Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Sabri Saeed Sanabani | -- | 2304 | 2023-10-09 15:21:20 | | | |
| 2 | Camila Xu | Meta information modification | 2304 | 2023-10-10 03:41:11 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Pessôa, R.; Clissa, P.B.; Sanabani, S.S. Gut Microbiome, Immunity, and Atopic Dermatitis. Encyclopedia. Available online: https://encyclopedia.pub/entry/49982 (accessed on 07 February 2026).
Pessôa R, Clissa PB, Sanabani SS. Gut Microbiome, Immunity, and Atopic Dermatitis. Encyclopedia. Available at: https://encyclopedia.pub/entry/49982. Accessed February 07, 2026.
Pessôa, Rodrigo, Patricia Bianca Clissa, Sabri Saeed Sanabani. "Gut Microbiome, Immunity, and Atopic Dermatitis" Encyclopedia, https://encyclopedia.pub/entry/49982 (accessed February 07, 2026).
Pessôa, R., Clissa, P.B., & Sanabani, S.S. (2023, October 09). Gut Microbiome, Immunity, and Atopic Dermatitis. In Encyclopedia. https://encyclopedia.pub/entry/49982
Pessôa, Rodrigo, et al. "Gut Microbiome, Immunity, and Atopic Dermatitis." Encyclopedia. Web. 09 October, 2023.
Copy Citation
Atopic dermatitis (AD), also known as atopic eczema, is a common inflammatory skin disease affecting 7–10% of adults and up to 25% of young children.
microbial dysbiosis
intestinal permeability
immuno-regulation
short-chain fatty acid
1. Introduction
Atopic dermatitis (AD), also known as atopic eczema, is a common inflammatory skin disease affecting 7–10% of adults and up to 25% of young children [1][2]. AD results from a complex interaction between the host immune system and various environmental factors in genetically susceptible individuals [3][4][5]. The prevalence of AD in different genders during childhood and adolescence remains inconclusive. According to some authors, the natural history of AD suggests that males predominate in childhood and females after puberty [6]. Studies in Europe have suggested an increase in the incidence and prevalence of AD in the 21st century compared to the 20th century [7][8], possibly due to various factors such as genetic and epigenetic factors, impaired skin barrier integrity, autoimmunity, viral infections, gut microbiome composition, dietary habits, and lifestyle factors [7][9][10][11][12][13][14][15][16]. Recent research has shown functional links between genetic variants in the IL-17 promoter, the gut microbiome, and AD [17]. The A allele of the IL-17 mutation rs2275913 has been associated with higher expression of IL-17 and dysbiosis, leading to intestinal and systemic inflammation and early onset of AD. The hygiene hypothesis has been proposed to explain the significant increase in AD in developing countries, where lack of exposure to infectious agents may affect immune maturation [18].
2. Genetic Predisposition to AD
Esparza-Gordillo et al. published the first AD genome-wide association study (GWAS) in May 2009. The study included 939 cases, 975 German controls, and 275 nuclear families, each having two affected siblings [19]. This study confirmed that the filaggrin locus (FLG) is a risk factor for AD and found a novel susceptibility region on chromosome 11q13.5, 38 kb upstream of C11orf30. In 2011, Sun et al. published the results of a GWAS in the Chinese Han population [20]. In this study, the FLG region was validated in the Chinese population, and two novel loci were discovered at 5q22.1 and 20q13.33. The validation of these two loci was performed in 1806 cases and 3256 controls from Germany. A meta-analysis of GWAS in Europe by Paternoster et al. identified three additional novel risk loci for AD (11q31.1, 19p13.2, 5q31) [21]. In 2015, Paternoster et al. conducted a multi-ancestry GWAS of the AD cohort with the best statistical power to date, including 21,000 patients and 95,000 controls, and validated the results in 32,059 cases and 228,648 controls [22]. This analysis confirmed previously reported AD risk loci and identified 11 additional new AD risk loci. The newly discovered loci include candidate genes for CD207 (langerin), PPP2R3C, IL-7R, STAT3, and ZBTB10, known to be involved in T-cell control and innate host defense. In another study by Esparza-Gordillo et al., the association of IL6R rs2228145 (C) genotype with higher plasma levels of soluble IL-6R in AD and sustained AD status was confirmed using two independent population-based cohorts [23]. Heritability studies demonstrate the importance of both general genetic factors related to atopy and disease-specific AD genes. According to studies in twins, the heritability of AD ranges from 71% to 84% [24]. However, these genetic factors are not 100% predictive of AD, suggesting a complex interaction between genetic determinants and environmental factors such as the gut microbiota [13][17].
