Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Everardo López-Romero | -- | 2208 | 2023-09-28 04:20:40 | | | |
| 2 | Jessie Wu | Meta information modification | 2208 | 2023-09-28 08:25:30 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Ortiz-Ramírez, J.A.; Cuéllar-Cruz, M.; Villagómez-Castro, J.C.; López-Romero, E. Sporothrix and Sporotrichosis. Encyclopedia. Available online: https://encyclopedia.pub/entry/49748 (accessed on 07 February 2026).
Ortiz-Ramírez JA, Cuéllar-Cruz M, Villagómez-Castro JC, López-Romero E. Sporothrix and Sporotrichosis. Encyclopedia. Available at: https://encyclopedia.pub/entry/49748. Accessed February 07, 2026.
Ortiz-Ramírez, Jorge A., Mayra Cuéllar-Cruz, Julio C. Villagómez-Castro, Everardo López-Romero. "Sporothrix and Sporotrichosis" Encyclopedia, https://encyclopedia.pub/entry/49748 (accessed February 07, 2026).
Ortiz-Ramírez, J.A., Cuéllar-Cruz, M., Villagómez-Castro, J.C., & López-Romero, E. (2023, September 28). Sporothrix and Sporotrichosis. In Encyclopedia. https://encyclopedia.pub/entry/49748
Ortiz-Ramírez, Jorge A., et al. "Sporothrix and Sporotrichosis." Encyclopedia. Web. 28 September, 2023.
Copy Citation
Sporothrix belongs to the fungal class Ascomycota and the order Ophiostomatales. It is a genus formed by filamentous fungi found in soil, plants, and decaying organic matter and includes pathogenic species for both humans and animals as well as environmental members.
fungal glycosidases
Sporothrix
substrates
glycoproteins
1. General Aspects
In general, members of the environmental clade do not infect mammals, except for some members of the S. pallida and S. stenoceras complexes [1][2][3]. Species of the pathogenic clinical clade of the genus include S. schenckii sensu stricto, S. brasiliensis, S. globosa, and S. luriei, which are responsible for the mycosis known as sporotrichosis of humans and animals, such as cats and dogs [4][5][6][7]. These species exhibit variations in virulence, transmission, and geographical distribution. Sporothrix is a true dimorphic fungus whose morphology depends on temperature, pH, and other factors, such as cyclic nucleotides [7][8]. At 25–28 °C and an acidic pH, it develops as mycelium, which is considered as the saprophytic phase whereas at higher temperatures (32–37 °C) and a pH of 7.0–7.5, it grows as yeast-like cells, which is the morphotype frequently isolated from infected tissues [7][8][9]. Hyphae form either primary or sympodial and secondary or sessile conidia [10][11][12][13][14]. Figure 1 shows the morphological stages of S. globosa, which are similar to other members of the genus.
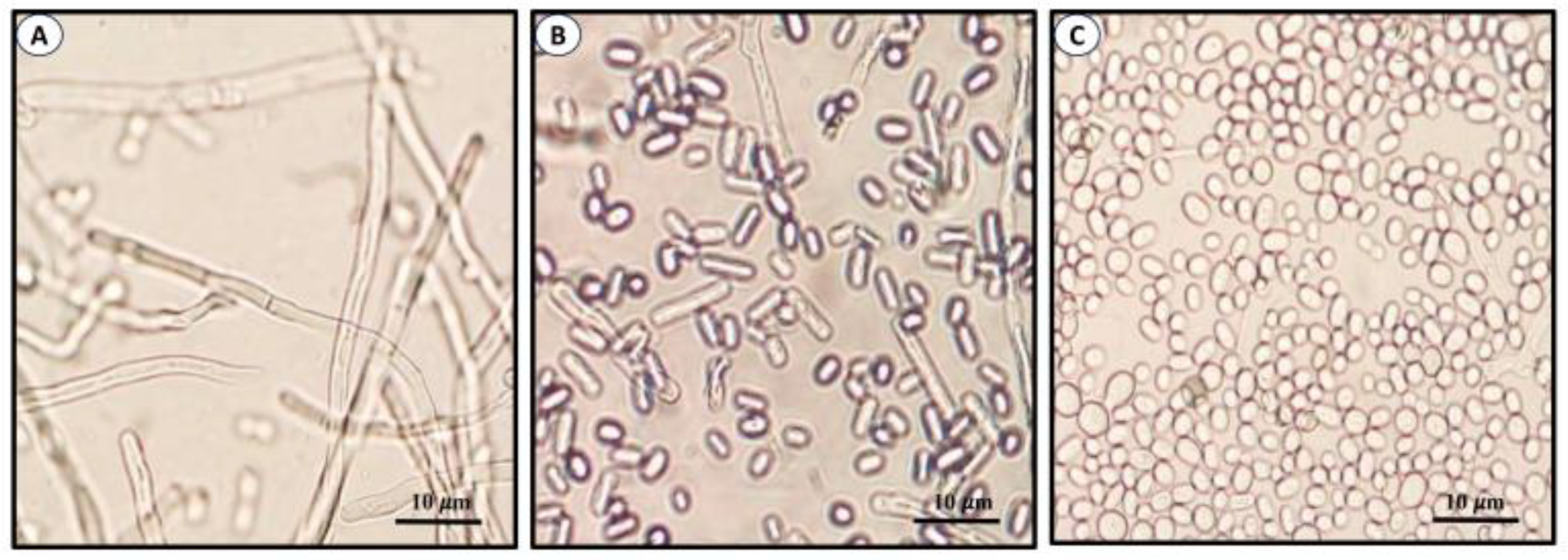
Figure 1. Morphological phases of S. globosa. Images show mycelia or filamentous cells (A), conidia (B), and yeast-like cells (C). Images produced in ELR laboratory.
Sporotrichosis is a cosmopolitan mycosis of humans and animals caused by members of the pathogenic clade of Sporothrix and is common mainly in intertropical areas. The pathogen is transmitted mainly in two ways: (a) the classical route, where soil- or decaying organic matter-borne fungal propagules (sapronosis) are inoculated through trauma and lacerations, and (b) an alternative route, in which the pathogen is inoculated via scratches or bites by infected animals. This route explains the horizontal transmission and zoonosis produced mainly by domestic cats [5][6][10]. On the other hand, infection by S. brasiliensis, an emerging fungal pathogen, can occur exclusively via bite, scratch, spore inhalation, direct contact with secretion, etc. [15].
