Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Juan Mitchell | -- | 2655 | 2023-10-05 01:51:07 | | | |
| 2 | Rita Xu | Meta information modification | 2655 | 2023-10-05 16:03:20 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Mitchell, J.; Lo, K.W. Cell Adhesion and Migration in Regenerative Medicine. Encyclopedia. Available online: https://encyclopedia.pub/entry/49839 (accessed on 06 March 2026).
Mitchell J, Lo KW. Cell Adhesion and Migration in Regenerative Medicine. Encyclopedia. Available at: https://encyclopedia.pub/entry/49839. Accessed March 06, 2026.
Mitchell, Juan, Kevin W.-H. Lo. "Cell Adhesion and Migration in Regenerative Medicine" Encyclopedia, https://encyclopedia.pub/entry/49839 (accessed March 06, 2026).
Mitchell, J., & Lo, K.W. (2023, October 05). Cell Adhesion and Migration in Regenerative Medicine. In Encyclopedia. https://encyclopedia.pub/entry/49839
Mitchell, Juan and Kevin W.-H. Lo. "Cell Adhesion and Migration in Regenerative Medicine." Encyclopedia. Web. 05 October, 2023.
Copy Citation
Cell adhesion and migration is essential for cell survival, communication, and regulation, and it is of fundamental importance in the development and maintenance of tissues. Cell adhesion has been widely explored due to its many important roles in the fields of tissue regenerative engineering and cell biology. With this in mind, researchers are employing novel strategies to harness the utility of cell adhesion—including the use of small molecules to promote adhesion and migration for improved tissue regeneration.
cell adhesion
regenerative medicine
tissue regeneration
small molecule
1. Introduction
With regard to the biological functioning of many different cell types, cellular adhesion is an essential regulatory mechanism of cell self-renewal and intercellular communication that is also of fundamental importance to the development and maintenance of tissues [1][2][3]. Cellular adhesion has been highlighted as a necessary prerequisite to cell longevity and unimpeded biological activity that effectively precludes apoptotic processes [4][5][6]. For instance, anchorage-dependent cells such as adult stem cells, neurons, osteoblasts, and epithelial cells all utilize cell adhesion as an important prerequisite for sustaining cell niches for subsequent integration, migration, survival, and cell specialization [7][8][9]. On the other hand, late attachment implies that cells have been in the system for longer before adhering to the surface, which could decrease their viability [10].
Cell adhesion plays a crucial role in tissue regeneration by supporting the structural integration of cells, facilitating cell migration and survival, guiding cell differentiation and determination of cell fate, and potentially influencing vascularization processes [4]. Therefore, the ability to regulate and control cell adhesion to biomaterial surfaces is of the utmost importance in the field of tissue regenerative engineering [11][12]. In fact, the ability of cells to rapidly attach to a biomaterial can significantly influence the success of clinical applications involving that biomaterial [13]. Such control requires an understanding of the basic molecular mechanisms underlying the adhesion between cells and their substratum. Two mechanisms of cell adhesion to biomaterials have been proposed: non-receptor-mediated cell adhesion to material surfaces via non-covalent bonding, such as electrostatic interactions, hydrogen bonding, or polar or non-polar interactions, and ionic interactions between various components or molecules on cell membrane and chemical functional groups on biomaterials [14].
Functional receptor-mediated and signal transmitting cell adhesion to a conventional biomaterial is mediated by extracellular matrix (ECM) molecules that are imperative to the in vivo and in vitro survival of adherent cells, such as fibronectin, proteoglycans, integrins, cadherins, vitronectin, elastin, collagen, laminin, and other classes of adhesion molecules [15]. In fact, a growing number of ECM molecules are now implicated in tissue homeostasis, and they are good potential targets for clinical interventions [16]. Tissue regeneration often uses ECM components as a key factor in tissue repair. The ECM provides not only the structural support for cells but also the biochemical cues needed to moderate cell physiology and phenotype [17]. To date, many strategies to control cell adhesion and homing have relied on controlling the placement of ECM proteins on biomaterial surfaces [18]. The most commonly used technique is to immobilize the recombinant ECM protein (i.e., fibronectin) on the biomaterial surface prior to plating the cells [19][20]. However, this is a costly manufacturing process because it requires specific techniques and reagents for immobilization [21]. Moreover, the instability of ECM proteins during immobilization is the most significant obstacle in developing surface-bound ECM biopolymers [22].
Another disadvantage of using recombinant ECM proteins is that they may elicit an undesirable immune response in the host [23]. Intriguingly, ECM-derived short peptides like Arg-Gly-Asp (RGD) have been proposed as alternatives for promoting cell adhesion [24]. However, their general lack of specificity and significantly decreased receptor binding affinities have proven detrimental in attempts to regulate the highly specific and integrated processes necessary for tissue regeneration [25][26][27][28][29]. Therefore, alternative strategies are being explored to overcome these limitations and achieve more precise control over the cellular responses involved in effective cell adhesion and tissue regeneration [30][31].
Another means of adhesion promotion includes natural and synthetic materials in the form of bioadhesives. In general, bioadhesives are substances that are designed to adhere to biological tissues. Bioadhesives function as especially adhesive compounds on tissues and grafts that amplify graft stability and wound closure [32]. Bioadhesives have been adopted as mediums for localized delivery of cells and growth factors to help facilitate soft tissue adhesion for enhanced wound repair, primarily in musculoskeletal tissues such as bone, cartilage, tendon, and intervertebral discs. However, it should be noted that bioadhesives are not without their own shortcomings that limit their clinical applications. Prominent bioadhesives like fibrin cannot be readily used at defect sites of high-tensile stress and are prone to degradation at accelerated rates [33]. In addition, many other common bioadhesive materials, such as cyanacrylate and PEG-based materials, also lack adequate in vivo analyses on their long-term efficacy and cytotoxicity [34], indicating the need for more thorough research and evaluation.