3. Gut Microbiome, Immunity, and AD
The gut microbiota is a diverse consortium of microorganisms that reside in the gastrointestinal tract, comprising bacteria, viruses, fungi, protozoa, and archaea [25]. It encompasses approximately 500–1000 different species, with the dominant phyla in healthy individuals being Bacteroidetes, Firmicutes, Actinobacteria, and Proteobacteria [26][27][28][29].The microbiome exerts various beneficial effects, including influencing energy balance, metabolism, gut epithelial cell health, immunologic activity, neurodevelopment, vitamin synthesis, and immune system maturation [26][30]. Additionally, byproducts of the gut microbiota can modulate host physiology and metabolism, aiding digestion and energy production from indigestible substrates. One example is the extraction of short-chain fatty acids (SCFAs) from indigestible fiber [26][27]. SCFAs fuel the intestinal mucosa, regulating immune responses and maintaining intestinal homeostasis to prevent inflammation and carcinogenesis [31][32].The composition of the gut microbiota is influenced by various factors such as changes in diet, increased stress, lifestyle behaviors, sex hormones, genetic makeup, and prolonged antibiotic use [33]. Alterations in the gut microbial composition have been implicated in the development of several diseases, including gastrointestinal disorders, cardiovascular conditions, atopic dermatitis (AD), diabetes, and obesity [13][34][35][36][37]. Remarkably, numerous studies have compared the microbiomes of individuals with AD or those genetically predisposed to the condition to that of healthy controls, revealing differences and suggesting the involvement of dysbiotic gut microbiota in the disease’s development [38][39][40].
3.1. Mode of Delivery and the Infant’s Gut Microbiota: Impact on the Risk of AD
Several studies have shown that the mode of delivery affects the composition of the infant’s gut microbiota. Vaginal delivery (VD) contributes to the normal colonization of the infant’s gut by exposing them to maternal vaginal microbiota, which includes Lactobacillus, Prevotella, Bacteroides, Escherichia, Shigella, and Bifidobacterium [41][42]. In contrast, delivery by cesarean section (CD) has been associated with delayed acquisition of vaginal microbiota, such as Bacteroides species. This delay has been linked to lower levels of Th-1-associated chemokines CXCL10 and CXCL11 in infants’ blood, suggesting the importance of this microbiota in promoting cytokine synthesis necessary for neonatal immunity [43][44]. Epidemiological evidence indicates that infants born via CD are more likely to develop AD compared to those born via VD [45][46][47]. Thus, the structure of the gut microbiome in early life appears critical for long-term health. It is crucial to gain a more precise understanding of neonatal gut ecology. Recent findings have noted an association between CD and reduced levels of microbial metabolites like riboflavin and folate. This association suggests that impaired folate biosynthesis may affect the immune function of natural killer cells against viral infections, potentially triggering AD [48]. Furthermore, antibiotic use during pregnancy and the early postnatal period elevates the risk of AD in infants [49]. These studies collectively highlight the critical role of a healthy microbiome in maintaining a strong immune system during early life.
3.2. Environmental Factors, the Gut Microbiome, and AD
It is generally believed that hereditary variables, such as a history of eczema in parents, influence the prevalence of eczema in their offspring [50][51]. Additionally, environmental factors have been shown to increase disease prevalence by affecting genetic predisposition [52][53]. Common allergens known to trigger AD symptoms include pollen, dust mites, and animal dander [54]. Extensive research has been conducted in recent years to identify environmental elements that increase the risk of AD and to find interventions to reduce or prevent the disease. Epidemiological data suggest a higher prevalence of AD in wealthy industrialized countries compared to developing countries [55][56][57][58]. This is believed to be attributed to stringent hygiene practices in developed countries, which limit exposure to beneficial microbes, impacting the education of the host immune system [51][59]. The cause of allergic diseases, including AD, is largely associated with abnormal immunological activation and reactivity, particularly in relation to early microbial exposure. This is especially relevant given the high prevalence of AD in younger age groups. Additionally, certain genes involved in the immune system may influence prenatal and neonatal interaction with intestinal bacteria [60]. The development of an individual’s gut flora, which influences susceptibility to AD, primarily occurs during infancy and early childhood. The gut microbiome may play a critical role in AD development by regulating the maturation of the immune system through interactions with the host, especially during infancy and early childhood [61][62][63]. Studies have reported increased abundance of Clostridium difficile, Escherichia coli, and Staphylococcus aureus (S. aureus), as well as decreased abundance of Bifidobacteria and Bacteroides, in the gut microbiome of AD patients compared to healthy controls (Figure 1). For instance, Watanabe et al. found significantly lower levels of Bifidobacteria in AD patients compared to healthy individuals [38]. Moreover, the abundance of bifidobacteria varies according to disease severity, with severe AD patients having lower levels compared to those with moderate atopy. A recent study by Fieten et al. identified a microbial signature that distinguishes between AD children with and without food allergies. AD children with food allergies showed increased levels of Bifidobacterium pseudocatenulatum (B. pseudocatenulatum) and Escherichia coli in their feces, but decreased levels of Bifidobacterium adolescentis (B. adolescentis), Bifidobacterium breve (B. breve), Faecalibacterium prausnitzii (F. prausnitzii), and Akkermansia muciniphila [40]. Additionally, several cohort studies have provided evidence that disrupted gut flora may precede the development of AD. Infants with mild AD or healthy infants have been found to have a higher prevalence of butyrate-producing bacteria such as Coprococcus eutactus compared to infants with severe AD [64]. Coprococcus