2. Clinical Manifestations of Sporotrichosis
Sporotrichosis is a benign mycosis of humans. In general, it is limited to the epidermis where it forms lesions, the subcutaneous tissue, and the adjacent lymphatic vessels with occasional dissemination to bone tissue and internal organs. The severity and characteristics of the injuries depend on factors such as the depth of the lesion, the inoculum size, the inoculated morphology, virulence, comorbidities, and the immunological state of the host [16][17][18][19][20][21]. Sporotrichosis has been classified into four clinical categories: (a) fixed cutaneous, (b) lymphocutaneous, (c) multifocal, and (d) extracutaneous (systemic or visceral). It has a bad prognosis as it is associated with immunosuppression [18][19][20][22][23][24][25]. Figure 2 illustrates two of the most common clinical lesions of sporotrichosis.
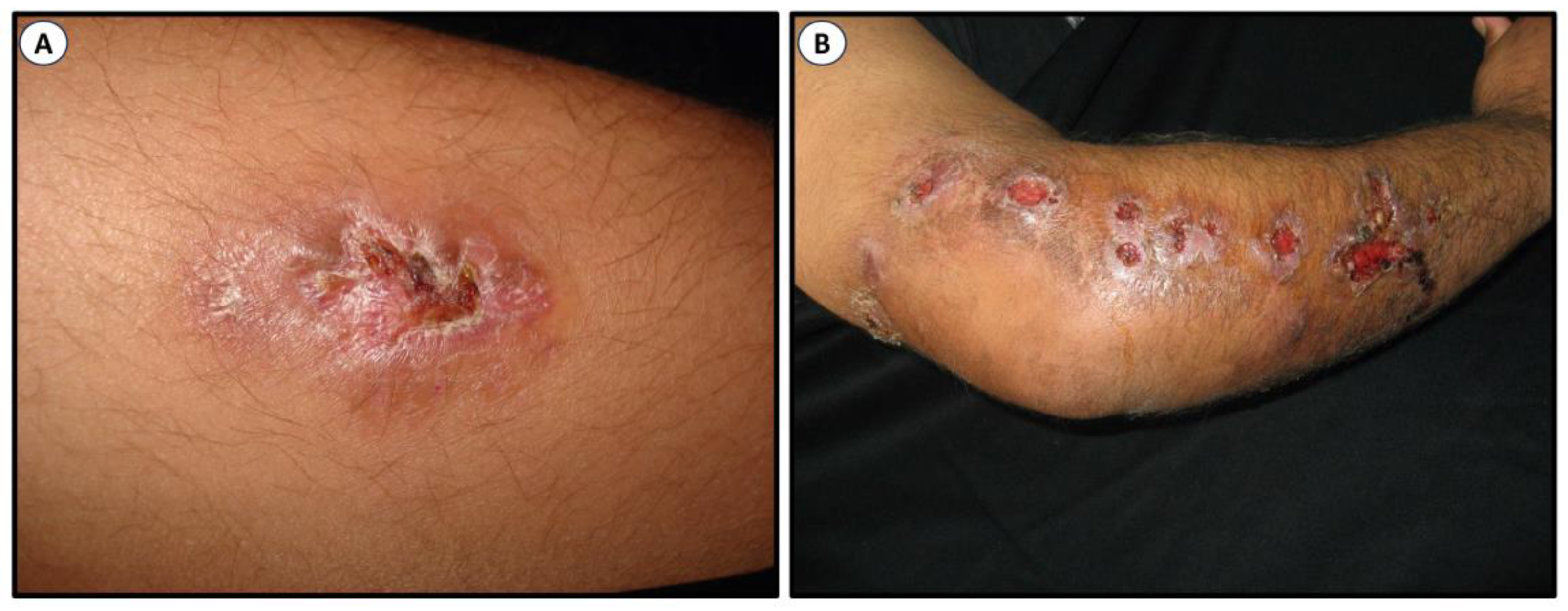
Figure 2. Clinical lesions of sporotrichosis. Fixed cutaneous (A) and classical lymphangitic or subcutaneous (B). Images were kindly provided by Dr. Alexandro Bonifaz, Mexico.
3. The Cell Wall
In most fungi, the cell wall (CW) plays a fundamental role in the pathogenicity and virulence of Sporothrix and other fungi, as it establishes the first contact with the host. It is formed by two layers: a relatively conserved internal layer containing β1,3- and β1,6-glucan, which is alkali-insoluble and linked to chitin by β1,4-linkages, forming a very dynamic exoskeleton that protects the cell from internal (cell membrane and cytoplasmic turgor) and external factors. The external layer is more heterogeneous and adaptable to physiological conditions. Apart from these components, the CW also contains galactomannans, glycoproteins, glycolipids, and melanin [26]. The fungal CW plays a vital role in host–pathogen interaction at most stages of infection, aiding in keeping cell integrity [27]. Various pathogen-associated molecular patterns (PAMPs) are present on the cell surface, which could be recognized by the host immune system’s pattern recognition receptors (PRRs), or they can function as virulence factors, contributing to the infection process and influencing the organism’s pathogenicity [28][29][30]. The proteins deriving from the Sporothrix CW remain insufficiently defined; however, multiple accounts exist regarding specific adhesins that attach to host extracellular matrix (ECM) proteins, including fibronectin, laminin, and type II collagen [31][32][33][34]. Pathogen adhesion to host cells plays a pivotal role in proper colonization and subsequent spread [34].