In the context of tissue engineering and regenerative medicine, the stability and biodegradation of small molecules can play a significant role. For example, if a small molecule with adhesive properties is integrated into a scaffold, its stability and degradation rate can influence the scaffold’s longevity and functionality. Several classes of small molecules demonstrate ample stability, allowing them to preserve their unique physical and chemical properties over extended periods of time. They also tout a significant degree of biodegradability across many intra- and extracellular environments. Citing such traits, a large number of small molecules have been consistently applied in many biomedical engineering fields, making tangible strides in research on stem cell differentiation and wound healing for severe tissue defects. With this in mind, researchers have directed their attention to the manipulation of intracellular signaling pathways using small molecules as bioactive modulators. The regulatory profiles of these compounds are capable of altering many pathways that are critical to biological growth and development, including but not limited to Sonic Hedgehog (SHH), BMP/Smad, MAPK/ERK, Epac, β-Runx1, and RANKL. For instance, preclinical studies have shown that the small molecule strontium ranelate influences bone remodeling pathways by decreasing osteoblast-induced RANKL/OPG synthesis within subchondral bone [35].
A plethora of clinical trials are also being conducted on these molecules to expand their practical portfolio in vivo [36]. Various forms of natural and synthetic scaffolds (e.g., hydrogels, ceramics, fibers, composites, etc.) have been coupled with small molecules to aid in the promotion of osteogenic signaling and sustained release, while also providing a matrix for osteoblast or stem cell adherence at defects sites [37]. Commonly used polymeric scaffolding materials like PLGA, polyurethane, calcium phosphate, and HA microspheres have all exhibited success in the delivery and sustained release of small molecules in vivo. Small molecule incorporation in these scaffolds often entails injection or crosslinking with hydrogels during polymerization, surface modification for external coating with molecules, and other conventional loading methods. Favorable characteristics such as mechanical rigidity, biocompatibility, supplementary adhesive strength, chemical moieties, and surface topography have made matrixial scaffolding an optimal medium for small molecule delivery [38][39]. Specially fabricated scaffolds are also capable of maintaining ECM integrity and avoiding immune-mediated rejection, which could hamper their clinical relevance [40][41][42][43]. With the list of cell adhesive small molecules continuously growing, these light-weight compounds may very well represent the next generation of therapeutics for targeted wound revitalization (see Figure 1).
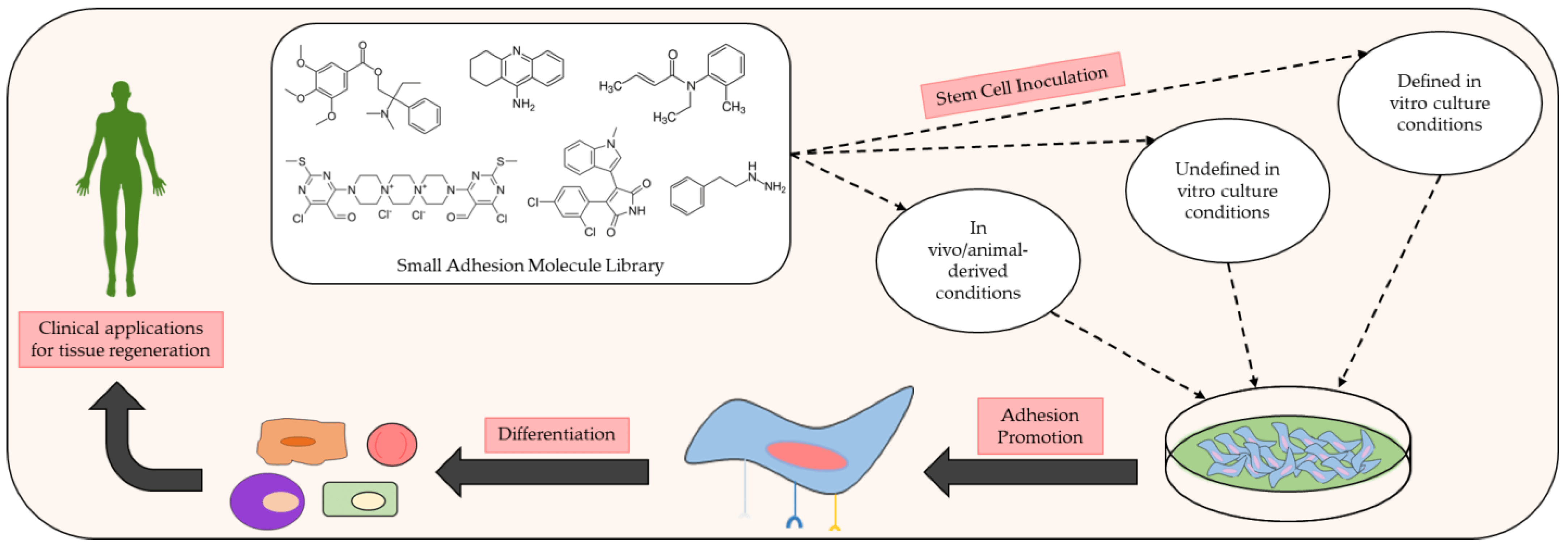
Figure 1. Schematic of the preclinical analysis of small molecules exhibiting beneficial adhesive properties and how they may be used to further develop and improve clinical methods of tissue regeneration. Many small adhesion molecules exert discernible effects on cellular adhesion and differentiation upon inoculation with various stem cell populations under specific in vivo and in vitro conditions. Common in vivo conditions for adhesion assays typically consist of animal models with controlled defects (e.g., injured rats). In vitro conditions for adhesion assays commonly implement defined culture conditions with known amounts of reagent and chemicals in the growth medium or undefined culture conditions in which the exact amounts of reagent and chemicals used in the growth medium are unknown.
