Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Xuefeng Liu | -- | 3998 | 2023-10-07 19:39:34 | | | |
| 2 | Jason Zhu | -1 word(s) | 3997 | 2023-10-08 04:07:30 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Daneshdoust, D.; Luo, M.; Li, Z.; Mo, X.; Alothman, S.; Kallakury, B.; Schlegel, R.; Zhang, J.; Guo, D.; Furth, P.A.; et al. Applications of Conditional Reprogramming in Breast Cancer Research. Encyclopedia. Available online: https://encyclopedia.pub/entry/49920 (accessed on 07 February 2026).
Daneshdoust D, Luo M, Li Z, Mo X, Alothman S, Kallakury B, et al. Applications of Conditional Reprogramming in Breast Cancer Research. Encyclopedia. Available at: https://encyclopedia.pub/entry/49920. Accessed February 07, 2026.
Daneshdoust, Danyal, Mingjue Luo, Zaibo Li, Xiaokui Mo, Sahar Alothman, Bhaskar Kallakury, Richard Schlegel, Junran Zhang, Deliang Guo, Priscilla A. Furth, et al. "Applications of Conditional Reprogramming in Breast Cancer Research" Encyclopedia, https://encyclopedia.pub/entry/49920 (accessed February 07, 2026).
Daneshdoust, D., Luo, M., Li, Z., Mo, X., Alothman, S., Kallakury, B., Schlegel, R., Zhang, J., Guo, D., Furth, P.A., Liu, X., & Li, J. (2023, October 07). Applications of Conditional Reprogramming in Breast Cancer Research. In Encyclopedia. https://encyclopedia.pub/entry/49920
Daneshdoust, Danyal, et al. "Applications of Conditional Reprogramming in Breast Cancer Research." Encyclopedia. Web. 07 October, 2023.
Copy Citation
Preclinical in vitro models play an important role in studying cancer cell biology and facilitating translational research, especially in the identification of drug targets and drug discovery studies. This is particularly relevant in breast cancer, where the global burden of disease is quite high based on prevalence and a relatively high rate of lethality. Predictive tools to select patients who will be responsive to invasive or morbid therapies (radiotherapy, chemotherapy, immunotherapy, and/or surgery) are relatively lacking. To be clinically relevant, a model must accurately replicate the biology and cellular heterogeneity of the primary tumor.
conditionally reprogrammed cells
breast cancer
precision medicine
1. Breast Cancer and Clinical Challenges
Breast cancer is the second most common type of cancer among women in the world, with an estimated 2.3 million new cases accounting for 11.7% of all cancer cases and approximately 700,000 deaths worldwide [1][2]. In the United States alone, more than 300,500 new cases and 43,700 deaths are reported annually [3]. Breast cancer is classified into four major molecular subtypes, namely luminal A (Estrogen Receptor (ER) and/or progesterone receptor (PR)-positive, HER2-negative, Ki-67<14%), luminal B ([luminal B (HER2-negative): ER and/or PR-positive, HER2-negative, Ki-67 >14%] [luminal B (HER2-positive): ER and/or PR-positive, HER2-over expressed or amplified, any Ki-67), HER2-enriched (ER/PR-negative, HER2-positive), and basal/triple negative (ER/PR/HER2-negative) based on human epidermal growth factor receptor-2 (HER2) receptor and hormonal status [4][5]. The management of breast cancer patients relies on the assessment of hormone receptor status, specifically PR, ER, and HER2 [6][7]. Hormonal therapies have proven effective for the majority of patients with hormone receptor-positive subtypes. For instance, selective estrogen modulators (SERMs) such as tamoxifen or selective estrogen receptor degraders (SERDs) such as fulvestrant, or aromatase inhibitors, sometimes in conjunction with CDK4/6 inhibitors, are used for ER-positive breast cancer, while the combination of Trastuzumab (Herceptin), Docetaxel (Taxotere), and Pertuzumab (Perjeta) can be employed for HER2-positive breast cancer [8][9][10][11]. Nevertheless, addressing the issue of both primary and acquired resistance to hormonal therapies continues to be a challenge [12]. Given the significant burden of cancer, researchers worldwide continue to strive toward understanding tumor growth and treatment outcomes in breast cancer using cutting-edge approaches.
A major obstacle hindering cancer research progress is the limited availability of cancer models [13]. Cancer cell lines have been widely used as effective models for drug discovery and preclinical studies. However, depending on the type and stage of the disease, the success rate for producing cancer cell lines is as low as 1–10% [14]. Although the number of available cell models has been increasing, they are still not enough to effectively study various types of cancers. Moreover, traditional cancer cell lines have a limitation in replicating the intricate heterogeneity of primary tumors, which considerably restricts the advancement of basic and translational medicine [15]. Animal models are widely used in laboratory cancer research and have made significant contributions to understanding of the biology of cancers [16]. Additionally, they have played a crucial role in preclinical studies of different types of cancers. In breast cancer, these models can take various forms, such as based on chemical carcinogenesis, genetically modified animals, xenograft models, syngeneic models, and other approaches and tools [17][18][19]. Animal models are frequently utilized in cancer research as a stand-in for humans. However, due to variations in genetics and biology between different species, the translation of experimental findings from animal models to clinical practice can be slow [20]. Creating feasible and innovative models for translational medicine and cancer research is crucial to tackling these challenges.