The β-glucan layer is joined to two classes of proteins: those containing internal repetitions (PIR) and those dependent on glycosil-phosphatidylinositol (GPI) that couple covalently to polysaccharides. Functions such as cell adhesion, masking, and immune blocking have been attributed to these extensions [35][36][37][38][39][40][41][42]. In S. schenckii and S. brasiliensis, the CW structure includes β-glucan microfibrils with β1,3, β1,4, and β1,6 linkages, as well as chitin and a peptidorhamnomannan (PRM), in addition to other homogeneous and heterogeneous polymers and manoproteins. Polysaccharides constitute approximately 80% of the dry weight of the CW, while glycoproteins constitute 20%. In some Sporothrix species, there may be variations, as some lack α-glucan. The absence of α-glucans in the CW of Sporothrix could have diverse consequences on the pathogen physiology, affecting its structural integrity, ability to withstand stress, interaction with the host immune system, colonization efficiency, and other aspects such as energy storage, virulence, and cell signaling [26][35][38][43][44][45][46][47][48][49][50]. Recently, the CW composition was analyzed in conidia, germlings, and yeast-like cells of S. globosa and compared with S. schenckii and S. brasiliensis. It was found that both conidia and yeast-like cells of S. globosa had a higher amount of chitin in their CWs, and all morphologies had more exposed β1,3-glucan on their surface compared to S. schenckii and S. brasiliensis [51]. The composition and structure of the fungal cell wall are influenced not only by environmental conditions but also by the cell cycle, changes in growth form, and other processes [52][53][54][55].
4. Pathogenicity and Virulence
Virulence factors have been recently reviewed in the hyphae, conidia, and yeast-like cells of S. schenckii [56]. Accordingly, while chitin and β1,3-glucans are associated with the virulence of conidia and hyphae, other components are responsible for the virulence of yeast-like cells. These include the antigenic PRM, CW proteins, melanin, secreted and intracellular proteases, extracellular vesicles, and some lipids. Many of the cell surface components are glycoproteins that allow Sporothrix to interact with the host and evade the immune response, thus allowing the pathogen to survive. Some of these virulence factors are discussed below.
Adhesion of the pathogen to target cells is a required condition for fungal infection to occur. Thus, blocking this primary step represents a potential target to prevent it. Some of the CW proteins, generically known as adhesins, bind to host extracellular matrix (ECM) proteins such as laminin, elastin, fibrinogen, fibrinonectin, and others. One of the best-known adhesins is Gp70, which is one of the major antigenic components of the CW and an important immunogenic factor against Sporothrix. Gp70 adhesin was purified, shown to contain 5.7% N-linked oligosaccharides, and shown to be involved in the adhesion of yeast cells to mouse tail dermis [57]. Its presence was further demonstrated in S. schenckii, S. brasiliensis, and S. globosa [58][59]. Later, recombinant Gp70 was expressed in E. coli, and its gene was predicted to encode for a 43 kDa protein. It was immunogenic and contrary to previous findings and contained a much higher amount of oligosaccharide [60]. Thus, Gp70 seems to play the dual role of an adhesin and antigen.
A major antigenic component of S. schenckii CW is PRM, a complex glycoconjugate that was purified from the yeast morphotype and shown to contain 57% mannose, 33.5% rhamnose, 14.2% proteins, and 1% galactose [47][61]. Whereas the sugar composition of RPM has been fully characterized, only a few of the constituent 325 proteins have been identified. Of these, recombinant GroEL/Hsp 60 and the uncharacterized protein Pap1 showed adhesion to ECM proteins and were more abundant in the yeast morphotype during interaction with HeLa cells [22].
A very important virulence factor is dimorphism, which is defined as the ability of certain fungi to switch between unicellular yeast and multicellular filamentous growth, depending on some environmental conditions. Seemingly, the change in the saprophyte to the parasitic form in pathogenic fungi obeys the need of the organism to adapt, grow, and disseminate in internal organs. The fungus enters the host in the form of conidia or short hyphae which, after some time, transforms into yeast-like cells. It is not clear whether this shift occurs extracellularly and then yeast cells are phagocyted or if conidia and hyphae are first internalized and the dimorphic transition occurs inside the cell. Some results suggest that the conversion of conidia to yeast and/or mycelium can occur inside macrophages [62].
In studies to investigate the compensatory cell responses to damage of the CW by perturbing agents, it was observed that 15 μM Congo red inhibits conidia germination of S. schenckii under conditions set for yeast development but not for mycelial growth, even at a 10-fold higher concentration. When the dye was added to yeast cells pre-grown in its absence, cells rapidly differentiated into mycelial cells, suggesting that this shift may be a strategy to evade the noxious effect of the dye [63]. Further studies confirmed the same behavior in S. globosa and showed that hypha returned to yeast-like cells as soon as the dye disappeared. Cell compensatory responses also included significant variations in the activity of glucosamine-6-phosphate synthase, a critical regulatory enzyme of UDP-GlcNAc levels [64][65].
Melanin is a factor of virulence present in the CW of Sporothrix and many other pathogenic fungi. For its importance in pathogenesis, this pigment has been studied from different aspects including its role in cutaneous sporotrichosis [66], synthesis and assembly in fungi [67], structure [68], biosynthesis in pathogenic species of Sporothrix [69], its relationship with the mammalian immune system [70], its synthesis pathway in fungi as a source for fungal toxins [71], and its role as a factor of virulence in S. schenckii [56]. Melanins are structurally complex dark pigment polymers present in all biological systems [72][73] and in fungi, they are synthesized by two pathways, either from 1,8-dihydronaphthalene (DHN) or L-3,4-dihydrophenylalanine (L-DOPA) [70]. The synthesis of pyomelanin in fungi involves oxidizing and polymerizing aromatic amino acids, like tyrosine, forming a three-dimensional melanin network. Pyomelanin provides protective and adaptive properties, helping the fungus resist environmental factors and antifungal agents. Precise synthesis details can vary by fungal species and environmental conditions [74]. In one study, nonpigmented S. schenckii was phagocyted more readily by human monocytes and murine macrophages than its melanized counterpart. It was also observed that the pigment protects the fungus against radiation [75]. In the same line, several characteristics of sporotrichosis induced in rats with wild type (MEL+) and mutant (MEL-) strains of S. schenckii were compared. Among other observed differences, the pigmented strain showed greater tissue invasion giving rise to multifocal granulomas, while the mutant cells restricted the fungus to the core of the granulomas [66]. Melanin also protects fungal cells from reactive oxygen species and nitric oxide released during phagocytosis [56]. Moreover, it has been observed in some fungi that melanin interferes with the immune system as it reduces the effectiveness of phagocytes, binds effector molecules and antifungals, and alters complement and antibody responses [70].
