layers, which can lead to low precision along the z-axis. To address this issue, Chen’s team [30] introduced a groundbreaking solution known as dynamic optical projection stereolithography (DOPsL) based on DLP. In DOPsL, the printing platform synchronizes its movements with the digital micromirror device (DMD) in the DLP system (Figure 2), enabling continuous high-resolution printing. This approach enables the rapid fabrication of microstructures with smooth sidewalls. By using DOPsL, the research group successfully printed an array of geometric shapes with improved z-axis printing accuracy. The geometric shapes included curved micro-hole structures, floral patterns, and spiral formations, all confined within a chip measuring 4.6 mm in length and 3.5 mm in width.

Figure 2. Schematic diagram of DOPsL.
2.3. Continuous Liquid Interface Production, CLIP
Compared to SLA, DLP significantly reduces printing time by solidifying an entire layer at once. However, in DLP printing, following the completion of each layer, the printing platform needs to be adjusted—raised or lowered—to allow the refilling of bioresin into the vat. Subsequently, the platform is immersed in the bioresin to continue the printing. This process is repeated again and again until the 3D printing is completed. When printing small-sized structures, the cumulative time taken for platform movement has a minimal impact on efficiency. However, when it comes to the printing of large 3D objects, the increased number of printing layers extends the time needed for the up-and-down motion of the printing platform, thereby significantly decreasing printing efficiency. Consequently, this presents challenges to the practical viability of commercial DLP applications.
To address this challenge, the DeSimone team [31] customized DLP printers and developed the CLIP technique, which enhanced the speed of DLP printing by a factor of 1000. While oxygen inhibition is generally avoided in photopolymerization due to its tendency to cause incomplete photopolymerization and surface tackiness when conducted in the presence of air, DeSimone’s team takes advantage of this effect. As depicted in Figure 1c, the conventional release film at the bottom of the vat of the DLP printer was replaced by an oxygen-permeable polytetrafluoroethylene (PTFE) film. Oxygen permeating from the bottom of the vat inhibits the crosslinking of the bioresin, creating a thin layer of a ‘dead zone’ with a thickness of only several tens of micrometers. Bioresin above this dead zone can still be photopolymerized. This approach blurs the concept of layers and significantly shortens the printing time. The Mirkin team [31] introduced another continuous printing technology called high-area rapid printing (HARP), which involves incorporating a fluorinated oil layer with a thickness of approximately 7 mm between the photoactive resin and the transparent release film. The interface between the resin and the fluorinated oil established a stable solid-liquid boundary, leading to a decrease in adhesive forces between the crosslinked layer and the release film at the bottom. This innovation enables the printing of a 38 cm × 61 cm × 76 cm construct within only 105 min, enabling continuous large-area and rapid vertical printing. The HARP technique is versatile and applicable to various materials. For instance, grid-like structures were printed using polybutadiene rubber elastomer, which can completely regain its original shape after compression.
2.4. Computed Axial Lithography, CAL
Despite the rapidity and high precision advantages offered by SLA, DLP, and CLIP, the printing process still follows a layer-by-layer manufacturing approach, which imposes limitations on the achievable speed. Inspired by the principles of computed tomography (CT) imaging, Taylor’s team [32] developed CAL, where arbitrary geometries can be fabricated volumetrically through photopolymerization. As shown in Figure 1e,f, While the photoactive resin container revolves around the vertical axis, a precomputed digital pattern sequence of light is projected into the photoactive resin. As the rotation progresses, numerous diverse projections are emitted into the resin. Over time, the cumulative light exposure solidifies the regions surpassing the photopolymerization threshold while leaving the portions below the threshold unpolymerized. This process enables the creation of the desired 3D structures in a single printing step.
At present, volumetric additive manufacturing has demonstrated its capability to create intricate components at a notable throughput (>105 mm3 per hour) and is suitable for a diverse array of materials [33][34][35]. Looking ahead, there is a clear trajectory for the continued advancement of volumetric stereolithography. It is anticipated to encompass several aspects, including enhancements of printing resolution, finetuning of the manufacturing process, optimization of the physical system, and the exploration of its integration across diverse domains.