2. Adhesamine
Recent research has revealed that adhesamine can accelerate the differentiation of hippocampal neurons, mediated by the heparin sulfate-binding mechanism [44]. Adhesamine is the first organic non-peptidic small molecule that has been shown to promote physiological adhesion and growth of cultured cells through surface modulation and selective binding to heparin sulfate on the cell surface [45]. Specifically, it was found that this process involves both the focal adhesion kinase (FAK) and mitogen-activated protein kinase (MAPK) signal transduction pathways [44]. These intercellular pathways are known to link cell surface receptor signals to targets in the cytoplasm or nucleus, promoting activities such as cell migration and morphing, growth, proliferation, and differentiation. FAK plays a primary role in the process of cell adhesion to the extracellular matrix and is intertwined with MAPK. MAPK/ERK [extracellular signal-regulated kinase] kinase (MEK) phosphorylates MAPK, translocating it to the nucleus and allowing it to activate transcription factors that control the expression of genes related to cell growth and differentiation [46].
Yamazoe et al. treated human hepatoma (HepG2) cells and Jurkat cells (human T-lymphocytes) with varying concentration of adhesamine (0.6–60 µM) and performed assays for adherence to culture plates following a 3 h or 24 h incubation period [45]. The authors noted that adhesamine enhanced the adhesion of HepG2 cells to the bottom of culture plates by up to two-fold, exhibiting a dose-dependent effect on cellular adhesion with increasing concentrations. Similarly, 30% and 60% of the treated Jurkat cells attached to the culture plates in the presence of 6 mM and 60 mM of adhesamine, respectively (see Figure 2). However, no intercellular adhesion between HepG2 or Jurkat cells within culture plates was observed after the treatment. In their structure–activity relationship studies, six derivatives of the adhesamine molecule with unique modified moieties were fabricated and tested for their individual adhesive effects on Jurkat cells. The six modifications made