The most important factor of the virulence of Sporothix and many other organisms is the formation of biofilms. These are highly organized structures formed by sessile cells and a variable amount of extracellular polymeric substances (EPS), which function as an impermeable protecting coat that limits the diffusion of chemical substances, leading to recurring infections and resistance to antifungals [76][77][78]. Since the discovery of biofilms, it has been learned that about 90% of microorganisms possess this ability. The formation of biofilms is a complex process that occurs in four phases, namely adhesion, aggregation, maturation, and disaggregation. These colonies of sessile cells can form either on biotic or abiotic surfaces [79][80][81][82], such as medical devices, including catheters, prothesis, pacemakers, and others. A number of properties of biofilms have been studied in several pathogens, particularly Candida albicans and other pathogenic species of the genus [81][82][83][84][85] A biofilm formed by C. albicans is shown in Figure 3A. Recently, a review of materials used to prevent adhesion, growth, and biofilm formation of Candida species [86] was published. Information on biofilm formation by Sporothrix is rather limited. An image of a Sporothrix schenckii biofilm is depicted in Figure 3B. In one study, the ability of Sporothrix spp. to form biofilms in vitro was investigated, as well as the growth, morphology, and sensitivity of sessile cells to antifungals. Results corroborated previous findings observed in other organisms, i.e., that S. schenckii formed well-structured biofilms and the growth of sessile cells was less sensitive to antifungals than planktonic cells [87][88]. Later, it was demonstrated that biofilms formed in vitro by both mycelia and the yeast-like cells of the S. schenckii complex are inhibited by potassium iodide and miltefosine, an antiparasitary drug effective against helminthiasis [89]. Chitosan exhibits antimicrobial activity against many organisms, a function that largely depends on its molecular weight (MW) and deacetylation degree (DD) [90]. In one experiment, low, medium, and high MW chitosans with DD of 75–85% were tested in ten isolates of S. brasiliensis in the filamentous phase. Their effect was measured on planktonic cells, initial adhesion for biofilm formation, and mature biofilms. Low MW chitosan was more inhibitory of both planktonic cells and biofilm formation than the other chitosans, suggesting that low MW chitosan can penetrate and interact with the biofilm more easily than the high MW form, leading to the destruction of the biofilm structure more efficiently [91].
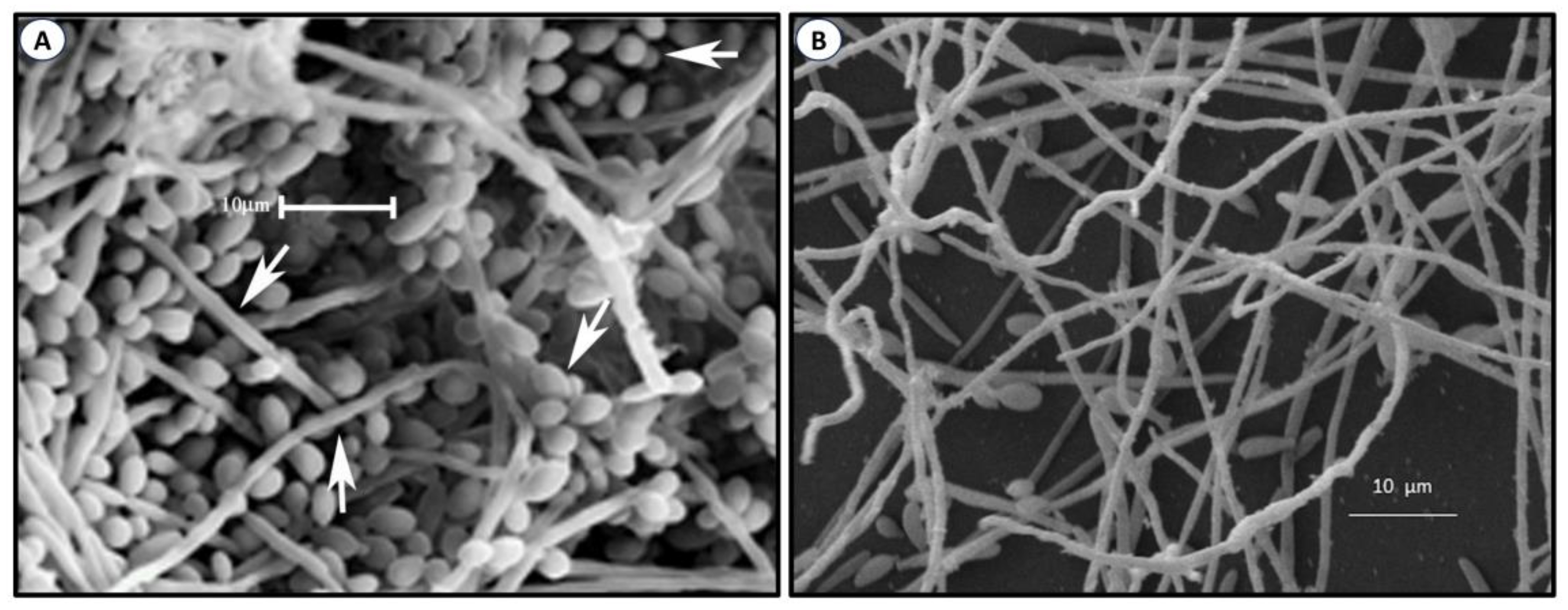
Figure 3. Biofilm formed by C. albicans (A). Adapted with permission from [83], under an open access Creative Common License Deed (CC BY 3.0). Copyright 2015 Hindawi. S. schenckii (B) was produced in the JCVC laboratory.
References
- Nesseler, A.; Schauerte, N.; Geiger, C.; Kaerger, K.; Walther, G.; Kurzai, O.; Eisenberg, T. Sporothrix humicola (Ascomycota: Ophiostomatales)—A soil-borne fungus with pathogenic potential in the eastern quoll (Dasyurus viverrinus). Med. Mycol. Case Rep. 2019, 25, 39–44.
- Rodrigues, A.M.; Della Terra, P.P.; Gremião, I.D.; Pereira, S.A.; Orofino-Costa, R.; de Camargo, Z.P. The threat of emerging and re-emerging pathogenic Sporothrix species. Mycopathologia 2020, 185, 813–842.
- de Carvalho, J.A.; Beale, M.A.; Hagen, F.; Fisher, M.C.; Kano, R.; Bonifaz, A.; Toriello, C.; Negroni, R.; Rego, R.S.d.M.; Gremião, I.D.F.; et al. Trends in the molecular epidemiology and population genetics of emerging Sporothrix species. Stud. Mycol. 2021, 100, 100129.