are bacteria that produce SCFAs, along with Bifidobacterium, Blautia, Eubacterium, and Propionibacterium, all of which have been detected in lower amounts in individuals with AD compared to those without AD [65]. SCFAs such as butyrate, propionate, and acetate are critical for maintaining host health by exerting anti-inflammatory effects through multiple mechanisms, including the maintenance of the mucus layer and epithelial integrity [66]. Therefore, decreased production of SCFAs may be responsible for the observed intestinal barrier breakdown, increased intestinal permeability, and inflammation in AD [67]. In the large intestine of healthy humans, acetate is produced by Bifidobacteria and certain Firmicutes and Bacteroidetes species [68], while propionate is produced by Bacteroidetes, Negativicutes (Firmicutes strain), and Lachnospiraceae [69]. Butyrate is produced by Faecalibacterium prausnitzii, Eubacterium rectale, Eubacterium hallii [70]. Reduced species diversity of the intestinal flora and delayed colonization with Bacteroidetes have also been associated with AD, particularly in infants at one month of age [63]. According to the concept of “microbial deprivation syndromes of affluence”, lower intensity and diversity of microbial stimulation during early childhood inhibit Th1 induction and Th2 suppression [71]. These results suggest a possible link between AD and a Th2-type immune response to skin allergens triggered by a dysbiotic gut microbiota, dysregulated gut inflammation, and a disrupted epithelial barrier. The gut–skin axis, which refers to the influence of gut flora on bacteria residing on the skin, plays a crucial role in AD. Although the exact mechanism remains unclear, researchers suspect that an imbalanced microbiota contributes to the inflammation and immunological responses observed in AD. Fecal microbiota transplantation (FMT) is the preferred approach for restoring the balance of the gut microbiota compared to probiotics. This is because FMT involves transferring the entire functional community of the gut microbiota from healthy donors to recipients [72]. FMT has shown effectiveness in treating various clinical conditions, including gastroenterological, metabolic, and autoimmune diseases [73]. Limited evidence from experimental studies in both humans and mice suggests that FMT can restore the gut microbiota and immune balance (Th1/Th2) while also suppressing allergic reactions associated with AD [74][75][76]. As a result, FMT holds promise as a potential new therapy for AD. Additionally, there is evidence that dietary interventions, such as probiotics and prebiotics, can modulate the gut microbiome and improve symptoms in individuals with AD [77][78]. In AD, there is a hypothesis of molecular mimicry occurring between environmental allergens, such as pollen or dust mites, and self-antigens in the skin [79][80]. For instance, the protein profilaggrin, vital for maintaining the integrity of the skin barrier, has been identified as a potential self-antigen that the immune system of individuals with AD may target [81]. Studies have shown that allergens, such as dust mites and pollen, contain molecules that are structurally similar to profilaggrin and other skin proteins [82][83]. When these allergens come into contact with the skin, they can trigger an immune response against both the allergen and the self-antigen, leading to inflammation and tissue damage [84].
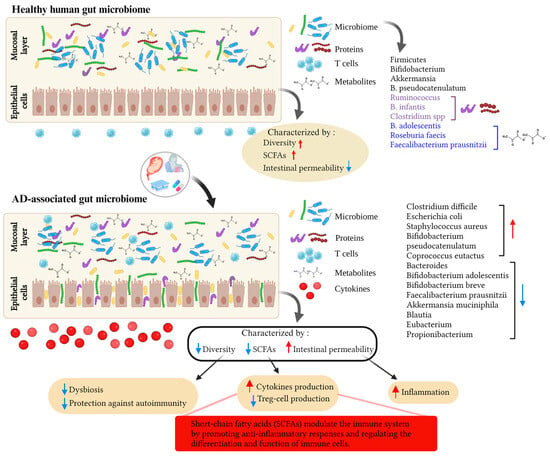
Figure 1. This figure illustrates the effects of environmental factors on the gut microbiota and their potential role in the development of atopic dermatitis (AD). Various environmental factors, including diet, hygiene, allergen exposure, and antibiotic use, can influence the composition and diversity of the gut microbiota. Alterations in the composition of the gut microbiota can disrupt gut barrier function and allow translocation of microbial metabolites and activation of the immune system. These processes contribute to the pathogenesis of AD and highlight the complex interplay of environmental factors, gut microbiota, and AD development. The red arrow pointing up means an increase, the blue arrow pointing down means a decrease. Created with BioRender.com.
3.3. Gut Virome, Mycobiome, and AD
The gut virome plays a crucial role in maintaining a healthy immune system by modulating the production of cytokines, which are important signaling molecules involved in inflammation and immune responses. In a recent study by Xiang et al. [13], the composition of the gut virome in 21 AD patients and 12 healthy controls was examined to better understand the effects of the gut microenvironment on AD. The results of this study did not reveal a clear pattern distinguishing the characteristics of the viral intestinal community between the two groups. Regarding diversity, the study confirmed that the alpha diversity of the AD patient group was significantly lower than that of the healthy control group, and the beta diversity showed significant differences between the groups. Gut mycobiomes in AD were investigated in a unique study by Mok and colleagues [85] in a group of 9- to 12-month-old infants who were divided into two groups based on their symptoms (cured or still experiencing symptoms). The authors employed metagenomic and metaproteomic approaches and concluded that mycobiome diversity was higher in the group still experiencing symptoms. Infants with gastrointestinal dysbiosis (GI) showed an increase in Rhodotorula sp. and a decrease in the Ascomycota/Basidiomycota ratio, whereas Wickerhamomyces and Kodamaea species increased significantly in the healthy group. Interestingly, microorganisms of the genera Acremonium and Rhizopus were more abundant in the healthy group. Additionally, the authors identified five fungi as biomarkers for each sample group and utilized a metaproteomic approach to determine that the diseased cohort had a greater amount of fungal proteins, with Rhodotorula sp. being the major producer. Moreover, this yeast fungus, which is widely distributed in the environment, produces two unique proteins, RAN-binding protein 1 and glycerol kinase, which are specific to infants with AD, suggesting their potential involvement in the development of this syndrome. However, further studies are needed to fully understand the role of the gut mycobiome in AD, including its interactions with the gut microbiome and the immune system.