2. Modeling Diseases
Efficient and rapid establishment of CRCs has been achieved using various human normal and tumor specimens, including the breast, without the need for exogenous viruses or genetic manipulation. These CRCs can preserve the characteristics of their primary tissues. By removing these conditions, the cells’ differentiation ability can be restored. They also can be cultured in both 2D and 3D systems. Consequently, CR can serve as an ideal in vitro model for studying breast cancer. Mahajan et al. applied CR technology to conduct a comparative analysis between early-passage conditionally reprogrammed breast cancer cells and their corresponding primary tumors [21]. They evaluated the genomic characteristics of six newly established CRC cultures derived from invasive breast cancer and compared them to the original primary breast tumors. Simultaneous profiling of CRCs and their corresponding primary breast tumors was conducted using targeted next-generation sequencing, genome-wide array-CGH, and global miRNA expression analysis. This comprehensive approach aimed to determine the molecular similarities between the two in terms of gene mutations, copy number alterations (CNAs), and miRNA expression levels. Using flow cytometry, they also evaluated the ploidy and amount of epithelial cells in the CRCs. The study findings indicated that the CRCs retained the overall genomic signatures of the original primary breast tumor and demonstrated a similar pattern and level of CNAs. The array-CGH analysis showed a high level of overlap, ranging from 72 to 100%, between the CRCs and their corresponding primary breast tumors. Notably, the cytobands commonly affected by CNAs displayed more than 95% overlap between each CRC and its corresponding primary breast tumor. Furthermore, the copy number profiles of these CRCs exhibited non-random and recurrent CNAs that are typically associated with specific intrinsic subtypes of breast cancer. The analysis using targeted next-generation sequencing also showed that the established CRCs maintained the particular gene alterations that had been found in their original tumors. Analysis of three paired CRCs and primary breast tumors (Cases 2, 4, and 6) revealed that they share the same type of variants affecting the FLT3, TP53, CDKN2A, PIK3CA, KDR, and JAK3 genes. In the unpaired CRC (Case 3) that was sequenced, variants in the TP53, CDKN2A, KDR, and JAK3 genes were observed. Specifically, the same variant in the TP53 gene resulting in a codon change (cCc/cGc) and amino acid alteration (P72R) was detected in this CRC, similar to the other CRCs and their corresponding primary breast tumors. In summary, Mahajan and colleagues demonstrated that the breast cancer CRCs analyzed in their study preserved the overall gene mutations, copy number, and miRNA expression patterns of the respective tumor tissue they were derived from [21].
Ductal carcinoma in situ (DCIS) is a non-invasive form of breast cancer, and currently, there are no reliable predictors to determine its progression into an invasive disease. Therefore, the majority of patients undergo radiation and/or hormone therapy following surgical removal, often resulting in overtreatment. Two commercially available cell lines for studying DCIS, namely SUM225CWN and MCF10DCIS.COM, are not ideal due to their origins that are not derived directly from primary DCIS tumors [22][23][24][25][26]. Five cell lines were generated from a patient in Singapore. These cell lines underwent genetic manipulation through hTERT transfection, a process that enhances their lifespan, but they are not commercially available [26].
Brown et al. sought to develop new models of DCIS by culturing primary DCIS tissue from patients following lumpectomy or mastectomy [27]. Following mechanical and enzymatic dissociation, primary DCIS cells were cultured from 19 patients using CR technology. The resulting cultures consisted predominantly of cytokeratin 8- and EpCAM-positive luminal, as well as cytokeratin 5-, cytokeratin 14-, and p63-positive basal mammary epithelial cells. This composition suggests the maintenance of cellular heterogeneity in the in vitro culture system. Moreover, the cellular identities of these cells were preserved both during the ‘conditionally reprogrammed’ proliferative state and after the withdrawal of conditioned media and the ROCK inhibitor, as indicated by the expression of basal and luminal markers [27].
3. Precision Medicine and Drug Discovery
Precision medicine is a recently developed strategy for characterizing malignancies biologically and is seen as a new frontier in the treatment and prevention of cancer. Precision medicine has a wide range of applications, including those for prevention, diagnosis, prognosis, monitoring treatment response, and early treatment resistance discovery. Precision medicine aims to do away with the “one size fits all” approach to managing cancer patients. Genetic analysis has recently revolutionized how different malignancies are classified and treated. Precision medicine is built on targeted therapy, a therapeutic approach that uses drugs to specifically target proteins and genes linked to the growth and survival of tumor cells [28]. Due to the lack of suitable in vitro models for breast cancer, this is a major concern in studying drug resistance and treatment response. A specific limitation in recognizing effective drugs for breast cancer is that the results are only based on studies in long-term cultured cell lines or xenograft models; thus, the consequences of most clinical research are usually unsatisfactory. At present, the use of patient-derived models (PDMs) with the features of maintained genotype, high immortality, throughput screening, and xenotransplantation is urgently needed for drug screening, drug discovery, and targeted therapy. The CR method can be used in primary cell cultures from normal and tumor tissues of different types of tissues to retain the genotypic and phenotypic characteristics and heterogeneity of the primary species. CR cells can also be used in cultures under 3D conditions and establishment xenografts in animals (zebrafish or mouse).