- Romeo, O.; Criseo, G. What lies beyond genetic diversity in Sporothrix schenckii species complex? Virulence 2013, 4, 203–206.
- Zhang, Y.; Hagen, F.; Stielow, B.; Rodrigues, A.M.; Samerpitak, K.; Zhou, X.; Feng, P.; Yang, L.; Chen, M.; Deng, S.; et al. Phylogeography and evolutionary patterns in Sporothrix spanning more than 14,000 human and animal case reports. Persoonia 2015, 35, 1–20.
- Huang, L.; Gao, W.; Giosa, D.; Criseo, G.; Zhang, J.; He, T.; Huang, X.; Sun, J.; Sun, Y.; Huang, J.; et al. Whole-genome sequencing and in silico analysis of two strains of Sporothrix globosa. Genome Biol. Evol. 2016, 8, 3292–3296.
- Del Valle, N.R.; Rosario, M.; Torres-Blasini, G. Effects of pH, temperature, aeration and carbon source on the development of the mycelial or yeast forms of Sporothrix schenckii from conidia. Mycopathologia 1983, 82, 83–88.
- Del Valle, N.R.; Debs-Elias, N.; Alsina, A. Effects of caffeine, cyclic 3’,5’ adenosine monophosphate and cyclic 3’,5’ guanosine monophosphate in the development of the mycelial form of Sporothrix schenckii. Mycopathologia 1984, 86, 29–33.
- López-Romero, E.; Reyes-Montes, M.D.R.; Pérez-Torres, A.; Ruiz-Baca, E.; Villagómez-Castro, J.C.; Mora-Montes, H.M.; Flores-Carreón, A.; Toriello, C. Sporothrix schenckii complex and sporotrichosis, an emerging health problem. Future Microbiol. 2011, 6, 85–102.
- Rodrigues, A.M.; De Hoog, G.S.; De Camargo, Z.P. Feline sporotrichosis. In Emerging and Epizootic Fungal Infections in Animals; Seyedmousavi, S., Sybren de Hoog, G., Guillot, J., Verweij, P.E., Eds.; Springer: Cham, Switzerland, 2018; pp. 199–231.
- Marimon, R.; Cano, J.; Gené, J.; Sutton, D.A.; Kawasaki, M.; Guarro, J. Sporothrix brasiliensis, S. globosa, and S. mexicana, three new Sporothrix species of clinical interest. J. Clin. Microbiol. 2007, 45, 3198–3206.
- Moussa, T.A.A.; Kadasa, N.M.S.; Al Zahrani, H.S.; Ahmed, S.A.; Feng, P.; van den Ende, A.H.G.G.; Zhang, Y.; Kano, R.; Li, F.; Li, S.; et al. Origin and distribution of Sporothrix globosa causing sapronoses in Asia. J. Med. Microbiol. 2017, 66, 560–569.
- Yu, X.; Wan, Z.; Zhang, Z.; Li, F.; Li, R.; Liu, X. Phenotypic and molecular identification of Sporothrix isolates of clinical origin in northeast China. Mycopathologia 2013, 176, 67–74.
- Ghosh, A.; Maity, P.K.; Hemashettar, B.M.; Sharma, V.K.; Chakrabarti, A. Physiological characters of Sporothrix schenckii isolates. Mycoses 2002, 45, 449–454.
- Rossow, J.A.; Queiroz-Telles, F.; Caceres, D.H.; Beer, K.D.; Jackson, B.R.; Pereira, J.G.; Gremião, I.D.F.; Pereira, S.A. A One Health Approach to Combatting Sporothrix Brasiliensis: Narrative Review of an Emerging Zoonotic Fungal Pathogen in South America. J. Fungi 2020, 6, 247.
- Rodrigues, A.M.; Fernandes, G.F.; De Camargo, Z.P. Sporotrichosis. In Emerging and Re-Emerging Infectious Diseases of Livestock; Bayry, J., Ed.; Springer: Cham, Switzerland, 2017; pp. 391–421.
- Almeida-Paes, R.; De Oliveira, L.C.; Oliveira, M.M.E.; Gutierrez-Galhardo, M.C.; Nosanchuk, J.D.; Zancopé-Oliveira, R.M. Phenotypic characteristics associated with virulence of clinical isolates from the Sporothrix complex. Biomed. Res. Int. 2015, 2015, 212308.
- Zhang, Y.Q.; Xu, X.G.; Zhang, M.; Jiang, P.; Zhou, X.Y.; Li, Z.Z.; Zhang, M.F. Sporotrichosis: Clinical and Histopathological Manifestations. Am. J. Dermatopathol. 2011, 33, 296–302.
- de Lima Barros, M.B.; de Almeida Paes, R.; Schubach, A.O. Sporothrix schenckii and sporotrichosis. Clin. Microbiol. Rev. 2011, 24, 354–633.
- Ramírez Soto, M.C. Differences in clinical ocular outcomes between exogenous and endogenous endophthalmitis caused by Sporothrix: A systematic review of published literature. Br. J. Ophthalmol. 2018, 102, 977–982.
- Orofino-Costa, R.; Rodrigues, A.M.; de Macedo, P.M.; Bernardes-Engemann, A.R. Sporotrichosis: An update on epidemiology, etiopathogenesis, laboratory and clinical therapeutics. An. Bras. Dermatol. 2017, 92, 606–620.
- García-Carnero, L.C.; Salinas-Marín, R.; Lozoya-Pérez, N.E.; Wrobel, K.; Wrobel, K.; Martínez-Duncker, I.; Niño-Vega, G.A.; Mora-Montes, H.M. The heat shock protein 60 and Pap1 participate in the Sporothrix schenckii-host interaction. J. Fungi 2021, 7, 960.
- Orellana, Á.; Moreno-Coutiño, G.; Poletti, E.; Vega, M.E.; Arenas, R. A fixed sporotrichosis with an asteroid body next to a vegetal fragment. Rev. Iberoam. Micol. 2009, 26, 250–251.
- Bravo, T.C. Esporotricosis: Avances recientes en el diagnóstico de laboratorio, histopatología y la epidemiología en México. Rev. Mex. Patol. Clin. Med. Lab. 2012, 59, 147–171.