2.5. 3D Printing Based on TPP
The fabrication resolution of the aforementioned VP methods based on one-photon absorption photopolymerization is limited by the optical diffraction limit. This limitation poses significant challenges in achieving high-resolution 3D structures at the submicron scale [36]. Different from other VP printing methods such as SLA, DLP, CLIP, and CAL, TPP utilizes a near-infrared femtosecond laser, which enables the fabrication of intricate and exceptionally precise 3D microstructures with high resolution, not only at the microscale but also at the nanoscale [37][38][39]. A TPP 3D printer primarily consists of a femtosecond laser pulse system and a platform that can move with sub-micron precision (Figure 1g) [37]. The femtosecond laser is used to generate laser pulses of wavelengths in the range of 600–1000 nm, while the motion platform is responsible for carrying the printing material and 3D focal scanning [40]. The initiation of TPP occurs through third-order nonlinear absorption within the focal area. 3D constructs can be fabricated by guiding the focused laser beam within the photoactive materials along a CAD path, achieving resolution that surpasses the optical diffraction limit. The application of TPP technology has spread across various fields, including microelectromechanical systems (MEMS) [41], microfluidics [42], microoptics [43], biomedicine [44], sensors [45], and microrobotics [46][47]. Since the printing resolution plays a crucial role in replicating the native microenvironment of the ECM for hydrogel constructs, TPP is considered an ideal method for creating tissue scaffolds. Extensive research has been conducted on TPP for hydrogel fabrication [48][49]. Yu et al. [50] prepared a series of scaffolds tailored to different stiffness and configurations using TPP. PEGDA was used as a monomer, and pentaerythritol triacrylate (PE-3A) was used as the crosslinker (Figure 3a,b). By adjusting the concentrations of PEGDA and PE-3A to 40 wt % and 60 wt %, respectively, a fine, intricate 3D structure with a resolution of 80 nm was fabricated (Figure 3c,d). By adjusting the concentration of PE-3A, scaffolds with Young’s modulus ranging from 1.4 to 11.9 MPa were achieved. To improve the biocompatibility of the scaffolds, 2 wt % of chitosan and sodium hyaluronate were incorporated into the PEGDA/PE-3A prepolymer, respectively. Results showed that chitosan improved adhesion of murine fibroblast (L929) cells, while sodium hyaluronate ensured the biocompatibility of the prepolymer.
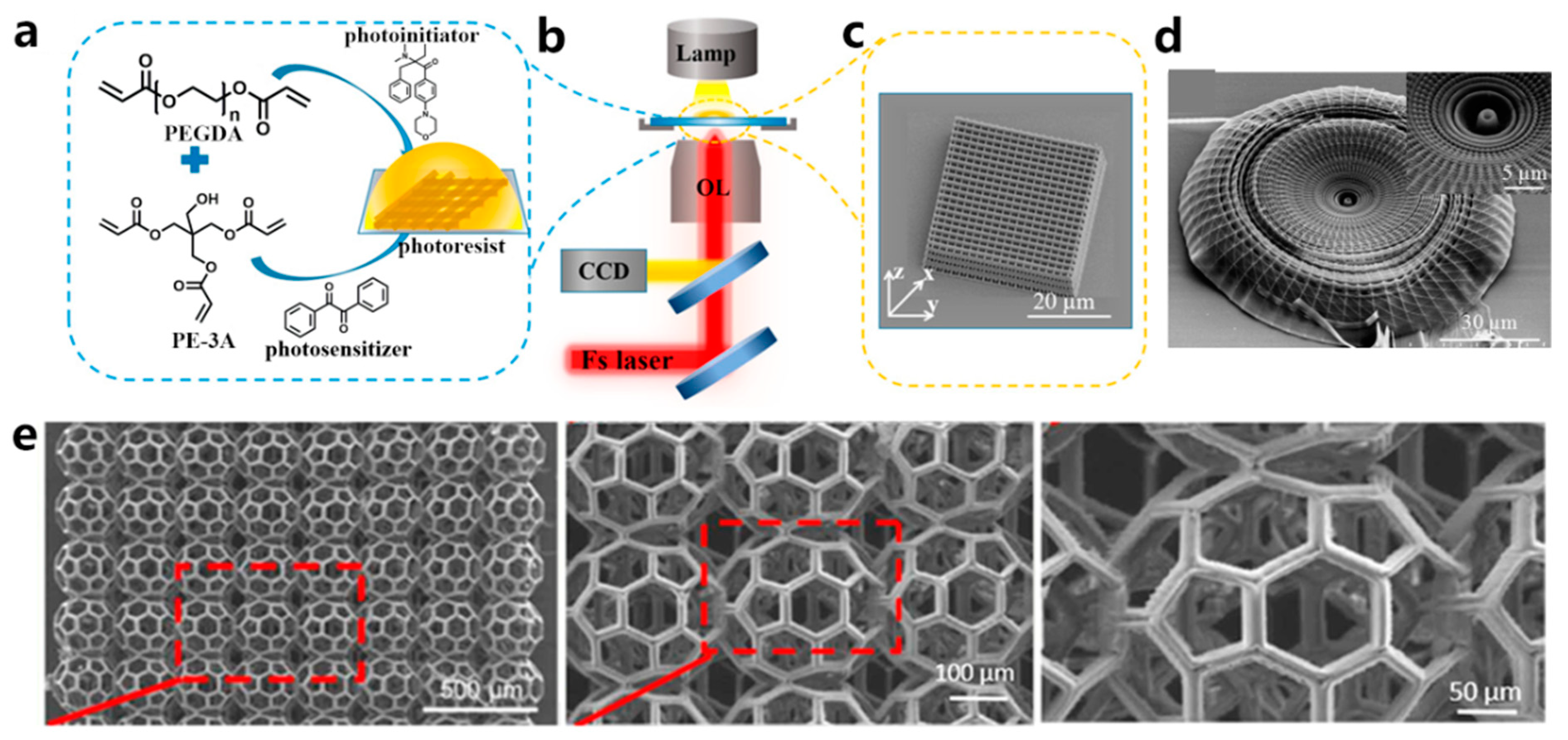
Figure 3. (a) The structure of the main components in the bioresin. (b) Illustration of the TPP technique used to fabricate 3D structures. (c) SEM photo of a TPP-printed woodpile structure. (d) SEM photo of a TPP-printed hydrogel scaffold with a straw hat shape. Inset is the magnified photo. (e) SEM images display a scaffold in the shape of a biodegradable buckyball.
2.6. Light Source for VP