to the primary adhesamine structure included: substitution of the dispirotripiperazine moiety with an amine linker, dipiperazylethane (molecule 2), methylation of the two nitrogen atoms in the dipiperazylethane linker of molecule 2 (molecule 3), removal of the two terminal pyrimidine rings on the primary adhesamine structure (molecule 4), reduction of the aldehyde groups in the pyrimidine rings to hydroxyl groups (molecule 5), introduction of a fluorescent dansyl group at the C5 position (molecule 6), and introduction of a dansyl conjugate of dispirotripiperazine (molecule 7). Of these derivatives, only molecules 5 and 6 retained their adhesion-promoting activity, while the remaining molecules saw a reduced or complete loss of adhesive qualities.
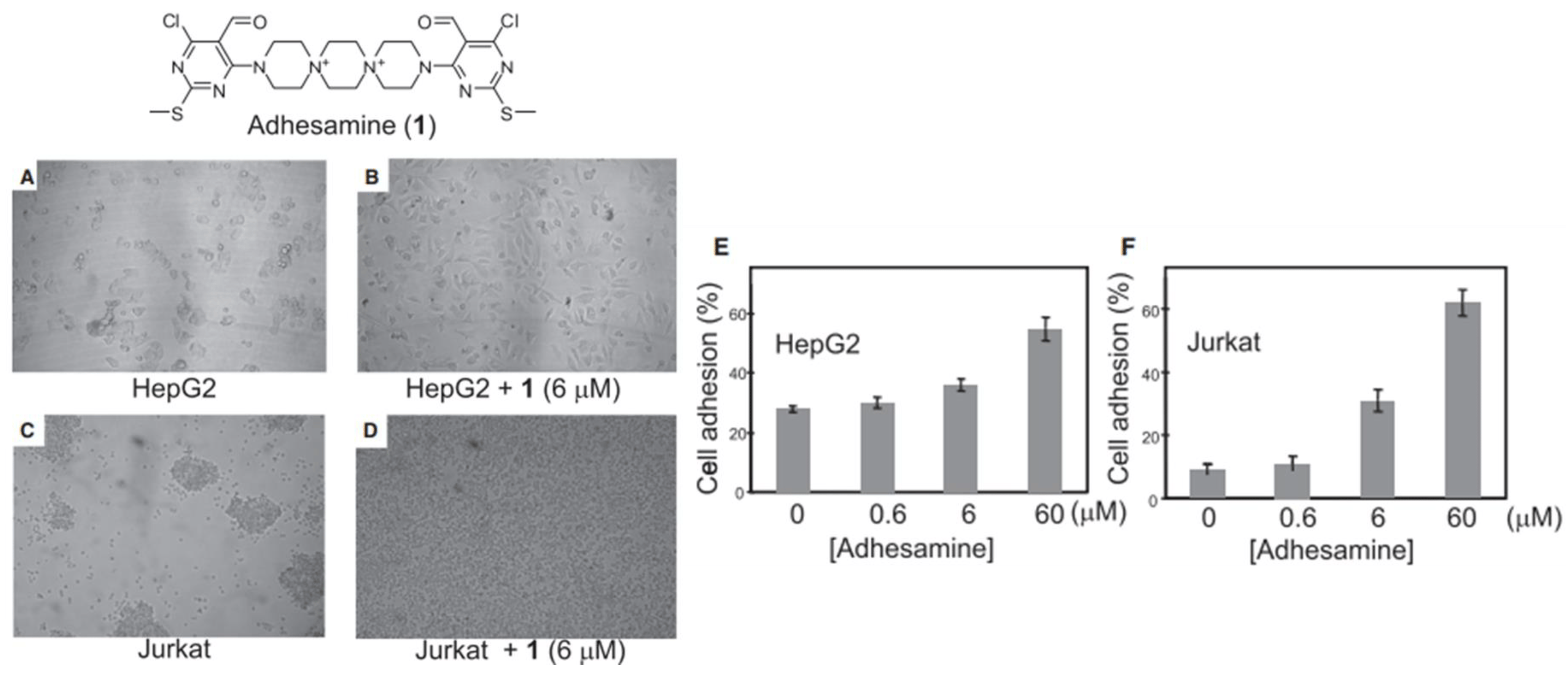
Figure 2. Cell adhesion assay for HepG2 and Jurkat cell lines incubated with 1% (v/v) DMSO alone or 6 μM of adhesamine (A–D). Rates of HepG2 cell adhesion to culture plates in varied concentrations of adhesamine, 0–60 μM (E). Rates of Jurkat cell adhesion to plates in varied concentrations of adhesamine, 0–60 μM (F).
To further characterize adhesamine’s cellular target sites, the subcellular localization of molecules 6 and 7 in HepG2 cells was determined under fluorescent microscopic observation. For this localization, HepG2 cells were incubated for a 3 h period in media containing 6 mM of molecules 6 or 7 and variants of heparan degradation enzymes. Incorporation of heparinase and heparitinases I and II in the solution medium allowed for verification of the targeting of surface heparan sulfate by adhesamine for cellular adhesion (see Figure 3).
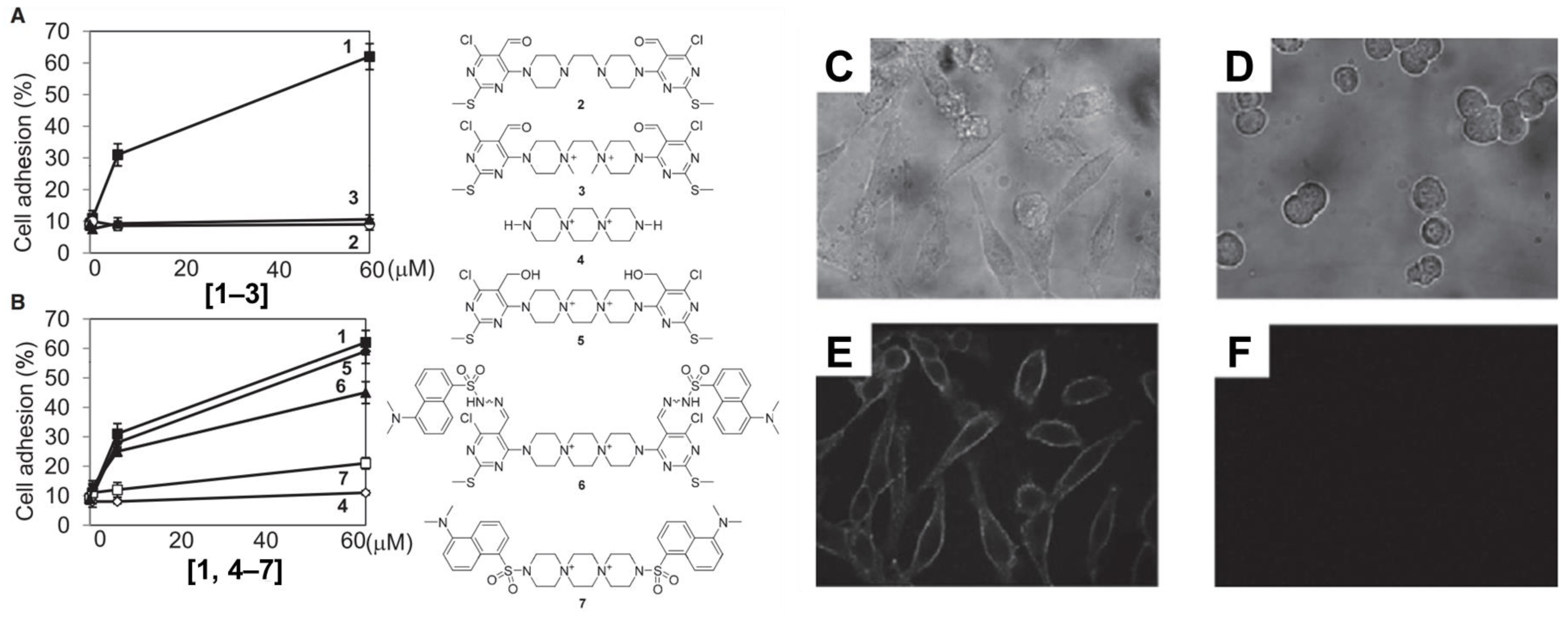
Figure 3. Structure–activity relationship studies surveying the ability of six adhesamine derivatives to promote cell adhesion of Jurkat cells (A,B). Subcellular localization of fluorescent probes on molecules 6 and 7 using brightfield (C,D) and confocal (E,F) imaging analysis for HepG2 cells. HepG2 cells were incubated with heparan degradation enzymes, 6 mM of molecule 6 (C,E), and 6 mM of molecule 7 (D,F) for 3 h after seeding.