References
- Weidinger, S.; Beck, L.A.; Bieber, T.; Kabashima, K.; Irvine, A.D. Atopic dermatitis. Nat. Rev. Dis. Primers 2018, 4, 1.
- Eichenfield, L.F.; Tom, W.L.; Berger, T.G.; Krol, A.; Paller, A.S.; Schwarzenberger, K.; Bergman, J.N.; Chamlin, S.L.; Cohen, D.E.; Cooper, K.D.; et al. Guidelines of care for the management of atopic dermatitis: Section 2. Management and treatment of atopic dermatitis with topical therapies. J. Am. Acad. Dermatol. 2014, 71, 116–132.
- Margolis, D.J.; Gupta, J.; Apter, A.J.; Ganguly, T.; Hoffstad, O.; Papadopoulos, M.; Rebbeck, T.R.; Mitra, N. Filaggrin-2 variation is associated with more persistent atopic dermatitis in African American subjects. J. Allergy Clin. Immunol. 2014, 133, 784–789.
- Wollenberg, A.; Barbarot, S.; Bieber, T.; Christen-Zaech, S.; Deleuran, M.; Fink-Wagner, A.; Gieler, U.; Girolomoni, G.; Lau, S.; Muraro, A.; et al. Consensus-based European guidelines for treatment of atopic eczema (atopic dermatitis) in adults and children: Part I. J. Eur. Acad. Dermatol. Venereol. JEADV 2018, 32, 657–682.
- De Bruyn Carlier, T.; Badloe, F.M.S.; Ring, J.; Gutermuth, J.; Kortekaas Krohn, I. Autoreactive T cells and their role in atopic dermatitis. J. Autoimmun. 2021, 120, 102634.
- Chen, W.; Mempel, M.; Schober, W.; Behrendt, H.; Ring, J. Gender difference, sex hormones, and immediate type hypersensitivity reactions. Allergy 2008, 63, 1418–1427.
- Hadi, H.A.; Tarmizi, A.I.; Khalid, K.A.; Gajdács, M.; Aslam, A.; Jamshed, S. The Epidemiology and Global Burden of Atopic Dermatitis: A Narrative Review. Life 2021, 11, 936.
- Bylund, S.; Kobyletzki, L.B.; Svalstedt, M.; Svensson, A. Prevalence and Incidence of Atopic Dermatitis: A Systematic Review. Acta Derm. Venereol. 2020, 100, adv00160.
- Nedoszytko, B.; Reszka, E.; Gutowska-Owsiak, D.; Trzeciak, M.; Lange, M.; Jarczak, J.; Niedoszytko, M.; Jablonska, E.; Romantowski, J.; Strapagiel, D.; et al. Genetic and Epigenetic Aspects of Atopic Dermatitis. Int. J. Mol. Sci. 2020, 21, 6484.
- Martin, M.J.; Estravis, M.; Garcia-Sanchez, A.; Davila, I.; Isidoro-Garcia, M.; Sanz, C. Genetics and Epigenetics of Atopic Dermatitis: An Updated Systematic Review. Genes 2020, 11, 442.
- de Sousa, T.R.; Fagundes, B.O.; Nascimento, A.; Fernandes, L.A.; Sgnotto, F.d.R.; Orfali, R.L.; Aoki, V.; Duarte, A.J.d.S.; Sanabani, S.S.; Victor, J.R. IgG from Adult Atopic Dermatitis (AD) Patients Induces Thymic IL-22 Production and CLA Expression on CD4+ T Cells: Possible Epigenetic Implications Mediated by miRNA. Int. J. Mol. Sci. 2022, 23, 6867.
- Abreu, D.; Kim, B.S. Innate Immune Regulation of Dermatitis. Immunol. Allergy Clin. N. Am. 2021, 41, 347–359.
- Lu, X.; Wang, H.; Zhang, J.; Jin, K.; Ma, L.; Wang, Y.; Yang, S.; Wang, X.; Shen, Q.; Zhou, T.; et al. Comparison of Gut Viral Communities in Atopic Dermatitis and Healthy Children. Front. Med. 2022, 9, 835467.
- Kantor, R.; Silverberg, J.I. Environmental risk factors and their role in the management of atopic dermatitis. Expert Rev. Clin. Immunol. 2017, 13, 15–26.
- Alves, E.; Gregorio, J.; Rijo, P.; Rosado, C.; Monteiro Rodrigues, L. Kefir and the Gut–skin Axis. Int. J. Environ. Res. Public Health 2022, 19, 13791.
- Liu, Y.; Sun, S.; Zhang, D.; Li, W.; Duan, Z.; Lu, S. Effects of Residential Environment and Lifestyle on Atopic Eczema Among Preschool Children in Shenzhen, China. Front. Public Health 2022, 10, 844832.