Though TPP is excellent at fabricating complex structures with high resolution, its primary drawback is its comparatively low throughput. To finish the printing within a reasonable timeframe, the TPP-printed structures tend to be rather small. Another limitation is that the biomaterials suitable for TPP are rather inadequate. Thus, Weisgrab et al. [51] modified poly(trimethylene-carbonate) (PTMC) with methacrylic anhydride (MAA) to synthesize the highly photoactive macromer PTMC-MA. Through a systematic study of the printing parameters, they successfully fabricated highly porous scaffolds, PTMAC-MA (96% porosity), with unprecedented large sizes (Figure 3e). After 28 days of cultivation, human adipose-derived mesenchymal stem cells (hADSCs) encapsulated within the porous scaffolds exhibited strong adhesion, proliferation, and differentiation towards their osteogenic and chondrogenic lineages. This work suggests that TPP has the potential to fabricate intricate, large-scale structures. Maibohm et al. [52] proposed a TPP approach that could manufacture large-sized constructs with rapid production and scalability. A fixed diffraction optical element (DOE) was equipped in the traditional TPP apparatus to establish a parallelized, multi-beamlet (nine beamlets) TPP printing process. Using this newly established TPP printing process, they successfully achieved the printing of large periodic 3D microstructure arrays with a high aspect ratio at the sub-micro level, spanning an area of several hundred square micrometers.
As mentioned in previous sections, in VP-based bioprinting, the selection of light sources plays a crucial role in achieving a balance between printing speed and cell viability. The wavelength of the light source should overlap with the absorption spectra of the PIs in the bioresin formulations to initiate the photopolymerization of bioresins. The most frequently used light sources for one photopolymerization fall within the UV and visible light ranges (especially in the UV-A and near-UV visible-light ranges), while TPP-based bioprinting employs NIR laser pulses with wavelengths ranging from 600 to 1000 nm [49][53][54][55]. Thus far, photopolymerization of bioresins has primarily been achieved by employing UV light, as most highly efficient PIs function under UV light [56]. However, UV light may induce genetic mutations and even cause cell death. Consequently, visible light sources are increasingly being employed in VP-based bioprinting to safeguard cell viability and mitigate the potential harm to encapsulated cells [57]. Substituting UV light with visible light not only enhances cell compatibility and expands the application of hydrogel systems, but also provides greater penetration depth, which is beneficial for fabricating hydrogels with more uniform structures [58][59]. Visible light-crosslinked hydrogels have been increasingly employed in diverse fields, including tissue engineering [60], 3D cell encapsulation [61], and drug delivery [62].