3. L1CAM and L1 Agonists or Mimetics
L1CAM is a transmembrane neuronal cell adhesion molecule within the L1 family with strong implications in cell adhesion, migration, survival, neuritogenesis, synapse formation, and plasticity [47]. The L1 peptide, known to directly facilitate mechanisms of cellular adhesion, has been associated with the activation of multiple signaling pathways. Some researchers have studied the use of molecules to upregulate these L1 pathways and enhance their functions (e.g., Erk). L1CAM is naturally expressed in a plethora of cell types, including but not limited to neurons and Schwann cells [48]. The study by Kataria et al. screened small molecules libraries of known drugs for L1 agonists and evaluated the effects of “hit” compounds via thorough in vitro and in vivo analyses [49]. Specifically, in vitro cell-based assays pinpointed eight “hits” that were identified as small molecule L1 agonists that stimulate neuronal migration, survival, and neurite outgrowth. These included well-established compounds like duloxetine, phenelzine sulfate, tacrine, ethinyl estradiol, crotamiton, honokiol, trimebutine-maleate, and piceid. The authors demonstrated that these small molecular compounds were capable of competitively binding to L1 peptides in the presence of the L1-binding substrate antibody 557, successfully reducing antibody 557-L1–peptide interactions during their molecule screening assay. Immunostaining assays administered using the selected L1 agonists showed that each small molecule tested significantly enhanced L1 expression along the surface and within the cytoplasm of cerebellar neurons. The highest levels of L1 surface expression were observed in neurons treated with duloxetine, phenelzine sulfate, tacrine, ethinyl estradiol, honokiol, and piceid. The highest nuclear L1 levels were observed in neurons treated with duloxetine, tacrine, honokiol, and piceid (see Figure 4). These molecules also supplemented L1-mediated functions such as neuronal outgrowth and cellular migration to the site of injury. In relation to neuron migration, the authors found that piceid and phenelzine sulfate aided migration the most out of all molecules tested. The remaining six molecules also prompted neuron migration to a notably higher extent than the control (143–172% vs. the control group’s 100%).
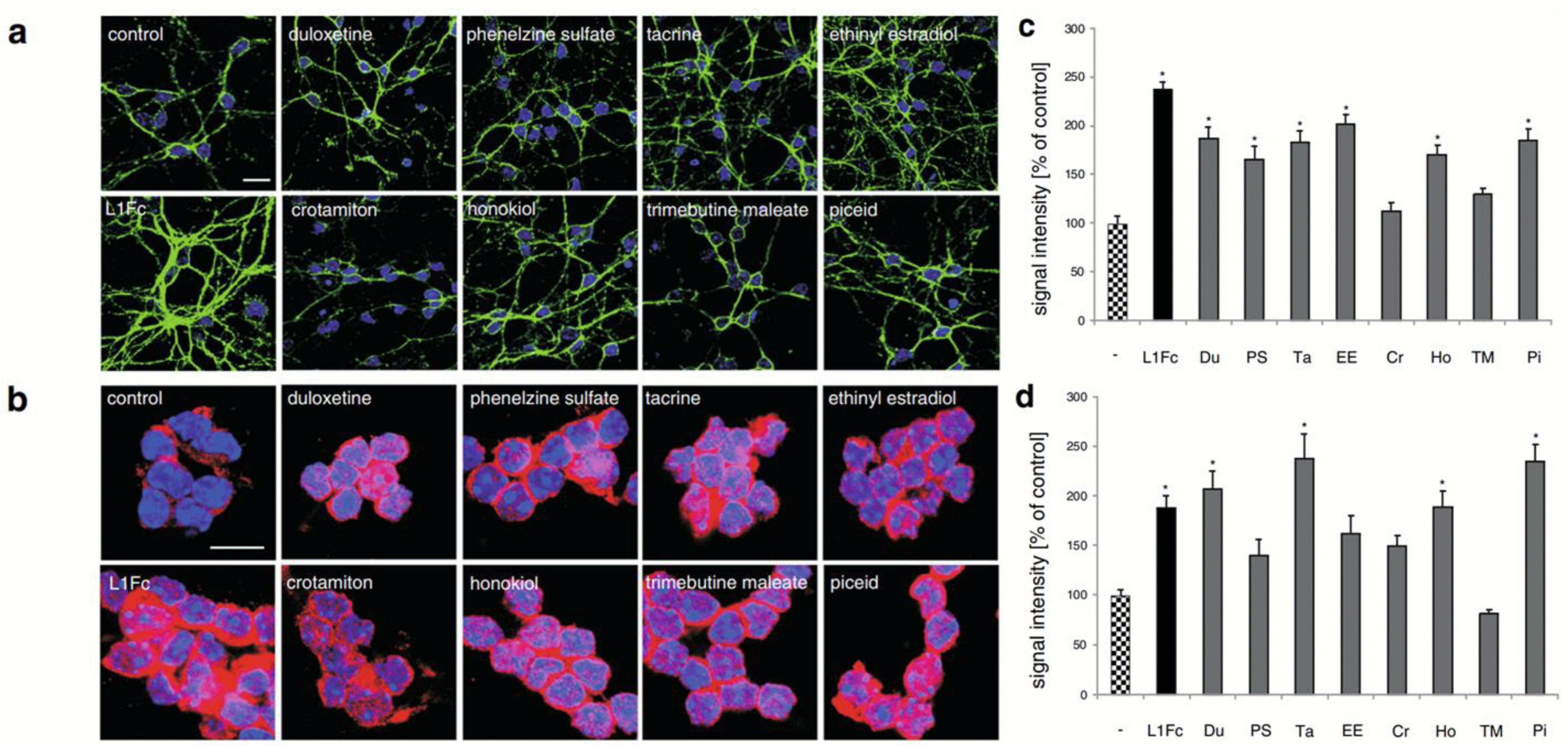
Figure 4. Immunostaining assay displaying the effects of small molecule L1 agonists on the extracellular domain and cytoplasmic expression of L1 protein indicating cell surface L1 and total L1 levels, respectively, in cerebellar neurons. Cells were treated with L1Fc or L1 agonists for 48 h and immunostained with antibody 555 (green) against the extracellular domain of L1 (a) or with antibody 172-R against the cytoplasmic domain of L1 (b); nuclei were stained with DAPI (blue). Signal quantification of cell surface L1 (c) and nuclear L1 (d) expression. Statistical significance of difference to untreated control determined at p < 0.05; *denotes significance in figure data.