- Kang, M.-J.; Lee, S.-Y.; Park, Y.-M.; Kim, B.-S.; Lee, M.-J.; Kim, J.-H.; Jeong, S.; Lee, S.-H.; Park, M.J.; Rhee, E.-S.; et al. Interactions Between IL-17 Variants and Streptococcus in the Gut Contribute to the Development of Atopic Dermatitis in Infancy. Allergy Asthma Immunol. Res. 2021, 13, 404–419.
- Diepgen, T. Epidemiology and job-related problems for the eczema patient. Acta Derm. Venereol. Suppl. 2005, 85, 41–44.
- Esparza-Gordillo, J.; Weidinger, S.; Folster-Holst, R.; Bauerfeind, A.; Ruschendorf, F.; Patone, G.; Rohde, K.; Marenholz, I.; Schulz, F.; Kerscher, T.; et al. A common variant on chromosome 11q13 is associated with atopic dermatitis. Nat. Genet. 2009, 41, 596–601.
- Sun, L.-D.; Xiao, F.-L.; Li, Y.; Zhou, W.-M.; Tang, H.-Y.; Tang, X.-F.; Zhang, H.; Schaarschmidt, H.; Zuo, X.-B.; Foelster-Holst, R.; et al. Genome-wide association study identifies two new susceptibility loci for atopic dermatitis in the Chinese Han population. Nat. Genet. 2011, 43, 690–694.
- Paternoster, L.; Standl, M.; Chen, C.M.; Ramasamy, A.; Bonnelykke, K.; Duijts, L.; Ferreira, M.A.; Alves, A.C.; Thyssen, J.P.; Albrecht, E.; et al. Meta-analysis of genome-wide association studies identifies three new risk loci for atopic dermatitis. Nat. Genet. 2011, 44, 187–192.
- EArly Genetics and Lifecourse Epidemiology (EAGLE) Eczema Consortium. Multi-ancestry genome-wide association study of 21,000 cases and 95,000 controls identifies new risk loci for atopic dermatitis. Nat. Genet. 2015, 47, 1449–1456.
- Esparza-Gordillo, J.; Schaarschmidt, H.; Liang, L.; Cookson, W.; Bauerfeind, A.; Lee-Kirsch, M.A.; Nemat, K.; Henderson, J.; Paternoster, L.; Harper, J.I.; et al. A functional IL-6 receptor (IL6R) variant is a risk factor for persistent atopic dermatitis. J. Allergy Clin. Immunol. 2013, 132, 371–377.
- van Beijsterveldt, C.E.; Boomsma, D.I. Genetics of parentally reported asthma, eczema and rhinitis in 5-yr-old twins. Eur. Respir. J. 2007, 29, 516–521.
- Turnbaugh, P.J.; Ley, R.E.; Hamady, M.; Fraser-Liggett, C.M.; Knight, R.; Gordon, J.I. The human microbiome project. Nature 2007, 449, 804–810.
- Shreiner, A.B.; Kao, J.Y.; Young, V.B. The gut microbiome in health and in disease. Curr. Opin. Gastroenterol. 2015, 31, 69–75.
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. What is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms 2019, 7, 14.
- Canfora, E.E.; Blaak, E.E. Acetate: A diet-derived key metabolite in energy metabolism: Good or bad in context of obesity and glucose homeostasis? Curr. Opin. Clin. Nutr. Metab. Care 2017, 20, 477–483.
- Landman, C.; Quevrain, E. Gut microbiota: Description, role and pathophysiologic implications. La Rev. De Med. Interne 2016, 37, 418–423.
- Cho, I.; Blaser, M.J. The human microbiome: At the interface of health and disease. Nat. Rev. Genet. 2012, 13, 260–270.
- Wells, J.M.; Rossi, O.; Meijerink, M.; van Baarlen, P. Epithelial crosstalk at the microbiota-mucosal interface. Proc. Natl. Acad. Sci. USA 2011, 108 (Suppl. 1), 4607–4614.
- Smith, P.M.; Howitt, M.R.; Panikov, N.; Michaud, M.; Gallini, C.A.; Bohlooly-Y, M.; Glickman, J.N.; Garrett, W.S. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science 2013, 341, 569–573.
- Zhernakova, A.; Kurilshikov, A.; Bonder, M.J.; Tigchelaar, E.F.; Schirmer, M.; Vatanen, T.; Mujagic, Z.; Vila, A.V.; Falony, G.; Vieira-Silva, S.; et al. Population-based metagenomics analysis reveals markers for gut microbiome composition and diversity. Science 2016, 352, 565–569.
- Saffouri, G.B.; Shields-Cutler, R.R.; Chen, J.; Yang, Y.; Lekatz, H.R.; Hale, V.L.; Cho, J.M.; Battaglioli, E.J.; Bhattarai, Y.; Thompson, K.J.; et al. Small intestinal microbial dysbiosis underlies symptoms associated with functional gastrointestinal disorders. Nat. Commun. 2019, 10, 2012.