TPP-based bioprinting utilizes NIR femtosecond laser pulses, which penetrate more deeply due to the transparency of most bioresins to NIR light. Additionally, NIR light falls within the “biological window”, where light has its maximum depth of penetration in tissue, causing minimal damage to cells and enabling the in vivo 3D printing of cell-encapsulated structures [63]. While NIR femtosecond laser pulses offer deeper penetration for bioresins and provide high manufacturing resolution, a significant limitation of these laser sources is their high cost. Furthermore, the high energy density of the laser device can potentially have a detrimental impact on cell fate [64]. Therefore, it is essential to carefully adjust the energy density to achieve both good fidelity of the printing structure and high cell viability.
2.7. Strategies to Improve Printing Resolution
Enhancing the printing speed of VP-based bioprinting is of critical importance for the efficient fabrication of intricate and scalable 3D structures. The speed of photopolymerization is influenced by both the efficiency of PI in the bioresin formulations and the exposure dosage [18]. The exposure dosage is dependent on the irradiation time and the light intensity. One effective approach to increasing printing speed is to reduce the exposure time for each layer, thus reducing the overall printing time. However, this approach may result in lower printing resolution because shorter exposure times can lead to incomplete photopolymerization, further diminishing the fidelity of the printed structure. Another approach to consider is increasing the intensity of irradiation light, which can result in higher crosslinking density and a higher degree of polymerization. However, it is important to note that high-intensity incident light may generate a high density of radicals and lead to difficulties in their diffusion, potentially causing heterogeneity in the polymer network. Moreover, excessive exposure may lead to cell death [56]. Therefore, finding the right balance between cell viability and printing speed is essential for optimizing VP-based bioprinting.
In addition to hardware updates, it is also possible to enhance printing resolution while simultaneously increasing printing speed by fine-tuning the bioresin formulations or developing innovative material toolboxes. In a photopolymerization formulation, PI plays a critical role as it directly influences the photopolymerization kinetics of the bioresin. Choosing PIs with significant absorption overlap with the wavelength of the light source is advantageous for reducing exposure time, thereby facilitating rapid printing, as it allows for the optimization of the solidification time for each layer. Thus far, the most efficient PIs for one-photon polymerization are UV-irradiated and have low water solubility. PIs for two-photon polymerizations are also limited. Thus, developing efficient visible-light PI with good water solubility is urgent.
Other effective strategies, including changing bioresin solvents [65], designing functional hydrogels [66], and utilizing post-treatment [67], can also enhance the printing speed. Ge et al. [65] employed deep eutectic solvents (DES) as the solvent to dissolve N-hydroxyethyl acrylamide (HEAA) and zwitterionic N-(3-sulfopropyl)-N-(methacryloxyethyl)-N,N-dimethylammonium betaine (DMAPS), creating a mechanically robust photopolymerization ionogel with excellent biocompatibility. This formulation can be used in 3D printing, and the resulting structure exhibited excellent fidelity and high resolution. In VP-based bioprinting, multiple monomers are frequently blended to improve the mechanical properties and biocompatibility of the printed hydrogels. However, the formulation with multiple components might result in poor compatibility among the constituents, potentially leading to reduced printing fidelity. Gong et al. [67] introduced a complexation-induced resolution enhancement 3D printing approach known as “shrinking printing”. Anionic inks, including methacrylated hyaluronic acid (HAMA), GelMA, and chitosan at varying concentrations, were employed as bioinks. After the printing process, the 3D structures were subjected to post-treatment by immersing them in a polycationic chitosan solution. Through charge complexation and the expulsion of water from the hydrogels, the linear dimensions of the hydrogels were reduced to varying degrees.
References
- Ge, Q.; Chen, Z.; Cheng, J.; Zhang, B.; Zhang, Y.F.; Li, H.; He, X.; Yuan, C.; Liu, J.; Magdassi, S.; et al. 3D printing of highly stretchable hydrogel with diverse UV curable polymers. Sci. Adv. 2021, 7, eaba4261.