In relation to Schwann cell migration, six molecules effectively promoted migration (duloxetine, phenelzine sulfate, piceid, honokiol, ethinyl estradiol, and trimebutine maleate), while the remaining two were determined to be ineffective in promoting migration (tacrine and crotamiton). When assessing L1-activated signaling and phosphorylation events, the authors observed that all the compounds stimulated Erk phosphorylation except for tacrine. The small molecules duloxetine, phenelzine sulfate, and honokiol displayed the strongest stimulation of Erk phosphorylation. The findings of this study have partially illuminated the aptitude of such molecules for repurposing into therapeutic agents that promote tissue revitalization through increased adhesion. Another study with an identical library of L1 agonists and a similar experimental design found that polydatin, a small glycoside, also incited L1-mediated neurite outgrowth along with neuronal migration [50]. Perhaps more importantly, some in vivo studies have revealed that L1 agonist small molecules can discernibly enhance the functional regeneration and re-myelination of neurons in a femoral nerve injury mouse model. Moreover, the L1 agonists also improved recovery of motor functions in a spinal cord injury mouse model. In the in vivo study on young mice with spinal cord injuries conducted by Xu et al., the L1 agonists trimebutine and honokiol were surveyed for their influence on L1 levels following tail vein injection [51]. Six weeks post spinal cord injury, the authors observed a marked increase in the expression of L1, pCK2α (a regulator of endocytic trafficking of L1 and L1-stimulated axon growth), and mTOR (proproliferative) proteins as well as phosphorylation in neurons localized to the spinal cord lesion centers, especially in mice treated with trimebutine. No substantial change in L1 expression was noted for the group treated with honokiol. However, both trimebutine and honokiol were observed to initiate L1-activated signaling cascades within cerebellar granule cells (trimebutine prompting pathway induction at 5 nM and honokiol prompting induction at 50 or 100 nM). While it was inevitably determined that trimebutine had a much more profound impact on axonal regrowth and restoration of locomotor activity than honokiol, both small molecules still enhanced neuronal regeneration in injured mice.
References
- Janiszewska, M.; Primi, M.C.; Izard, T. Cell adhesion in cancer: Beyond the migration of single cells. Perspect. Surg. 2020, 295, 2495–2505.
- Wu, S.; Du, W.; Duan, Y.; Zhang, D.; Liu, Y.; Wu, B.; Gao, C. Regulating the migration of smooth muscle cells by a vertically distributed poly(2-hydroxyethyl methacrylate) gradient on polymer brushes covalently immobilized with RGD peptides. Acta Biomater. 2018, 75, 75–92.
- Bolívar-Monsalve, E.J.; Alvarez, M.M.; Hosseini, S.; Espinosa-Hernandez, M.A.; Ceballos-González, C.F.; Sanchez-Dominguez, M.; Shin, S.R.; Cecen, B.; Hassan, S.; Di Maio, E.; et al. Engineering bioactive synthetic polymers for biomedical applications: A review with emphasis on tissue engineering and controlled release. Mater. Adv. 2021, 2, 4447–4478.
- Khalili, A.A.; Ahmad, M.R. A Review of Cell Adhesion Studies for Biomedical and Biological Applications. Int. J. Mol. Sci. 2015, 16, 18149–18184.
- Yang, W.; Sun, L.; Cai, S.; Chen, Y.; Liang, W.; Zhou, P.; Liu, L. Dynamically directing cell organization via micro-hump structure patterned cell-adhered interfaces. Lab Chip 2020, 20, 2447–2452.
- Kukumberg, M.; Yao, Y.; Goh, S.H.; Neo, D.J.H.; Yao, J.Y.; Yim, E.K.F. Evaluation of the Topographical Influence on the Cellular Behavior of Human Umbilical Vein Endothelial Cells. Adv. Biosyst. 2018, 2, 1700217.
- Chen, S.; Lewallen, M.; Xie, T. Adhesion in the stem cell niche: Biological roles and regulation. Development 2013, 140, 255–265.