- Vásquez-del-Mercado, E.; Arenas, R.; Padilla-Desgarenes, C. Sporotrichosis. Clin. Dermatol. 2012, 30, 437–443.
- Travassos, L.R.; Lloyd, K.O. Sporothrix schenckii and related species of Ceratocystis. Microbiol. Rev. 1980, 44, 683.
- Mora-Montes, H.M.; Ponce-Noyola, P.; Villagómez-Castro, J.C.; Gow, N.A.R.; Flores-Carreón, A.; López-Romero, E. Protein glycosylation in Candida. Future Microbiol. 2009, 4, 1167–1183.
- García-Carnero, L.C.; Pérez-García, L.A.; Martínez-Álvarez, J.A.; Reyes-Martínez, J.E.; Mora-Montes, H.M. Current Trends to Control Fungal Pathogens: Exploiting Our Knowledge in the Host–Pathogen Interaction. Infect. Drug. Resist. 2018, 11, 903–913.
- Lin, B.; Qing, X.; Liao, J.; Zhuo, K. Role of Protein Glycosylation in Host-Pathogen Interaction. Cells 2020, 9, 1022.
- Retanal, C.; Ball, B.; Geddes-Mcalister, J. Post-Translational Modifications Drive Success and Failure of Fungal–Host Interactions. J. Fungi 2021, 7, 124.
- Lima, O.C.; Figueiredo, C.C.; Pereira, B.A.S.; Coelho, M.C.P.; Morandi, V.; Lopes-Bezerra, L.M. Adhesion of the Human Pathogen Sporothrix schenckii to Several Extracellular Matrix Proteins. Braz. J. Med. Biol. Res. 1999, 32, 651–657.
- Lima, O.C.; Figueiredo, C.C.; Previato, J.O.; Mendonça-Previato, L.; Morandi, V.; Lopes Bezerra, L.M. Involvement of Fungal Cell Wall Components in Adhesion of Sporothrix schenckii to Human Fibronectin. Infect. Immun. 2001, 69, 6874.
- Lima, O.C.; Bouchara, J.P.; Renier, G.; Marot-Leblond, A.; Chabasse, D.; Lopes-Bezerra, L.M. Immunofluorescence and Flow Cytometry Analysis of Fibronectin and Laminin Binding to Sporothrix schenckii Yeast Cells and Conidia. Microb. Pathog. 2004, 37, 131–140.
- Teixeira, P.A.C.; de Castro, R.A.; Nascimento, R.C.; Tronchin, G.; Torres, A.P.; Lazéra, M.; de Almeida, S.R.; Bouchara, J.P.; Loureiro y Penha, C.V.; Lopes-Bezerra, L.M. Cell Surface Expression of Adhesins for Fibronectin Correlates with Virulence in Sporothrix schenckii. Microbiology 2009, 155, 3730–3738.
- Kollár, R.; Reinhold, B.B.; Petráková, E.; Yeh, H.J.C.; Ashwell, G.; Drgonová, J.; Kapteyn, J.C.; Klis, F.M.; Cabib, E. Architecture of the yeast cell wall. Beta(1-->6)-glucan interconnects mannoprotein, Beta(1-->)3-glucan, and chitin. J. Biol. Chem. 1997, 272, 17762–17775.
- Klis, F.M.; Mol, P.; Hellingwerf, K.; Brul, S. Dynamics of cell wall structure in Saccharomyces cerevisiae. FEMS Microbiol. Rev. 2002, 26, 239–256.
- Beauvais, A.; Latgé, J.P. Special Issue: Fungal Cell Wall. J. Fungi 2018, 4, 91.
- Gow, N.A.R.; Latge, J.-P.; Munro, C.A. The fungal cell wall: Structure, biosynthesis, and function. Microbiol. Spectr. 2017, 5, 1–25.
- Latgé, J.P. The cell wall: A carbohydrate armour for the fungal cell. Mol. Microbiol. 2007, 66, 279–290.
- Wheeler, R.T.; Kombe, D.; Agarwala, S.D.; Fink, G.R. Dynamic, morphotype-specific Candida albicans β-glucan exposure during infection and drug treatment. PLoS Pathog. 2008, 4, e1000227.
- Yadav, U.; Khan, M.A. Targeting the GPI biosynthetic pathway. Pathog. Glob. Health 2018, 112, 115–122.
- Garcia-Rubio, R.; de Oliveira, H.C.; Rivera, J.; Trevijano-Contador, N. The fungal cell wall: Candida, Cryptococcus, and Aspergillus species. Front. Microbiol. 2020, 10, 492056.
- Previato, J.O.; Gorin, P.A.J.; Travassos, L.R. Cell wall composition in different cell types of the dimorphic species Sporothrix schenckii. Exp. Mycol. 1979, 3, 83–91.
- Ruiz-Baca, E.; Alba-Fierro, C.A.; Pérez-Torres, A. Components and virulence factors of the Sporothrix schenckii species complex. In Sporotrichosis: New Developments and Future Prospects; Springer: Cham, Switzerland, 2015; pp. 37–59.
- Fernandes, G.F.; dos Santos, P.O.; Rodrigues, A.M.; Sasaki, A.A.; Burger, E.; de Camargo, Z.P. Characterization of virulence profile, protein secretion and immunogenicity of different Sporothrix schenckii sensu stricto isolates compared with S. globosa and S. brasiliensis species. Virulence 2013, 4, 241–249.
- Ruiz-Baca, E.; Hernández-Mendoza, G.; Cuéllar-Cruz, M.; Toriello, C.; López-Romero, E.; Gutiérrez-Sánchez, G. Detection of 2 immunoreactive antigens in the cell wall of Sporothrix brasiliensis and Sporothrix globosa. Diagn. Microbiol. Infect. Dis. 2014, 79, 328–330.
- Lopes-Bezerra, L.M. Sporothrix schenckii cell wall peptidorhamnomannans. Front. Microbiol. 2011, 2, 16636.
- Alba-Fierro, C.A.; Pérez-Torres, A.; López-Romero, E.; Cuéllar-Cruz, M.; Ruiz-Baca, E. Cell call proteins of Sporothrix schenckii as immunoprotective agents. Rev. Iberoam. Micol. 2014, 31, 86–89.