To investigate heterogeneity and drug sensitivity, Mimoto and colleagues generated CRCs from patients with recurrent HR+/HER2- (hormone receptor-positive/human epidermal receptor 2-negative) breast cancer [29]. In CR cells, the pathological features and mutation status were retained, but the RNA expression was different from the original tumor cells. To investigate heterogeneity and drug sensitivity, Mimoto and colleagues generated CRCs from individuals with recurring HR+/HER2- breast cancer. In CR cells, the pathological features and mutation status were preserved, but the RNA expression was different from the original tumor cells. They also evaluated the response of CR cells obtained from a liver metastasis that was ER+/PgR+/HER2- to a total of 224 drugs. Out of these, 66 drugs demonstrated a decrease in cell viability, which included SERD and CDK4/6 inhibitors. The patient initially received SERD and CDK4/6 inhibitors following mastectomy, and for a duration of 13 months, no recurrence was observed. These findings are consistent with the results obtained from the drug screening performed on the CR cells [29].
The findings highlight the potential application of CR cells for drug sensitivity tests and determining appropriate treatments. Their research represents a key step in innovative clinical tools development to aid in decision-making for patients with metastatic HR+/HER2- breast cancer. Phyllodes tumors of the breast are uncommon tumors that involve both stromal and epithelial components. Surgical removal is the primary recommended treatment for Phyllodes tumors. However, despite complete resection, the malignant form of Phyllodes tumors still carries a high recurrence risk, reaching up to 40%. Furthermore, there is currently no consensus regarding the most effective drugs for treating Phyllodes tumors. Recently, Urbaniak et al. applied CR technology to evaluate the response of phyllodes tumor of the breast to seven drugs namely Salinomycin, Bcl-2/Bcl-xL inhibitor ABT-263, paclitaxel, DOX, colchicine, vincristine, and cisplatin. Of these, ABT-263, Salinomycin, and DOX were found to be highly selective toward phyllodes tumor cells [30]. This revealed the feasibility of using CR technology for drug discovery, selectively targeting phyllodes tumor cells. Based on the metaplastic breast-carcinoma cell line with EGFR amplification from a patient using the CR method, Chung et al. discovered that the combination of EGFR inhibitor and paclitaxel was a promising strategy for metaplastic breast-carcinoma with EGFR amplification [31].
Customizing assays for tumor molecular phenotyping is essential due to variations in the differentiation status of tumors and normal tissues in different patients. In response to this challenge, Anjanappa and colleagues [32] employed a combination of the CR method and single-cell gene expression analysis. This approach aimed to investigate the tumor heterogeneity at an individual patient level. Their study involved 420 tumor cells and 284 adjacent normal cells, focusing on the expression of 93 genes. These genes encompassed the PAM50-intrinsic subtype classifier and genes associated with stemness. Notably, normal and tumor cells marked by ALDH+/CD49f+/EpCAM+ exhibited different clustering compared to unselected normal and tumor cells. Through PAM50 gene-set analysis, they effectively identified both minor and major tumor cell subgroups within the ALDH+/CD49f+/EpCAM+ population. The major clone resembled the tumor’s clinical characteristics. Additionally, by utilizing a gene set linked to stemness, they detected varying activations of stemness pathways within different clones of the same tumor. This refined profiling technique enabled the differentiation between genes truly deregulated in cancer from genes indicating potential precursors of tumor cells. The assays used in their study offer a heightened ability to pinpoint genes deregulated in cancer with greater accuracy [32].
Recent progress in DNA sequencing technology have made it feasible to sequence a considerable number of tumor samples at a reasonable cost. Nonetheless, to enhance its clinical applicability, it is crucial to decrease sequencing errors and identify rare mutations that might exist within a small subset of tumor cells. Tumor complexity and intratumor heterogeneity contribute to the diversity of subclones. Despite advancements in next-generation sequencing, pinpointing low frequency mutations in primary tumors and metastases that contribute to subclonal diversity remains a challenge in precision genomics. To address this, Anjanappa and colleagues [33] employed CR technology for short-term cultivation of epithelial cells derived from primary and metastatic tumors. This approach enabled them to expand minor clones and gather epithelial cell-specific DNA/RNA for quantitative next-generation sequencing analysis. Comparative analysis of DNA from unprocessed breast tumors and tumor cells cultured from the same tumors was carried out using the AmpliSeq Comprehensive Cancer Panel. They revealed previously uncharacterized mutations found exclusively in the cultured tumor cells, with some of these mutations reported in brain metastases but not primary breast tumors. Moreover, whole-genome sequencing highlighted mutations enriched in liver metastases across different cancer types. Notably, Notch pathway mutations and chromosomal inversions were detected in all five liver metastases, regardless of the type of cancer. Mutations and rearrangements in the FHIT gene, involved in purine metabolism, were identified in four out of five liver metastases. Additionally, the same set of four liver metastases shared mutations in 32 genes. Among these were mutations in various HLA-DR family members, impacting the OX40 signaling pathway, potentially influencing the immune response to metastatic cells. Pathway analysis of all mutated genes in liver metastases indicated abnormal signaling of tumor necrosis factor and transforming growth factor in these metastatic cells. CR technology employed in their study indicated an improved capacity for identifying mutations in both primary and metastatic cancer cells [33]. Therefore, CR technology offers a new tool for assessing the toxicity and effectiveness of new drugs and developing personalized treatment strategies for breast cancer.
4. Noninvasive Diagnosis and Surveillance
Early diagnosis is crucial for effective breast cancer treatment and plays an important role in reducing mortality rates [34]. While mammography is considered the gold standard for imaging breast cancer, its limitations in detecting tumors in dense breast tissue necessitate the use of supplementary detection methods. One commonly employed complementary method is ultrasound. However, ultrasound has its limitations as it may not always detect microcalcifications and can potentially miss early signs of tumors [35]. To achieve early detection of breast cancer, it is imperative to utilize fast, simple, and cost-effective blood-based biomarkers in conjunction with mammography. These biomarkers serve as valuable tools in identifying breast cancer at its early stages.