- Anselme, K. Osteoblast adhesion on biomaterials. Biomaterials 2000, 21, 667–681.
- Rao, S.S.; Winter, J.O. Adhesion molecule-modified biomaterials for neural tissue engineering. Front. Neuroeng. 2009, 2, 6.
- Özkaya, A.B.; Geyik, C. From viability to cell death: Claims with insufficient evidence in high-impact cell culture studies. PLoS ONE 2022, 17, e0250754.
- Kim, H.S.; Kumbar, S.G.; Nukavarapu, S.P. Biomaterial-directed cell behavior for tissue engineering. Curr. Opin. Biomed. Eng. 2021, 17, 100260.
- Das, S.; Jacob, R.S.; Patel, K.; Singh, N.; Maji, S.K. Amyloid Fibrils: Versatile Biomaterials for Cell Adhesion and Tissue Engineering Applications. Biomacromolecules 2018, 19, 1826–1839.
- Lee, C.; Hu, S.; Christy, J.; Chou, F.; Ramli, T.C.; Chen, H. Biointerface Coatings With Structural and Biochemical Properties Modifications of Biomaterials. Adv. Mater. Interfaces 2023, 10, 2202286.
- Neděla, O.; Slepička, P.; Švorčík, V. Surface Modification of Polymer Substrates for Biomedical Applications. Materials 2017, 10, 1115.
- Pang, X.; He, X.; Qiu, Z.; Zhang, H.; Xie, R.; Liu, Z.; Cui, Y. Targeting integrin pathways: Mechanisms and advances in therapy. Signal Transduct. Target. Ther. 2023, 8, 1.
- Järveläinen, H.; Sainio, A.; Koulu, M.; Wight, T.N.; Penttinen, R. Extracellular matrix molecules: Potential targets in pharmacotherapy. Pharmacol. Rev. 2009, 61, 198–223.
- Nicolas, J.; Magli, S.; Rabbachin, L.; Sampaolesi, S.; Nicotra, F.; Russo, L. 3D Extracellular Matrix Mimics: Fundamental Concepts and Role of Materials Chemistry to Influence Stem Cell Fate. Biomacromolecules 2020, 21, 1968–1994.
- Tallawi, M.; Rosellini, E.; Barbani, N.; Cascone, M.G.; Rai, R.; Saint-Pierre, G.; Boccaccini, A.R. Strategies for the chemical and biological functionalization of scaffolds for cardiac tissue engineering: A review. J. R. Soc. Interface 2015, 12, 20150254.
- Parisi, L.; Toffoli, A.; Ghezzi, B.; Mozzoni, B.; Lumetti, S.; Macaluso, G.M. A glance on the role of fibronectin in controlling cell response at biomaterial interface. Jpn. Dent. Sci. Rev. 2020, 56, 50–55.
- Xing, H.; Lee, H.; Luo, L.; Kyriakides, T.R. Extracellular matrix-derived biomaterials in engineering cell function. Biotechnol. Adv. 2020, 42, 107421.
- Hassan, M.E.; Yang, Q.; Xiao, Z.; Liu, L.; Wang, N.; Cui, X.; Yang, L. Impact of immobilization technology in industrial and pharmaceutical applications. 3 Biotech 2019, 9, 440.
- Wang, Z.; Wang, Z.; Lu, W.W.; Zhen, W.; Yang, D.; Peng, S. Novel biomaterial strategies for controlled growth factor delivery for biomedical applications. NPG Asia Mater. 2017, 9, e435.
- Kasravi, M.; Ahmadi, A.; Babajani, A.; Mazloomnejad, R.; Hatamnejad, M.R.; Shariatzadeh, S.; Niknejad, H. Immunogenicity of decellularized extracellular matrix scaffolds: A bottleneck in tissue engineering and regenerative medicine. Biomater. Res. 2023, 27, 10.
- Yamada, Y.; Onda, T.; Wada, Y.; Hamada, K.; Kikkawa, Y.; Nomizu, M. Structure–Activity Relationships of RGD-Containing Peptides in Integrin αvβ5-Mediated Cell Adhesion. ACS Omega 2023, 8, 4687–4693.
- Haggag, Y.A. Peptides as Drug Candidates: Limitations and Recent Development Perspectives. Biomed. J. Sci. Tech. Res. 2018, 8, 6659–6662.
- Klimek, K.; Ginalska, G.G. Proteins and Peptides as Important Modifiers of the Polymer Scaffolds for Tissue Engineering Applications—A Review. Polymers 2020, 12, 844.
- Ren, X.; Zhao, M.; Lash, B.; Martino, M.M.; Julier, Z. Growth Factor Engineering Strategies for Regenerative Medicine Applications. Front. Bioeng. Biotechnol. 2020, 7, 469.
- Wang, H.-J.; Di, L.; Ren, Q.-S.; Wang, J.-Y. Applications and Degradation of Proteins Used as Tissue Engineering Materials. Materials 2009, 2, 613–635.
- Wang, L.; Wang, N.; Zhang, W.; Cheng, X.; Yan, Z.; Shao, G.; Fu, C. Therapeutic peptides: Current applications and future directions. Signal Transduct. Target. Ther. 2022, 7, 48.
- Barker, T.H. The role of ECM proteins and protein fragments in guiding cell behavior in regenerative medicine. Biomaterials 2011, 32, 4211–4214.
- Carson, A.E.; Barker, T.H. Emerging concepts in engineering extracellular matrix variants for directing cell phenotype. Regen. Med. 2009, 4, 593–600.