- Lopes-Bezerra, L.M.; Walker, L.A.; Niño-Vega, G.; Mora-Montes, H.M.; Neves, G.W.P.; Villalobos-Duno, H.; Barreto, L.; Garcia, K.; Franco, B.; Martínez-Álvarez, J.A.; et al. Cell Walls of the Dimorphic Fungal Pathogens Sporothrix schenckii and Sporothrix brasiliensis Exhibit Bilaminate Structures and Sloughing of Extensive and Intact Layers. PLoS Negl. Trop. Dis. 2018, 12, e0006169.
- Arroyo, J.; Farkaš, V.; Sanz, A.B.; Cabib, E. Strengthening the fungal cell wall through chitin–glucan cross-links: Effects on morphogenesis and cell integrity. Cell Microbiol. 2016, 18, 1239–1250.
- García-Carnero, L.C.; Martínez-Duncker, I.; Gómez-Gaviria, M.; Mora-Montes, H.M. Differential recognition of clinically relevant Sporothrix species by human mononuclear cells. J. Fungi 2023, 9, 448.
- Latgé, J.P. Tasting the fungal cell wall. Cell Microbiol. 2010, 12, 863–872.
- Klis, F.M.; Boorsma, A.; De Groot, P.W.J. Cell wall construction in Saccharomyces cerevisiae. Yeast 2006, 23, 185–202.
- Ruiz-Herrera, J.; Victoria Elorza, M.; Valentín, E.; Sentandreu, R. Molecular organization of the cell wall of Candida albicans and its relation to pathogenicity. FEMS Yeast Res. 2006, 6, 14–29.
- Sorais, F.; Barreto, L.; Leal, J.A.; Bernabé, M.; San-Blas, G.; Niño-Vega, G.A. Cell wall glucan synthases and GTPases in Paracoccidioides brasiliensis. Med. Mycol. 2010, 48, 35–47.
- García-Carnero, L.C.; Martínez-Álvarez, J.A. Virulence factors of Sporothrix schenckii. J. Fungi 2022, 8, 318.
- Ruiz-Baca, E.; Toriello, C.; Pérez-Torres, A.; Sabanero-Lopez, M.; Villagómez-Castro, J.C.; López-Romero, E. Isolation and some properties of a glycoprotein of 70 kDa (Gp70) from the cell wall of Sporothrix schenckii involved in fungal adherence to dermal extracellular matrix. Med. Mycol. 2009, 47, 185–196.
- Rodrigues, A.M.; Fernandes, G.F.; Araujo, L.M.; Della Terra, P.P.; dos Santos, P.O.; Pereira, S.A.; Schubach, T.M.P.; Burger, E.; Lopes-Bezerra, L.M.; de Camargo, Z.P. Proteomics-based characterization of the humoral immune response in sporotrichosis: Toward discovery of potential diagnostic and vaccine antigens. PLoS Negl. Trop. Dis. 2015, 9, e0004016.
- Rodrigues, A.M.; Kubitschek-Barreira, P.H.; Fernandes, G.F.; de Almeida, S.R.; Lopes-Bezerra, L.M.; de Camargo, Z.P. Immunoproteomic analysis reveals a convergent humoral response signature in the Sporothrix schenckii complex. J. Proteomics 2015, 115, 8–22.
- Martínez-Álvarez, J.A.; García-Carnero, L.C.; Kubitschek-Barreira, P.H.; Lozoya-Pérez, N.E.; Belmonte-Vázquez, J.L.; De Almeida, J.R.F.; De Gómez-Infante, A.J.; Curty, N.; Villagómez-Castro, J.C.; Peña-Cabrera, E.; et al. Analysis of some immunogenic properties of the recombinant Sporothrix schenckii Gp70 expressed in Escherichia coli. Future Med. 2019, 14, 397–410.
- Lloyd, K.O.; Bitoon, M.A. Isolation and purification of a peptido-rhamnomannan from the yeast form of Sporothrix schenckii. structural and immunochemical Studies. J. Immunol. 1971, 107, 663–671.
- Guzman-Beltran, S.; Perez-Torres, A.; Coronel-Cruz, C.; Torres-Guerrero, H. Phagocytic receptors on macrophages distinguish between different Sporothrix schenckii morphotypes. Microbes Infect. 2012, 14, 1093–1101.
- Sánchez-López, J.F.; González-Ibarra, J.; Macías-Segoviano, J.I.; Cuéllar-Cruz, M.; Álvarez-Vargas, A.; Cano-Canchola, C.; López-Romero, E. Congo Red affects the growth, morphology and activity of glucosamine-6-phosphate synthase in the human pathogenic fungus Sporothrix schenckii. Arch. Microbiol. 2019, 201, 135–141.
- Ortiz-Ramírez, J.A.; Cuéllar-Cruz, M.; López-Romero, E. Responses of Sporothrix globosa to the cell wall perturbing agents Congo Red and Calcofluor White. Antonie Van Leeuwenhoek 2021, 114, 609–624.
- Ortiz-Ramírez, J.A.; Cuéllar-Cruz, M.; López-Romero, E. Cell Compensatory responses of fungi to damage of the cell wall induced by Calcofluor White and Congo Red with emphasis on Sporothrix schenckii and sporothrix globosa. A review. Front. Cell Infect. Microbiol. 2022, 12, 976924.
- Madrid, I.M.; Xavier, M.O.; Mattei, A.S.; Fernandes, C.G.; Guim, T.N.; Santin, R.; Schuch, L.F.D.; Nobre, M.d.O.; Araújo Meireles, M.C. Role of melanin in the pathogenesis of cutaneous sporotrichosis. Microbes Infect. 2010, 12, 162–165.
- Eisenman, H.C.; Casadevall, A. Synthesis and assembly of fungal melanin. Appl. Microbiol. Biotechnol. 2012, 93, 931–940.
- Nosanchuk, J.D.; Stark, R.E.; Casadevall, A. Fungal melanin: What do we know about structure? Front. Microbiol. 2015, 6, 173098.
- Almeida-Paes, R.; Borba-Santos, L.P.; Rozental, S.; Marco, S.; Zancopé-Oliveira, R.M.; da Cunha, M.M.L. Melanin biosynthesis in pathogenic species of Sporothrix. Fungal Biol. Rev. 2017, 31, 50–59.