Liquid biopsies are non-invasive and safe methods for tumor detection. These minimally invasive sampling techniques utilize circulating biomarkers to analyze tumors and deliver reliable diagnostic information. Liquid biopsies encompass various components, such as exosomes [36], microRNA (miRNA) [37], circulating tumor cells (CTCs) [38], circulating tumor DNA [39], etc. CTCs are cells that detach from the primary tumor and enter the bloodstream. Some of these cells manage to evade the body’s immune system and undergo a process known as epithelial-mesenchymal transition. These highly invasive processes, including the acquisition of tumor DNA information, genomic data, and proteomic information, allow for the dynamic monitoring of tumor activity.
In 1869, Thomas R. Ashworth first identified CTCs in the blood of a cancer patient through a comparison of CTC morphology with different tumor cells [40]. Despite their discovery dating back approximately 150 years, there was limited research focused on CTCs until the mid-1990s. This can be attributed to the fact that CTCs are exceptionally rare in the bloodstream, with only a few CTCs present among billions of erythrocytes and millions of leukocytes [41][42]. Hence, the detection of CTCs is technically challenging. Jeong et al. applied CR technology to detect CTCs in breast cancer patients [43]. Their objective was to assess the efficacy of the CR system in detecting CTCs in breast cancer. CTCs were isolated from the peripheral blood of breast cancer patients and cultured using the protocol described by Liu et al. [44]. Subsequently, total RNA was extracted from the cultured CTCs, and reverse transcription PCR (RT-PCR) was performed to amplify the MAGE A1-6 and hTERT genes. RNA extraction was also directly extracted from blood samples, and only RT-PCR was used to analyze the expression of these two genes. Following CRC culture, CTC growth was observed in seven out of the samples (23.3% detection rate). The detection rates of CTCs using RT-PCR for the MAGE A1-6 and hTERT genes in CTCs grown through the CRC culture method were 10.0% and 26.7%, respectively. The positive expression rates for the MAGE and hTERT genes in CTCs assessed only by RT-PCR were 23.5% and 44.1%, respectively. By combining the positive expression rates from RT-PCR alone and CRC culture for the MAGE A1-6 and hTERT genes, the CTC detection rates increased to 23.3% and 53.3%, respectively. Furthermore, when the positive expression rates of the two genes were combined using either method, the CTC detection rate reached its highest value [43]. Their study demonstrated the potential of CRC culture in detecting CTCs in breast cancer. Additionally, the combination of CRC culture and RT-PCR for the MAGE A1 6 and hTERT genes proved to be beneficial in enhancing the detection rate of CTCs in the blood.
5. Disparity of Breast Cancer
Researchers recently established a CRC-derived biobank that can be used to explore the genetic diversity and in vitro behavior of high risk non-cancer breast epithelial cells. While age-associated decreased viability impacted collection, Researchers successfully isolated non-cancer high risk ipsilateral breast epithelial cells from a wide range of ages, breast cancer subtypes, and pathological stages [45]. Interestingly, It is found that MYC and ribosome related genes were expressed at significantly higher levels in samples from women identifying as black or African-American. Genetic variations linked to geographically defined ancestry have been hypothesized to contribute to the disparity in breast cancer outcome women who identify as black or African-American experience as compared to women who identify as white or Caucasian [46]. To explore if non-cancer ipsilateral breast cells from women who identify as black as compared to white showed any significant differences in gene expression, DEGs between samples obtained from women who self-identified as black (n = 6) versus white (n = 15) were identified (padj<0.05). One hundred seventy-seven genes were significantly up-regulated and one hundred forty-four genes significantly down-regulated in non-cancer ipsilateral cells from women identifying as black as compared to white (Figure 1A). GSEA analysis of these DEGs showed significant associations with ribosome biology (Figure 1B) and up-regulation of genes associated with MYC (Figure 1C) with MYC itself also higher expressed (padj = 0.052) (Figure 1D). Significantly, the division was not absolute between women identifying as black versus white as a similar pattern of down-regulated ribosome-related and up-regulated MYC-related genes was seen in n = 3 women identifying as white (Figure 1D). The methodology used for isolation of cells, preparation of RNA, processing for RNA-seq and GSEA analysis was the same as presented in a previous publication (67). The results suggest that biological differences, particularly Myc-associated pathways, of mammary epithelial cells may contribute to BC disparity, especially BC initiation in addition to social-economic factors. Since Myc induces a multigenic program that involves changes in intracellular calcium signalling and fatty acid metabolism, previous collaborative study suggested key roles for fatty acid transporters (CD36), lipases (LPL), and kinases (PDGFRB, CAMKK2, and AMPK), each of which contributes to promoting fatty acid oxidation (FAO) in human mammary epithelial cells transduced with Myc [47]. Thus, it is possible that dysregulation of fatty acid metabolism by both biological and social/economic factors contribute disparities of BC in African American population.