- Emoto, T.; Yamashita, T.; Sasaki, N.; Hirota, Y.; Hayashi, T.; So, A.; Kasahara, K.; Yodoi, K.; Matsumoto, T.; Mizoguchi, T.; et al. Analysis of Gut Microbiota in Coronary Artery Disease Patients: A Possible Link between Gut Microbiota and Coronary Artery Disease. J. Atheroscler. Thromb. 2016, 23, 908–921.
- Islam, F.; Mitra, S.; Nafady, M.H.; Rahman, M.T.; Tirth, V.; Akter, A.; Emran, T.B.; Mohamed, A.A.; Algahtani, A.; El-Kholy, S.S. Neuropharmacological and Antidiabetic Potential of Lannea coromandelica (Houtt.) Merr. Leaves Extract: An Experimental Analysis. Evid. Based Complement. Altern. Med. Ecam 2022, 2022, 6144733.
- Turnbaugh, P.J.; Ley, R.E.; Mahowald, M.A.; Magrini, V.; Mardis, E.R.; Gordon, J.I. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006, 444, 1027–1031.
- Watanabe, S.; Narisawa, Y.; Arase, S.; Okamatsu, H.; Ikenaga, T.; Tajiri, Y.; Kumemura, M. Differences in fecal microflora between patients with atopic dermatitis and healthy control subjects. J. Allergy Clin. Immunol. 2003, 111, 587–591.
- Song, H.; Yoo, Y.; Hwang, J.; Na, Y.C.; Kim, H.S. Faecalibacterium prausnitzii subspecies–level dysbiosis in the human gut microbiome underlying atopic dermatitis. J. Allergy Clin. Immunol. 2016, 137, 852–860.
- Fieten, K.B.; Totte, J.E.E.; Levin, E.; Reyman, M.; Meijer, Y.; Knulst, A.; Schuren, F.; Pasmans, S. Fecal Microbiome and Food Allergy in Pediatric Atopic Dermatitis: A Cross-Sectional Pilot Study. Int. Arch. Allergy Immunol. 2018, 175, 77–84.
- Dominguez-Bello, M.G.; Costello, E.K.; Contreras, M.; Magris, M.; Hidalgo, G.; Fierer, N.; Knight, R. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc. Natl. Acad. Sci. USA 2010, 107, 11971–11975.
- Shao, Y.; Forster, S.C.; Tsaliki, E.; Vervier, K.; Strang, A.; Simpson, N.; Kumar, N.; Stares, M.D.; Rodger, A.; Brocklehurst, P.; et al. Stunted microbiota and opportunistic pathogen colonization in caesarean-section birth. Nature 2019, 574, 117–121.
- Jakobsson, H.E.; Abrahamsson, T.R.; Jenmalm, M.C.; Harris, K.; Quince, C.; Jernberg, C.; Bjorksten, B.; Engstrand, L.; Andersson, A.F. Decreased gut microbiota diversity, delayed Bacteroidetes colonisation and reduced Th1 responses in infants delivered by caesarean section. Gut 2014, 63, 559–566.
- Arboleya, S.; Suarez, M.; Fernandez, N.; Mantecon, L.; Solis, G.; Gueimonde, M.; de Los Reyes-Gavilan, C.G. C-section and the Neonatal Gut Microbiome Acquisition: Consequences for Future Health. Ann. Nutr. Metab. 2018, 73 (Suppl. 3), 17–23.
- Negele, K.; Heinrich, J.; Borte, M.; von Berg, A.; Schaaf, B.; Lehmann, I.; Wichmann, H.E.; Bolte, G.; for the LISA Study Group. Mode of delivery and development of atopic disease during the first 2 years of life. Pediatr. Allergy Immunol. Off. Publ. Eur. Soc. Pediatr. Allergy Immunol. 2004, 15, 48–54.
- Debley, J.S.; Smith, J.M.; Redding, G.J.; Critchlow, C.W. Childhood asthma hospitalization risk after cesarean delivery in former term and premature infants. Ann. Allergy Asthma Immunol. Off. Publ. Am. Coll. Allergy Asthma Immunol. 2005, 94, 228–233.
- Laubereau, B.; Filipiak-Pittroff, B.; von Berg, A.; Grubl, A.; Reinhardt, D.; Wichmann, H.E.; Koletzko, S.; for the GINI Study Group. Caesarean section and gastrointestinal symptoms, atopic dermatitis, and sensitisation during the first year of life. Arch. Dis. Child. 2004, 89, 993–997.
- Mack, M.R.; Brestoff, J.R.; Berrien-Elliott, M.M.; Trier, A.M.; Yang, T.B.; McCullen, M.; Collins, P.L.; Niu, H.; Bodet, N.D.; Wagner, J.A.; et al. Blood natural killer cell deficiency reveals an immunotherapy strategy for atopic dermatitis. Sci. Transl. Med. 2020, 12, eaay1005.
- Mubanga, M.; Lundholm, C.; D’Onofrio, B.M.; Stratmann, M.; Hedman, A.; Almqvist, C. Association of Early Life Exposure to Antibiotics With Risk of Atopic Dermatitis in Sweden. JAMA Netw. Open 2021, 4, e215245.
- Apfelbacher, C.J.; Diepgen, T.L.; Schmitt, J. Determinants of eczema: Population-based cross-sectional study in Germany. Allergy 2011, 66, 206–213.