- Ligon, S.C.; Liska, R.; Stampfl, J.; Gurr, M.; Mulhaupt, R. Polymers for 3D Printing and Customized Additive Manufacturing. Chem. Rev. 2017, 117, 10212–10290.
- Jorgensen, A.M.; Yoo, J.J.; Atala, A. Solid Organ Bioprinting: Strategies to Achieve Organ Function. Chem. Rev. 2020, 120, 11093–11127.
- Howard, D.; Buttery, L.D.; Shakesheff, K.M.; Roberts, S.J. Tissue engineering: Strategies, stem cells and scaffolds. J. Anat. 2008, 213, 66–72.
- Li, T.; Chang, J.; Zhu, Y.; Wu, C. 3D Printing of Bioinspired Biomaterials for Tissue Regeneration. Adv. Healthc. Mater. 2020, 9, e2000208.
- Lee, S.C.; Gillispie, G.; Prim, P.; Lee, S.J. Physical and Chemical Factors Influencing the Printability of Hydrogel-based Extrusion Bioinks. Chem. Rev. 2020, 120, 10834–10886.
- dos Santos, B.C.; Noritomi, P.Y.; da Silva, J.V.L.; Maia, I.A.; Manzini, B.M. Biological multiscale computational modeling: A promising tool for 3D bioprinting and tissue engineering. Bioprinting 2022, 28, e00234.
- Grigoryan, B.; Paulsen, S.J.; Corbett, D.C.; Sazer, D.W.; Fortin, C.L.; Zaita, A.J.; Greenfield, P.T.; Calafat, N.J.; Gounley, J.P.; Ta, A.H.; et al. Multivascular networks and functional intravascular topologies within biocompatible hydrogels. Science 2019, 364, 458–464.
- Ge, G.; Wang, Q.; Zhang, Y.Z.; Alshareef, H.N.; Dong, X. 3D Printing of Hydrogels for Stretchable Ionotronic Devices. Adv. Funct. Mater. 2021, 31, 2107437.
- Fu, Z.; Naghieh, S.; Xu, C.; Wang, C.; Sun, W.; Chen, X. Printability in extrusion bioprinting. Biofabrication 2021, 13, 033001.
- Li, X.; Liu, B.; Pei, B.; Chen, J.; Zhou, D.; Peng, J.; Zhang, X.; Jia, W.; Xu, T. Inkjet Bioprinting of Biomaterials. Chem. Rev. 2020, 120, 10793–10833.
- Vanaei, S.; Parizi, M.S.; Vanaei, S.; Salemizadehparizi, F.; Vanaei, H.R. An Overview on Materials and Techniques in 3D Bioprinting Toward Biomedical Application. Eng. Regen. 2021, 2, 1–18.
- Hölzl, K.; Lin, S.; Tytgat, L.; Van Vlierberghe, S.; Gu, L.; Ovsianikov, A. Bioink properties before, during and after 3D bioprinting. Biofabrication 2016, 8, 032002.
- Miri, A.K.; Mirzaee, I.; Hassan, S.; Mesbah Oskui, S.; Nieto, D.; Khademhosseini, A.; Zhang, Y.S. Effective bioprinting resolution in tissue model fabrication. Lab. Chip 2019, 19, 2019–2037.
- Xu, H.Q.; Liu, J.C.; Zhang, Z.Y.; Xu, C.X. A review on cell damage, viability, and functionality during 3D bioprinting. Mil. Med. Res. 2022, 9, 70.
- Zhang, F.; Zhu, L.; Li, Z.; Wang, S.; Shi, J.; Tang, W.; Li, N.; Yang, J. The recent development of vat photopolymerization: A review. Addit. Manuf. 2021, 48, 102423.
- Mobaraki, M.; Ghaffari, M.; Yazdanpanah, A.; Luo, Y.; Mills, D.K. Bioinks and bioprinting: A focused review. Bioprinting 2020, 18, e00080.
- Lee, M.; Rizzo, R.; Surman, F.; Zenobi-Wong, M. Guiding Lights: Tissue Bioprinting Using Photoactivated Materials. Chem. Rev. 2020, 120, 10950–11027.
- Sun, Y.; Yu, K.; Nie, J.; Sun, M.; Fu, J.; Wang, H.; He, Y. Modeling the printability of photocuring and strength adjustable hydrogel bioink during projection-based 3D bioprinting. Biofabrication 2021, 13, 035032.