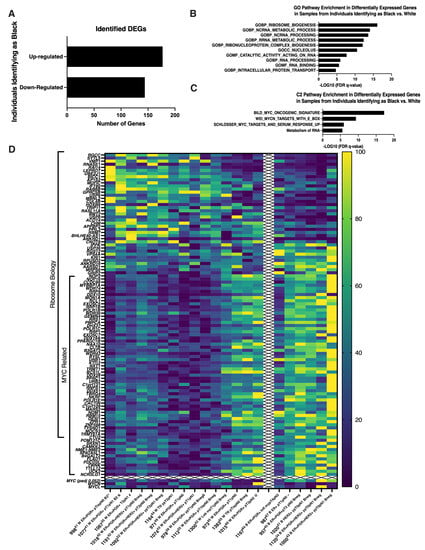
Figure 1. Self-identification as Black or African-American was associated with enrichment in ribosome and MYC biology-related genes. A. Bar graph indicating numbers of up-regulated and down-regulated DEGs identified in non-cancer ipsilateral samples from women identifying as Black or African-American versus white. B. Bar graph presenting the top ten GO gene sets with the lowest significant FDR q-values identified from the MSigDB Collection utilizing identified DEGs in non-cancer ipsilateral samples from women identifying as Black or African-American versus white. C. Bar graph presenting three C2 gene sets with FDR q-values <0.05 identified from the MSigDB Collection utilizing identified DEGs in non-cancer ipsilateral samples from women identifying as Black or African-American versus white. D. Heat map illustrating relative expression levels of identified DEGs enriched in samples from individuals identifying as Black or African-American or white. DEGs enriched in Ribosome biology or MYC related are indicated. DEG: differentially expressed gene, at Padj ≤ 0.05. FDR: false discovery rate. MsigDB, Molecular Signatures Database, v7.5.1 [48][49][50] Color coding: Dark blue to yellow with increasing expression.
Using CR technology, Nakshatri and colleagues [51] found a distinct subgroup of cells in a majority of African American women that exhibited higher CD44 expression but lacked CD24 or EpCAM. These cells displayed elevated expression levels of genes associated with stemness and epithelial-mesenchymal transition. Thus, CR technology allows the studying of basic biological factors of BC disparity using live cells from different populations. Together with traditional genetic and other -omics based studies, these will transform disparity biology studies using live normal or cancer cells from healthy donors and/or BC patients.
6. Heterogeneity of Breast Cancer
Breast cancer is a category of diseases with high heterogeneity. The heterogeneity is attributed to differences in the genomic, epigenomic, transcriptomic, and proteomic characteristics of the cancer cells, these factors also contribute biological properties of cancer cells such as proliferation, apoptosis, metastasis, and therapeutic response.
Breast cancer heterogeneity can be observed in tumor tissues among individual patients (different patients) or intertumor heterogeneity (different tumors), or intratumor heterogeneity (within the same tumor tissues). To study micro- or molecular heterogeneity of breast cancer, researchers established cell-derived clones using CR technology (Figure 2A). Nine cell clones were generated from one needle biopsy of breast cancer tissue. Results from whole genome sequencing and transcriptome analyses showed complexity at genomic and transcriptome levels (Figure 2B), while transcriptome analysis indicated two categories of profiling of gene expression in nine single cell-derived clones.
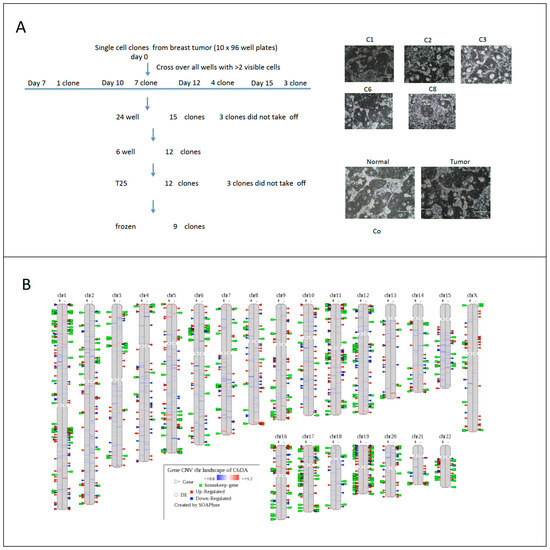
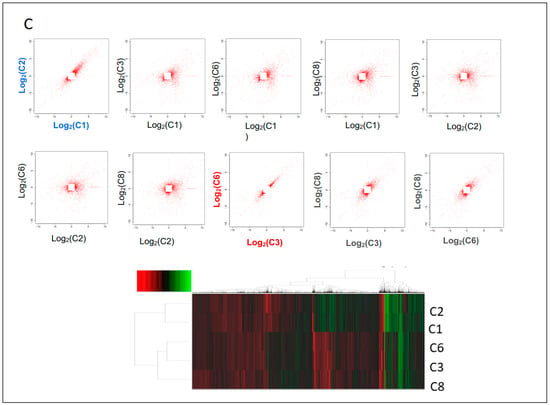
Figure 2. Micro-heterogeneity of breast cancer using CR and single cell derived clones. Needle biopsy from breast cancer tissue was processed and digested. Single cell suspension was diluted to 1 cell per 200 ul and 100 ul were plated in each well in ten 96-well plates. All wells were evaluated under light microcopy; wells with two or more than two cells were labeled and left out for the next step. Nine clones were expanded after replating to 24, 12 well-plates and T25 flasks (A). DNA and RNA were isolated from 5 clones, adjacent normal and tumor CRCs for whole genome sequencing and transcriptome analyses. CNVs were labeled on the left side of each chromosome of clone number 6 and differential gene expressions were labeled on the right side of chromosome compared to normal cells from same patient (B). Correlation of profiling of gene expression of 5 clones indicated two categories of breast cancer cell types (C).