- Schultz Larsen, F. Atopic dermatitis: A genetic-epidemiologic study in a population-based twin sample. J. Am. Acad. Dermatol. 1993, 28, 719–723.
- Loset, M.; Brown, S.J.; Saunes, M.; Hveem, K. Genetics of Atopic Dermatitis: From DNA Sequence to Clinical Relevance. Dermatology 2019, 235, 355–364.
- Lee, J.-Y.; Seo, J.-H.; Kwon, J.-W.; Yu, J.; Kim, B.-J.; Lee, S.-Y.; Kim, H.-B.; Kim, W.-K.; Kim, K.-W.; Shin, Y.-J.; et al. Exposure to gene-environment interactions before 1 year of age may favor the development of atopic dermatitis. Int. Arch. Allergy Immunol. 2012, 157, 363–371.
- Jedrychowski, W.; Perera, F.; Maugeri, U.; Mrozek-Budzyn, D.; Miller, R.L.; Flak, E.; Mroz, E.; Jacek, R.; Spengler, J.D. Effects of prenatal and perinatal exposure to fine air pollutants and maternal fish consumption on the occurrence of infantile eczema. Int. Arch. Allergy Immunol. 2011, 155, 275–281.
- Odhiambo, J.A.; Williams, H.C.; Clayton, T.O.; Robertson, C.F.; Asher, M.I.; ISAAC Phase Three Study Group. Global variations in prevalence of eczema symptoms in children from ISAAC Phase Three. J. Allergy Clin. Immunol. 2009, 124, 1251–1258 e1223.
- Schultz Larsen, F.; Diepgen, T.; Svensson, A. The occurrence of atopic dermatitis in north Europe: An international questionnaire study. J. Am. Acad. Dermatol. 1996, 34, 760–764.
- Belyhun, Y.; Amberbir, A.; Medhin, G.; Erko, B.; Hanlon, C.; Venn, A.; Britton, J.; Davey, G. Prevalence and risk factors of wheeze and eczema in 1-year-old children: The Butajira birth cohort, Ethiopia. Clin. Exp. Allergy J. Br. Soc. Allergy Clin. Immunol. 2010, 40, 619–626.
- Shaw, T.E.; Currie, G.P.; Koudelka, C.W.; Simpson, E.L. Eczema prevalence in the United States: Data from the 2003 National Survey of Children’s Health. J. Investig. Dermatol. 2011, 131, 67–73.
- Harada, Y.; Sakamoto, T.; Shinomura, T.; Takamoto, K.; Senda, T.; Tsuda, M. Total synthesis of a gene for octopus rhodopsin and its preliminary expression. J. Biochem. 1991, 110, 501–507.
- Kim, B.-J.; Lee, S.-Y.; Kim, H.-B.; Lee, E.; Hong, S.-J. Environmental changes, microbiota, and allergic diseases. Allergy Asthma Immunol. Res. 2014, 6, 389–400.
- Wang, M.; Karlsson, C.; Olsson, C.; Adlerberth, I.; Wold, A.E.; Strachan, D.P.; Martricardi, P.M.; Aberg, N.; Perkin, M.R.; Tripodi, S.; et al. Reduced diversity in the early fecal microbiota of infants with atopic eczema. J. Allergy Clin. Immunol. 2008, 121, 129–134.
- Adlerberth, I.; Carlsson, B.; de Man, P.; Jalil, F.; Khan, S.R.; Larsson, P.; Mellander, L.; Svanborg, C.; Wold, A.E.; Hanson, L.A. Intestinal colonization with Enterobacteriaceae in Pakistani and Swedish hospital-delivered infants. Acta Paediatr. 1991, 80, 602–610.
- Abrahamsson, T.R.; Jakobsson, H.E.; Andersson, A.F.; Bjorksten, B.; Engstrand, L.; Jenmalm, M.C. Low diversity of the gut microbiota in infants with atopic eczema. J. Allergy Clin. Immunol. 2012, 129, 434–440.E2.
- Nylund, L.; Nermes, M.; Isolauri, E.; Salminen, S.; de Vos, W.M.; Satokari, R. Severity of atopic disease inversely correlates with intestinal microbiota diversity and butyrate-producing bacteria. Allergy 2015, 70, 241–244.
- Reichardt, N.; Duncan, S.H.; Young, P.; Belenguer, A.; McWilliam Leitch, C.; Scott, K.P.; Flint, H.J.; Louis, P. Phylogenetic distribution of three pathways for propionate production within the human gut microbiota. ISME J. 2014, 8, 1323–1335.
- Macia, L.; Thorburn, A.N.; Binge, L.C.; Marino, E.; Rogers, K.E.; Maslowski, K.M.; Vieira, A.T.; Kranich, J.; Mackay, C.R. Microbial influences on epithelial integrity and immune function as a basis for inflammatory diseases. Immunol. Rev. 2012, 245, 164–176.
- De Benedetto, A.; Rafaels, N.M.; McGirt, L.Y.; Ivanov, A.I.; Georas, S.N.; Cheadle, C.; Berger, A.E.; Zhang, K.; Vidyasagar, S.; Yoshida, T.; et al. Tight junction defects in patients with atopic dermatitis. J. Allergy Clin. Immunol. 2011, 127, 773–786.E7.