- Kim, J. Characterization of Biocompatibility of Functional Bioinks for 3D Bioprinting. Bioengineering 2023, 10, 457.
- Huang, J.; Qin, Q.; Wang, J. A Review of Stereolithography: Processes and Systems. Processes 2020, 8, 1138–1154.
- Chartrain, N.A.; Williams, C.B.; Whittington, A.R. A review on fabricating tissue scaffolds using vat photopolymerization. Acta Biomater. 2018, 74, 90–111.
- Yu, C.; Ma, X.; Zhu, W.; Wang, P.; Miller, K.L.; Stupin, J.; Koroleva-Maharajh, A.; Hairabedian, A.; Chen, S. Scanningless and continuous 3D bioprinting of human tissues with decellularized extracellular matrix. Biomaterials 2019, 194, 1–13.
- Piironen, K.; Haapala, M.; Talman, V.; Jarvinen, P.; Sikanen, T. Cell adhesion and proliferation on common 3D printing materials used in stereolithography of microfluidic devices. Lab. Chip 2020, 20, 2372–2382.
- Tian, X.; Jin, J.; Yuan, S.; Chua, C.K.; Tor, S.B.; Zhou, K. Emerging 3D-Printed Electrochemical Energy Storage Devices: A Critical Review. Adv. Energy Mater. 2017, 7, 1700127.
- Bao, Y. Recent Trends in Advanced Photoinitiators for Vat Photopolymerization 3D Printing. Macromol. Rapid Commun. 2022, 43, e2200202.
- Chan, V.; Zorlutuna, P.; Jeong, J.H.; Kong, H.; Bashir, R. Three-dimensional photopatterning of hydrogels using stereolithography for long-term cell encapsulation. Lab. Chip 2010, 10, 2062–2070.
- Pagac, M.; Hajnys, J.; Ma, Q.P.; Jancar, L.; Jansa, J.; Stefek, P.; Mesicek, J. A Review of Vat Photopolymerization Technology: Materials, Applications, Challenges, and Future Trends of 3D Printing. Polymers 2021, 13, 598–618.
- Gu, Z.; Fu, J.; Lin, H.; He, Y. Development of 3D bioprinting: From printing methods to biomedical applications. Asian J. Pharm. Sci. 2020, 15, 529–557.
- Zhang, A.P.; Qu, X.; Soman, P.; Hribar, K.C.; Lee, J.W.; Chen, S.; He, S. Rapid fabrication of complex 3D extracellular microenvironments by dynamic optical projection stereolithography. Adv. Mater. 2012, 24, 4266–4270.
- Tumbleston, J.R.; Shirvanyants, D.; Ermoshkin, N.; Janusziewicz, R.; Johnson, A.R.; Kelly, D.; Chen, K.; Pinschmidt, R.; Rolland, J.P.; Ermoshkin, A.; et al. Additive manufacturing. Continuous liquid interface production of 3D objects. Science 2015, 347, 1349–1352.
- Terrones, G.; Pearlstein, A.J. Diffusion-Induced Nonuniformity of Photoinitiation in a Photobleaching Medium. Macromolecules 2004, 37, 1565–1575.
- Loterie, D.; Delrot, P.; Moser, C. High-resolution tomographic volumetric additive manufacturing. Nat. Commun. 2020, 11, 852.
- Regehly, M.; Garmshausen, Y.; Reuter, M.; Konig, N.F.; Israel, E.; Kelly, D.P.; Chou, C.Y.; Koch, K.; Asfari, B.; Hecht, S. Xolography for linear volumetric 3D printing. Nature 2020, 588, 620–624.
- Madrid-Wolff, J.; Boniface, A.; Loterie, D.; Delrot, P.; Moser, C. Controlling Light in Scattering Materials for Volumetric Additive Manufacturing. Adv. Sci. 2022, 9, e2105144.
- Li, B.; Liao, C.; Cai, Z.; Zhou, J.; Zhao, C.; Jing, L.; Wang, J.; Xiong, C.; Xu, L.; Wang, Y.; et al. Femtosecond laser 3D printed micro objective lens for ultrathin fiber endoscope. Fundam. Res. 2022, in press.
- Xing, J.F.; Zheng, M.L.; Duan, X.M. Two-photon polymerization microfabrication of hydrogels: An advanced 3D printing technology for tissue engineering and drug delivery. Chem. Soc. Rev. 2015, 44, 5031–5039.