The heterogeneity found within the normal breast can impact the characterization of cancer stem cells. Nakshatri and colleagues [51] employed CR technology to document heterogeneity in healthy breast tissue profiles. Their study involved growing cells from over 60 primary samples using CR system and a combination of nine markers. These markers allowed the quantification of at least 20 cell types per individual. Moreover, the CR method enabled the growth of stem, progenitor, and mature cells, and the percentage of these cell types varied among individuals. They noticed a distinct subgroup of cells in a majority of African American women that exhibited higher CD44 expression but lacked CD24 or EpCAM. This difference in cell population between African American and Caucasian patients was significant. These cells displayed elevated expression levels of genes associated with stemness and epithelial-mesenchymal transition. Notably, this gene expression pattern closely resembled that of PROCR+/EpCAM- mammary stem cells [52]
References
- Giaquinto, A.N.; Sung, H.; Miller, K.D.; Kramer, J.L.; Newman, L.A.; Minihan, A.; Jemal, A.; Siegel, R.L. Breast Cancer Statistics, 2022. CA: A Cancer J. Clin. 2022, 72, 524–541.
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249.
- Siegel, R.L.; Wagle, N.S.; Cercek, A.; Smith, R.A.; Jemal, A. Colorectal cancer statistics, 2023. CA Cancer J Clin. 2023, 73, 233–254.
- van‘t Veer, L.J.; Dai, H.; van de Vijver, M.J.; He, Y.D.; Hart, A.A.; Mao, M.; Peterse, H.L.; van der Kooy, K.; Marton, M.J.; Witteveen, A.T.; et al. Gene expression profiling predicts clinical outcome of breast cancer. Nature 2002, 415, 530–536.
- Perou, C.M.; Sørlie, T.; Eisen, M.B.; van de Rijn, M.; Jeffrey, S.S.; Rees, C.A.; Pollack, J.R.; Ross, D.T.; Johnsen, H.; Akslen, L.A.; et al. Molecular portraits of human breast tumours. Nature 2000, 406, 747–752.
- Cardiff, R.D.; Kenney, N. A Compendium of the Mouse Mammary Tumor Biologist: From the Initial Observations in the House Mouse to the Development of Genetically Engineered Mice. Cold Spring Harb. Perspect. Biol. 2010, 3, a003111.
- Sørlie, T.; Perou, C.M.; Tibshirani, R.; Aas, T.; Geisler, S.; Johnsen, H.; Hastie, T.; Eisen, M.B.; van de Rijn, M.; Jeffrey, S.S.; et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc. Natl. Acad. Sci. USA 2001, 98, 10869–10874.
- Metcalfe, C.; Lauchle, J.O. Clinical Translation: Targeting the Estrogen Receptor. Nucl. Recept. Hum. Health Dis. 2022, 1390, 297–309.
- Piezzo, M.; Cocco, S.; Caputo, R.; Cianniello, D.; Gioia, G.D.; Lauro, V.D.; Fusco, G.; Martinelli, C.; Nuzzo, F.; Pensabene, M.; et al. Targeting Cell Cycle in Breast Cancer: CDK4/6 Inhibitors. Int. J. Mol. Sci. 2020, 21, 6479.
- Luque-Cabal, M.; García-Teijido, P.; Fernández-Pérez, Y.; Sánchez-Lorenzo, L.; Palacio-Vázquez, I. Mechanisms behind the Resistance to Trastuzumab in HER2-Amplified Breast Cancer and Strategies to Overcome It. Clin. Med. Insights: Oncol. 2016, 10s1, CMO.S34537–30.
- Swain, S.M.; Baselga, J.; Kim, S.B.; Ro, J.; Semiglazov, V.; Campone, M.; Ciruelos, E.; Ferrero, J.M.; Schneeweiss, A.; Heeson, S.; et al. Faculty Opinions recommendation of Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer. New Engl. J. Med. 2015, 372, 724–734.
- Portman, N.; Alexandrou, S.; Carson, E.; Wang, S.; Lim, E.; Caldon, C.E. Overcoming CDK4/6 inhibitor resistance in ER-positive breast cancer. Endocr. Relat. Cancer 2019. 26, r15–r30.
- Codenotti, S.; Mansoury, W.; Pinardi, L.; Monti, E.; Marampon, F.; Fanzani, A. Animal models of well-differentiated/dedifferentiated liposarcoma: Utility and limitations. OncoTargets Ther. 2019, ume 12, 5257–5268.
- Meijer, T.G.; Naipal, K.A.; Jager, A.; van Gent, D.C. Ex vivo tumor culture systems for functional drug testing and therapy response prediction. Futur. Sci. OA 2017, 3, FSO190.
- Gillet, J.-P.; Varma, S.; Gottesman, M.M. The Clinical Relevance of Cancer Cell Lines. PEDIATRICS 2013, 105, 452–458.
- Ito, R.; Takahashi, T.; Ito, M. Humanized mouse models: Application to human diseases. J. Cell. Physiol. 2017, 233, 3723–3728.
- Giles, E.D.; Wellberg, E.A. Preclinical Models to Study Obesity and Breast Cancer in Females: Considerations, Caveats, and Tools. J. Mammary Gland. Biol. Neoplasia 2020, 25, 237–253.
- Diaz-Cruz, E.S.; Cabrera, M.C.; Nakles, R.; Rutstein, B.H.; Furth, P.A. BRCA1 deficient mouse models to study pathogenesis and therapy of triple negative breast cancer. Breast Dis. 2011, 32, 85–97.