- Rey, F.E.; Faith, J.J.; Bain, J.; Muehlbauer, M.J.; Stevens, R.D.; Newgard, C.B.; Gordon, J.I. Dissecting the in vivo metabolic potential of two human gut acetogens. J. Biol. Chem. 2010, 285, 22082–22090.
- Louis, P.; Flint, H.J. Formation of propionate and butyrate by the human colonic microbiota. Environ. Microbiol. 2017, 19, 29–41.
- Morrison, D.J.; Preston, T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes 2016, 7, 189–200.
- Rodriguez, J.M.; Murphy, K.; Stanton, C.; Ross, R.P.; Kober, O.I.; Juge, N.; Avershina, E.; Rudi, K.; Narbad, A.; Jenmalm, M.C.; et al. The composition of the gut microbiota throughout life, with an emphasis on early life. Microb. Ecol. Health Dis. 2015, 26, 26050.
- Suez, J.; Zmora, N.; Zilberman-Schapira, G.; Mor, U.; Dori-Bachash, M.; Bashiardes, S.; Zur, M.; Regev-Lehavi, D.; Ben-Zeev Brik, R.; Federici, S.; et al. Post-Antibiotic Gut Mucosal Microbiome Reconstitution Is Impaired by Probiotics and Improved by Autologous FMT. Cell 2018, 174, 1406–1423 e1416.
- Qu, Z.; Tian, P.; Yang, B.; Zhao, J.; Wang, G.; Chen, W. Fecal microbiota transplantation for diseases: Therapeutic potential, methodology, risk management in clinical practice. Life Sci. 2022, 304, 120719.
- Mashiah, J.; Karady, T.; Fliss-Isakov, N.; Sprecher, E.; Slodownik, D.; Artzi, O.; Samuelov, L.; Ellenbogen, E.; Godneva, A.; Segal, E.; et al. Clinical efficacy of fecal microbial transplantation treatment in adults with moderate-to-severe atopic dermatitis. Immun. Inflamm. Dis. 2022, 10, e570.
- Kim, J.H.; Kim, K.; Kim, W. Gut microbiota restoration through fecal microbiota transplantation: A new atopic dermatitis therapy. Exp. Mol. Med. 2021, 53, 907–916.
- Jiang, X.; Liu, Z.; Ma, Y.; Miao, L.; Zhao, K.; Wang, D.; Wang, M.; Ruan, H.; Xu, F.; Zhou, Q.; et al. Fecal microbiota transplantation affects the recovery of AD-skin lesions and enhances gut microbiota homeostasis. Int. Immunopharmacol. 2023, 118, 110005.
- Moro, G.; Arslanoglu, S.; Stahl, B.; Jelinek, J.; Wahn, U.; Boehm, G. A mixture of prebiotic oligosaccharides reduces the incidence of atopic dermatitis during the first six months of age. Arch. Dis. Child. 2006, 91, 814–819.
- Weston, S.; Halbert, A.; Richmond, P.; Prescott, S.L. Effects of probiotics on atopic dermatitis: A randomised controlled trial. Arch. Dis. Child. 2005, 90, 892–897.
- Valenta, R.; Natter, S.; Seiberler, S.; Roschanak, M.; Mothes, N.; Mahler, V.; Eibensteiner, P. Autoallergy: A pathogenetic factor in atopic dermatitis? Curr. Probl. Dermatol. 1999, 28, 45–50.
- Schmid-Grendelmeier, P.; Fluckiger, S.; Disch, R.; Trautmann, A.; Wuthrich, B.; Blaser, K.; Scheynius, A.; Crameri, R. IgE-mediated and T cell-mediated autoimmunity against manganese superoxide dismutase in atopic dermatitis. J. Allergy Clin. Immunol. 2005, 115, 1068–1075.
- Kato, A.; Fukai, K.; Oiso, N.; Hosomi, N.; Murakami, T.; Ishii, M. Association of SPINK5 gene polymorphisms with atopic dermatitis in the Japanese population. Br. J. Dermatol. 2003, 148, 665–669.
- Zhang, J.; Chen, J.; Newton, G.K.; Perrior, T.R.; Robinson, C. Allergen Delivery Inhibitors: A Rationale for Targeting Sentinel Innate Immune Signaling of Group 1 House Dust Mite Allergens through Structure-Based Protease Inhibitor Design. Mol. Pharmacol. 2018, 94, 1007–1030.
- Chapman, M.D.; Pomes, A.; Breiteneder, H.; Ferreira, F. Nomenclature and structural biology of allergens. J. Allergy Clin. Immunol. 2007, 119, 414–420.
- Galli, S.J.; Tsai, M.; Piliponsky, A.M. The development of allergic inflammation. Nature 2008, 454, 445–454.
- Mok, K.; Suratanon, N.; Roytrakul, S.; Charoenlappanit, S.; Patumcharoenpol, P.; Chatchatee, P.; Vongsangnak, W.; Nakphaichit, M. ITS2 Sequencing and Targeted Meta-Proteomics of Infant Gut Mycobiome Reveal the Functional Role of Rhodotorula sp. during Atopic Dermatitis Manifestation. J. Fungi 2021, 7, 748.
More
Information
Subjects:
Dermatology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
574
Revisions:
2 times
(View History)
Update Date:
10 Oct 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