- Marschner, D.E.; Pagliano, S.; Huang, P.-H.; Niklaus, F. A methodology for two-photon polymerization micro 3D printing of objects with long overhanging structures. Addit. Manuf. 2023, 66, 103474.
- Dong, Y.; Lipu, L.; Hong, Y.; Qihuang, G.; Yan, L. Laser Micro-Nano Three-Dimensional Printing. Laser Optoelectron. Progress. 2018, 55, 011411.
- O’Halloran, S.; Pandit, A.; Heise, A.; Kellett, A. Two-Photon Polymerization: Fundamentals, Materials, and Chemical Modification Strategies. Adv. Sci. 2022, 10, e2204072.
- Lao, Z.; Xia, N.; Wang, S.; Xu, T.; Wu, X.; Zhang, L. Tethered and Untethered 3D Microactuators Fabricated by Two-Photon Polymerization: A Review. Micromachines 2021, 12, 465–494.
- Buchegger, B.; Tanzer, A.; Posch, S.; Gabriel, C.; Klar, T.A.; Jacak, J. STED lithography in microfluidics for 3D thrombocyte aggregation testing. J. Nanobiotechnol. 2021, 19, 23.
- Wang, H.; Zhang, W.; Ladika, D.; Yu, H.; Gailevičius, D.; Wang, H.; Pan, C.F.; Nair, P.N.S.; Ke, Y.; Mori, T.; et al. Two-Photon Polymerization Lithography for Optics and Photonics: Fundamentals, Materials, Technologies, and Applications. Adv. Funct. Mater. 2023, 33, 2214211.
- Jia, Y.; Spiegel, C.A.; Welle, A.; Heißler, S.; Sedghamiz, E.; Liu, M.; Wenzel, W.; Hackner, M.; Spatz, J.P.; Tsotsalas, M.; et al. Covalent Adaptable Microstructures via Combining Two-Photon Laser Printing and Alkoxyamine Chemistry: Toward Living 3D Microstructures. Adv. Funct. Mater. 2022, 33, 202207826.
- Epifanov, E.O.; Tarkhov, M.A.; Timofeeva, E.R.; Trofimov, I.V.; Asharchuk, I.M.; Obydennov, D.V.; Li, W.; Gonchukov, S.A.; Minaev, N.V. Fabrication of micro-optical connectors for electro-optical sensor devices by a combined femtosecond laser system. Laser Phys. Lett. 2021, 18, 036201.
- Kim, S.; Kubicek, R.; Bergbreiter, S. 3D-Printed Electrostatic Microactuators for Flexible Microsystems. Adv. Funct. Mater. 2023, 202304991.
- Jia, Y.; Zhu, Z.; Jing, X.; Lin, J.; Lu, M. Fabrication and performance evaluation of magnetically driven double curved conical ribbon micro-helical robot. Mater. Des. 2023, 226, 111651.
- Wang, X.; Wei, Z.; Baysah, C.Z.; Zheng, M.; Xing, J. Biomaterial-based microstructures fabricated by two-photon polymerization microfabrication technology. RSC Adv. 2019, 9, 34472–34480.
- Jing, X.; Fu, H.; Yu, B.; Sun, M.; Wang, L. Two-photon polymerization for 3D biomedical scaffolds: Overview and updates. Front. Bioeng. Biotechnol. 2022, 10, 994355.
- Yu, H.; Liu, J.; Zhao, Y.Y.; Jin, F.; Dong, X.Z.; Zhao, Z.S.; Duan, X.M.; Zheng, M.L. Biocompatible Three-Dimensional Hydrogel Cell Scaffold Fabricated by Sodium Hyaluronate and Chitosan Assisted Two-Photon Polymerization. ACS Appl. Bio Mater. 2019, 2, 3077–3083.
- Weisgrab, G.; Guillaume, O.; Guo, Z.; Heimel, P.; Slezak, P.; Poot, A.; Grijpma, D.; Ovsianikov, A. 3D Printing of large-scale and highly porous biodegradable tissue engineering scaffolds from poly(trimethylene-carbonate) using two-photon-polymerization. Biofabrication 2020, 12, 045036.
- Maibohm, C.; Silvestre, O.F.; Borme, J.; Sinou, M.; Heggarty, K.; Nieder, J.B. Multi-beam two-photon polymerization for fast large area 3D periodic structure fabrication for bioapplications. Sci. Rep. 2020, 10, 8740.