- Dabydeen, S.A.; Furth, P.A. Genetically engineered ERα-positive breast cancer mouse models. Endocr.-Relat. Cancer 2014, 21, R195–R208.
- Clevers, H. Modeling Development and Disease with Organoids. Cell 2016, 165, 1586–1597.
- Mahajan, A.S.; Sugita, B.M.; Duttargi, A.N.; Saenz, F.; Krawczyk, E.; McCutcheon, J.N.; Fonseca, A.S.; Kallakury, B.; Pohlmann, P.; Gusev, Y.; et al. Genomic comparison of early-passage conditionally reprogrammed breast cancer cells to their corresponding primary tumors. PLoS ONE 2017, 12, e0186190.
- Barnabas, N.; Cohen, D. Phenotypic and Molecular Characterization of MCF10DCIS and SUM Breast Cancer Cell Lines. Int. J. Breast Cancer 2013, 2013, 1–16.
- Kalaany, N.Y.; Sabatini, D.M. Tumours with PI3K activation are resistant to dietary restriction. Nature 2009, 458, 725–731.
- Miller, F.R.; Santner, S.J.; Tait, L.; Dawson, P.J. MCF10DCIS.com xenograft model of human comedo ductal carcinoma in situ. Perspect. Surg. 2000, 92, 1185–1186.
- Forozan, F.; Veldman, R.A.; Ammerman, C.; Parsa, N.Z.; Kallioniemi, A.; Kallioniemi, O.-P.; Ethier, S.P. Molecular cytogenetic analysis of 11 new breast cancer cell lines. Br. J. Cancer 1999, 81, 1328–1334.
- Yong, J.W.; Choong, M.L.; Wang, S.; Wang, Y.; Lim, S.Q.; Lee, M.A. Characterization of ductal carcinoma in situ cell lines established from breast tumor of a Singapore Chinese patient. Cancer Cell Int. 2014, 14, 94.
- Brown, D.D.; Dabbs, D.J.; Lee, A.V.; McGuire, K.P.; Ahrendt, G.M.; Bhargava, R.; Davidson, N.E.; Brufsky, A.M.; Johnson, R.R.; Oesterreich, S.; et al. Developing in vitro models of human ductal carcinoma in situ from primary tissue explants. Breast Cancer Res. Treat. 2015, 153, 311–321.
- Daneshdoust, D.; Yin, M.; Luo, M.; Sundi, D.; Dang, Y.; Lee, C.; Li, J.; Liu, X. Conditional Reprogramming Modeling of Bladder Cancer for Clinical Translation. Cells 2023, 12, 1714.
- Mimoto, R.; Yogosawa, S.; Saijo, H.; Fushimi, A.; Nogi, H.; Asakura, T.; Yoshida, K.; Takeyama, H. Clinical implications of drug-screening assay for recurrent metastatic hormone receptor-positive, human epidermal receptor 2-negative breast cancer using conditionally reprogrammed cells. Sci. Rep. 2019, 9, 1–8.
- Urbaniak, A.; Jousheghany, F.; Yuan, Y.; Huczyński, A.; Delgado, M.; Kieber-Emmons, T.; Monzavi-Karbassi, B.; Chambers, T.C.; Piña-Oviedo, S. The response of phyllodes tumor of the breast to anticancer therapy: An in vitro and ex vivo study. Oncol. Lett. 2019, 18, 5097–5106.
- Chung, P.-H.; Chen, T.-W.; Chang, D.-Y.; Lin, C.-H.; Huang, S.-M.; Lu, Y.-S. Abstract P4-06-09: Synergistic effect of EGFR1 inhibitor and paclitaxel in newly patient derived metaplastic carcinoma cell line which harbored EGFR gene amplification. Cancer Res. 2017, 77, 4–6.
- Anjanappa, M.; Cardoso, A.; Cheng, L.; Mohamad, S.; Gunawan, A.; Rice, S.; Dong, Y.; Li, L.; Sandusky, G.E.; Srour, E.F.; et al. Individualized Breast Cancer Characterization through Single-Cell Analysis of Tumor and Adjacent Normal Cells. Cancer Res 2017, 77, 2759–2769.
- Anjanappa, M.; Hao, Y.; Simpson, E.R.; Bhat-Nakshatri, P.; Nelson, J.B.A.; Tersey, S.; Mirmira, R.G.A.; Cohen-Gadol, A.; Saadatzadeh, M.R.; Li, L.; et al. A system for detecting high impact-low frequency mutations in primary tumors and metastases. Oncogene 2017, 37, 185–196.
- Miller, K.D.; Nogueira, L.; Mariotto, A.B.; Rowland, J.H.; Yabroff, K.R.; Alfano, C.M.; Jemal, A.; Kramer, J.L.; Siegel, R.L. Cancer treatment and survivorship statistics, 2019. CA Cancer J. Clin. 2019, 69, 363–385.
- Bhushan, A.; Gonsalves, A.; Menon, J.U. Current State of Breast Cancer Diagnosis, Treatment, and Theranostics. Pharmaceutics 2021, 13, 723.
- Naghibi, A.F.; Daneshdoust, D.; Taha, S.R.; Abedi, S.; Dehdezi, P.A.; Zadeh, M.S.; Dokoohaki, F.; Soleymani-Goloujeh, M. Role of cancer stem cell-derived extracellular vesicles in cancer progression and metastasis. Pathol.—Res. Pract. 2023, 247, 154558.