- Zhu, J.; Zhou, H.; Gerhard, E.M.; Zhang, S.; Rodríguez, F.I.P.; Pan, T. Smart bioadhesives for wound healing and closure. Bioact. Mater. 2023, 19, 360–375.
- Tarafder, S.; Park, G.Y.; Felix, J.; Lee, C.H. Bioadhesives for musculoskeletal tissue regeneration. Acta Biomater. 2020, 117, 77–92.
- Tzagiollari, A.; McCarthy, H.O.; Levingstone, T.J.; Dunne, N.J. Biodegradable and Biocompatible Adhesives for the Effective Stabilisation, Repair and Regeneration of Bone. Bioengineering 2022, 9, 250.
- Tat, S.K.; Pelletier, J.-P.; Mineau, F.; Caron, J.; Martel-Pelletier, J. Strontium ranelate inhibits key factors affecting bone remodeling in human osteoarthritic subchondral bone osteoblasts. Bone 2011, 49, 559–567.
- Hu, S.; Jiang, S.; Qi, X.; Bai, R.; Ye, X.; Xie, T. Races of small molecule clinical trials for the treatment of COVID-19: An up-to-date comprehensive review. Drug Dev. Res. 2022, 83, 16–54.
- Awale, G.M.; Barajaa, M.A.; Kan, H.-M.; Seyedsalehi, A.; Nam, G.H.; Hosseini, F.S.; Laurencin, C.T. Regenerative engineering of long bones using the small molecule forskolin. Proc. Nat. Acad. Sci. USA 2023, 120, e2219756120.
- Jiang, T.; Carbone, E.J.; Lo, K.W.-H.; Laurencin, C.T. Electrospinning of polymer nanofibers for tissue regeneration. Prog. Polym. Sci. 2015, 46, 1–24.
- Goonoo, N.; Bhaw-Luximon, A. Mimicking growth factors: Role of small molecule scaffold additives in promoting tissue regeneration and repair. RSC Adv. 2019, 9, 18124–18146.
- Moore, A.N.; Hartgerink, J.D. Self-Assembling Multidomain Peptide Nanofibers for Delivery of Bioactive Molecules and Tissue Regeneration. Accounts Chem. Res. 2017, 50, 714–722.
- Liu, C.; Yu, Q.; Yuan, Z.; Guo, Q.; Liao, X.; Han, F.; Feng, T.; Liu, G.; Zhao, R.; Zhu, Z.; et al. Engineering the viscoelasticity of gelatin methacryloyl (GelMA) hydrogels via small “dynamic bridges” to regulate BMSC behaviors for osteochondral regeneration. Bioact. Mater. 2022, 25, 445–459.
- Jeon, O.H.; Elisseeff, J. Orthopedic tissue regeneration: Cells, scaffolds, and small molecules. Drug Deliv. Transl. Res. 2016, 6, 105–120.
- Carbone, E.J.; Jiang, T.; Nelson, C.; Henry, N.; Lo, K.W.-H. Small molecule delivery through nanofibrous scaffolds for musculoskeletal regenerative engineering. Nanomed. Nanotechnol. Biol. Med. 2014, 10, 1691–1699.
- Hoshino, M.; Tsujimoto, T.; Yamazoe, S.; Uesugi, M.; Terada, S. Adhesamine, a new synthetic molecule, accelerates differentiation and prolongs survival of primary cultured mouse hippocampal neurons. Biochem. J. 2010, 427, 297–304.
- Yamazoe, S.; Shimogawa, H.; Sato, S.-I.; Esko, J.D.; Uesugi, M. A Dumbbell-Shaped Small Molecule that Promotes Cell Adhesion and Growth. Chem. Biol. 2009, 16, 773–782.
- Braicu, C.; Buse, M.; Busuioc, C.; Drula, R.; Gulei, D.; Raduly, L.; Berindan-Neagoe, I. A Comprehensive Review on MAPK: A Promising Therapeutic Target in Cancer. Cancers 2019, 11, 1618.
- Cau, F.; Fanni, D.; Manchia, M.; Gerosa, C.; Piras, M.; Murru, R.; Faa, G. Expression of L1 Cell Adhesion Molecule (L1CAM) in extracellular vesicles in the human spinal cord during development. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 6273–6282.
- Giger, R.J.; Hollis, E.R., 2nd; Tuszynski, M.H. Guidance Molecules in Axon Regeneration. Cold Spring Harb. Perspect. Biol. 2010, 2, a001867.
- Kataria, H.; Lutz, D.; Chaudhary, H.; Schachner, M.; Loers, G. Small Molecule Agonists of Cell Adhesion Molecule L1 Mimic L1 Functions In Vivo. Mol. Neurobiol. 2016, 53, 4461–4483.
- Nagaraj, V.; Kim, R.; Martianou, T.; Kurian, S.; Nayak, A.; Patel, M.; Schachner, M.; Theis, T. Effects of L1 adhesion molecule agonistic mimetics on signal transduction in neuronal functions. Biochem. Biophys. Res. Commun. 2023, 642, 27–34.
- Xu, J.; Hu, C.; Jiang, Q.; Pan, H.; Shen, H.; Schachner, M. Trimebutine, a small molecule mimetic agonist of adhesion molecule L1, contributes to functional recovery after spinal cord injury in mice. Dis. Models Mech. 2017, 10, 1117–1128.
More
Information
Subjects:
Allergy
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
856
Revisions:
2 times
(View History)
Update Date:
05 Oct 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