- Levato, R.; Dudaryeva, O.; Garciamendez-Mijares, C.E.; Kirkpatrick, B.E.; Rizzo, R.; Schimelman, J.; Anseth, K.S.; Chen, S.; Zenobi-Wong, M.; Zhang, Y.S. Light-based vat-polymerization bioprinting. Nat. Rev. Methods Primers 2023, 3, 47.
- Mauriello, J.; Maury, R.; Guillaneuf, Y.; Gigmes, D. 3D/4D Printing of Polyurethanes by Vat Photopolymerization. Adv. Mater. Technol. 2023, 2300366.
- Huang, Z.; Chi-Pong Tsui, G.; Deng, Y.; Tang, C.-Y. Two-photon polymerization nanolithography technology for fabrication of stimulus-responsive micro/nano-structures for biomedical applications. Nanotechnol. Rev. 2020, 9, 1118–1136.
- Elkhoury, K.; Zuazola, J.; Vijayavenkataraman, S. Bioprinting the future using light: A review on photocrosslinking reactions, photoreactive groups, and photoinitiators. SLAS Technol. 2023, 28, 142–151.
- Nieto, D.; Marchal Corrales, J.A.; Jorge de Mora, A.; Moroni, L. Fundamentals of light-cell-polymer interactions in photo-cross-linking based bioprinting. APL Bioeng. 2020, 4, 041502.
- Yang, D.H.; Chun, H.J. Visible Light-Curable Hydrogel Systems for Tissue Engineering and Drug Delivery. Adv. Exp. Med. Biol. 2020, 1249, 85–93.
- Petta, D.; Armiento, A.R.; Grijpma, D.; Alini, M.; Eglin, D.; D’Este, M. 3D bioprinting of a hyaluronan bioink through enzymatic-and visible light-crosslinking. Biofabrication 2018, 10, 044104.
- Wang, C.; Zhang, P.; Xiao, W.; Zhao, J.; Shi, M.; Wei, H.; Deng, Z.; Guo, B.; Zheng, Z.; Yu, Y. Visible-light-assisted multimechanism design for one-step engineering tough hydrogels in seconds. Nat. Commun. 2020, 11, 4694.
- Sharifi, S.; Sharifi, H.; Akbari, A.; Chodosh, J. Systematic optimization of visible light-induced crosslinking conditions of gelatin methacryloyl (GelMA). Sci. Rep. 2021, 11, 23276.
- Madzarevic, M.; Ibric, S. Evaluation of exposure time and visible light irradiation in LCD 3D printing of ibuprofen extended release tablets. Eur. J. Pharm. Sci. 2021, 158, 105688.
- Smith, A.M.; Mancini, M.C.; Nie, S. Bioimaging: Second window for in vivo imaging. Nat. Nanotechnol. 2009, 4, 710–711.
- Wang, K.; Pena, J.; Xing, J. Upconversion Nanoparticle-Assisted Photopolymerization. Photochem. Photobiol. 2020, 96, 741–749.
- Ge, G.; Mandal, K.; Haghniaz, R.; Li, M.; Xiao, X.; Carlson, L.; Jucaud, V.; Dokmeci, M.R.; Ho, G.W.; Khademhosseini, A. Deep Eutectic Solvents-based Ionogels with Ultrafast Gelation and High Adhesion in Harsh Environments. Adv. Funct. Mater. 2023, 33, 2207388.
- Zhou, T.; Yuk, H.; Hu, F.; Wu, J.; Tian, F.; Roh, H.; Shen, Z.; Gu, G.; Xu, J.; Lu, B.; et al. 3D printable high-performance conducting polymer hydrogel for all-hydrogel bioelectronic interfaces. Nat. Mater. 2023, 22, 895–902.
- Gong, J.; Schuurmans, C.C.L.; Genderen, A.M.V.; Cao, X.; Li, W.; Cheng, F.; He, J.J.; Lopez, A.; Huerta, V.; Manriquez, J.; et al. Complexation-induced resolution enhancement of 3D-printed hydrogel constructs. Nat. Commun. 2020, 11, 1267.
More
Information
Subjects:
Materials Science, Biomaterials
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
2.0K
Revisions:
2 times
(View History)
Update Date:
11 Oct 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