- İlhan, A.; Golestani, S.; Shafagh, S.G.; Asadi, F.; Daneshdoust, D.; Al-Naqeeb, B.Z.T.; Nemati, M.M.; Khalatbari, F.; Yaseri, A.F. The dual role of microRNA (miR)-20b in cancers: Friend or foe? Cell. Commun. Signal. 2023, 21, 26.
- Mahmoud, M.M.; Sanad, E.F.; Elshimy, R.A.A.; Hamdy, N.M. Competitive Endogenous Role of the LINC00511/miR-185-3p Axis and miR-301a-3p From Liquid Biopsy as Molecular Markers for Breast Cancer Diagnosis. Front. Oncol. 2021, 11, 749753.
- Buono, G.; Gerratana, L.; Bulfoni, M.; Provinciali, N.; Basile, D.; Giuliano, M.; Corvaja, C.; Arpino, G.; Del Mastro, L.; De Placido, S.; et al. Circulating tumor DNA analysis in breast cancer: Is it ready for prime-time? Cancer Treat Rev. 2019, 73, 73–83.
- Ashworth, T. A case of cancer in which cells similar to those in the tumours were seen in the blood after death. Aust. Med. J. 1869, 14, 146.
- Baccelli, I.; Schneeweiss, A.; Riethdorf, S.; Stenzinger, A.; Schillert, A.; Vogel, V.; Klein, C.; Saini, M.; Bäuerle, T.; Wallwiener, M.; et al. Identification of a population of blood circulating tumor cells from breast cancer patients that initiates metastasis in a xenograft assay. Nat. Biotechnol. 2013, 31, 539–544.
- Nellore, B.P.V.; Kanchanapally, R.; Pramanik, A.; Sinha, S.S.; Chavva, S.R.; Hamme, A.; Ray, P.C. Aptamer-Conjugated Graphene Oxide Membranes for Highly Efficient Capture and Accurate Identification of Multiple Types of Circulating Tumor Cells. Bioconjugate Chem. 2015, 26, 235–242.
- Jeong, Y.J.; Park, S.H.; Jeon, C.-H. Detection of circulating tumor cells in patients with breast cancer using the conditionally reprogrammed cell culture method and reverse transcription-PCR of hTERT and MAGE A1-6. Oncol. Lett. 2020, 20, 1.
- Liu, X.; Krawczyk, E.A.; Suprynowicz, F.; Palechor-Ceron, N.; Yuan, H.; Dakic, A.; Simic, V.; Zheng, Y.-L.; Sripadhan, P.; Chen, C.; et al. Conditional reprogramming and long-term expansion of normal and tumor cells from human biospecimens. Nat. Protoc. 2017, 12, 439–451.
- Alothman, S.J.; Kang, K.; Liu, X.; Krawczyk, E.; Azhar, R.I.; Hu, R.; Goerlitz, D.; Kallakury, B.V.; Furth, P.A. Characterization of transcriptome diversity and in vitro behavior of primary human high-risk breast cells. Sci. Rep. 2022, 12, 1–16.
- McAuliffe, P.F.; Evans, K.W.; Akcakanat, A.; Chen, K.; Zheng, X.; Zhao, H.; Eterovic, A.K.; Sangai, T.; Holder, A.M.; Sharma, C.; et al. Ability to Generate Patient-Derived Breast Cancer Xenografts Is Enhanced in Chemoresistant Disease and Predicts Poor Patient Outcomes. PLoS ONE 2015, 10, e0136851.
- Casciano, J.C.; Perry, C.; Cohen-Nowak, A.J.; Miller, K.D.; Voorde, J.V.; Zhang, Q.; Chalmers, S.; Sandison, M.E.; Liu, Q.; Hedley, A.; et al. MYC regulates fatty acid metabolism through a multigenic program in claudin-low triple negative breast cancer. Br. J. Cancer 2020, 122, 868–884.
- Subramanian, A.; Tamayo, P.; Mootha, V.K.; Mukherjee, S.; Ebert, B.L.; Gillette, M.A.; Paulovich, A.; Pomeroy, S.L.; Golub, T.R.; Lander, E.S.; et al. Faculty Opinions recommendation of Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA 2005, 102, 15545–15550.
- Liberzon, A.; Birger, C.; Thorvaldsdóttir, H.; Ghandi, M.; Mesirov, J.P.; Tamayo, P. The Molecular Signatures Database (MSigDB) hallmark gene set collection. Cell Syst. 2015, 1, 417–425.
- Liberzon, A.; Subramanian, A.; Pinchback, R.; Thorvaldsdóttir, H.; Tamayo, P.; Mesirov, J.P. Molecular signatures database (MSigDB) 3.0. Bioinformatics 2011, 27, 1739–1740.
- Nakshatri, H.; Anjanappa, M.; Bhat-Nakshatri, P. Ethnicity-Dependent and -Independent Heterogeneity in Healthy Normal Breast Hierarchy Impacts Tumor Characterization. Sci. Rep. 2015, 5, 13526.
- Wang, D.; Cai, C.; Dong, X.; Yu, Q.C.; Zhang, X.-O.; Yang, L.; Zeng, Y.A. Identification of multipotent mammary stem cells by protein C receptor expression. Nature 2014, 517, 81–84.
More
Information
Subjects:
Oncology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
443
Revisions:
2 times
(View History)
Update Date:
08 Oct 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