- Liu, S.; Youngchim, S.; Zamith-Miranda, D.; Nosanchuk, J.D. Fungal melanin and the mammalian immune system. J. Fungi 2021, 7, 264.
- Gao, J.; Wenderoth, M.; Doppler, M.; Schuhmacher, R.; Marko, D.; Fischer, R. Fungal melanin biosynthesis pathway as source for fungal toxins. mBio 2022, 13, e00219-22.
- Riley, P.A. Melanin. Int. J. Biochem. Cell Biol. 1997, 29, 1235–1239.
- Suwannarach, N.; Kumla, J.; Watanabe, B.; Matsui, K.; Lumyong, S. Characterization of melanin and optimal conditions for pigment production by an endophytic fungus, Spissiomyces endophytica SDBR-CMU319. PLoS ONE 2019, 14, e0222187.
- Almeida-Paes, R.; Figueiredo-Carvalho, M.H.G.; Brito-Santos, F.; Almeida-Silva, F.; Oliveira, M.M.E.; Zancopé-Oliveira, R.M. Melanins Protect Sporothrix brasiliensis and Sporothrix schenckii from the Antifungal Effects of Terbinafine. PLoS ONE 2016, 11, e0152796.
- Romero-Martinez, R.; Wheeler, M.; Guerrero-Plata, A.; Rico, G.; Torres-Guerrero, H. Biosynthesis and functions of melanin in Sporothrix schenckii. Infect. Immun. 2000, 68, 3696–3703.
- Allewell, N.M. Introduction to Biofilms Thematic Minireview Series. J. Biol. Chem. 2016, 291, 12527–12528.
- Nogueira Brilhante, R.S.; Correia, E.E.M.; De Melo Guedes, G.M.; Pereira, V.S.; De Oliveira, J.S.; Bandeira, S.P.; De Alencar, L.P.; De Andrade, A.R.C.; Collares Maia Castelo-Branco, D.d.S.; De Aguiar Cordeiro, R.; et al. Quantitative and structural analyses of the in vitro and ex vivo biofilm-forming ability of dermatophytes. J. Med. Microbiol. 2017, 66, 1045–1052.
- Ortega-Peña, S.; Hernández-Zamora, E. Microbial biofilms and their impact on medical areas: Physiopathology, diagnosis and treatment. Bol. Med. Hosp. Infant. Mex. 2018, 75, 79–88.
- Fanning, S.; Mitchell, A.P. Fungal biofilms. PLoS Pathog. 2012, 8, e1002585.
- Finkel, J.S.; Mitchell, A.P. Genetic control of Candida albicans biofilm development. Nat. Rev. Microbiol. 2011, 9, 109–118.
- Al-Fattani, M.A.; Douglas, L.J. Biofilm matrix of Candida albicans and Candida tropicalis: Chemical composition and role in drug resistance. J. Med. Microbiol. 2006, 55, 999–1008.
- Kernien, J.F.; Snarr, B.D.; Sheppard, D.C.; Nett, J.E. The Interface between fungal biofilms and innate immunity. Front. Immunol. 2018, 8, 328714.
- Serrano-Fujarte, I.; López-Romero, E.; Reyna-López, G.E.; Martínez-Gámez, M.A.; Vega-González, A.; Cuéllar-Cruz, M. Influence of culture media on biofilm formation by Candida species and response of sessile cells to antifungals and oxidative stress. Biomed. Res. Int. 2015, 2015, 783639.
- Nobile, C.J.; Johnson, A.D. Candida albicans biofilms and human Disease. Annu. Rev. Microbiol. 2015, 69, 71–92.
- Pemmaraju, S.C.; Padmapriya, K.; Pruthi, P.A.; Prasad, R.; Pruthi, V. Impact of oxidative and osmotic stresses on Candida albicans biofilm formation. Biofouling 2016, 32, 897–909.
- Tornero-Gutiérrez, F.; Ortiz-Ramírez, J.A.; López-Romero, E.; Cuéllar-Cruz, M. Materials used to prevent adhesion, growth and biofilm formation of Candida species. Med. Mycol. 2023, 61, myad065.
- Brilhante, R.S.N.; De Aguiar, F.R.M.; Da Silva, M.L.Q.; De Oliveira, J.S.; De Camargo, Z.P.; Rodrigues, A.M.; Pereira, V.S.; Serpa, R.; De Souza Collares Maia Castelo-Branco, D.; Correia, E.E.M.; et al. Antifungal susceptibility of Sporothrix schenckii complex biofilms. Med. Mycol. 2018, 56, 297–306.
- Brilhante, R.S.N.; Fernandes, M.R.; Pereira, V.S.; Costa, A.d.C.; de Oliveira, J.S.; de Aguiar, L.; Rodrigues, A.M.; de Camargo, Z.P.; Pereira-Neto, W.A.; Sidrim, J.J.C.; et al. Biofilm formation on cat claws by Sporothrix species: An ex vivo model. Microb. Pathog. 2021, 150, 104670.
- Brilhante, R.S.N.; Da Silva, M.L.Q.; Pereira, V.S.; De Oliveira, J.S.; Maciel, J.M.; Da Silva, I.N.G.; Garcia, L.G.S.; Guedes, G.M.D.M.; Cordeiro, R.D.A.; Pereira-Neto, W.D.A.; et al. Potassium iodide and miltefosine inhibit biofilms of Sporothrix schenckii species complex in yeast and filamentous forms. Med. Mycol. 2019, 57, 764–772.
- Goy, R.C.; De Britto, D.; Assis, O.B.G. A Review of the antimicrobial activity of chitosan. Polímeros 2009, 19, 241–247.
- Garcia, L.G.S.; de Melo Guedes, G.M.; Fonseca, X.M.Q.C.; Pereira-Neto, W.A.; Castelo-Branco, D.S.C.M.; Sidrim, J.J.C.; de Aguiar Cordeiro, R.; Rocha, M.F.G.; Vieira, R.S.; Brilhante, R.S.N. Antifungal activity of different molecular weight chitosans against planktonic cells and biofilm of Sporothrix brasiliensis. Int. J. Biol. Macromol. 2020, 143, 341–348.
More
Information
Subjects:
Microbiology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.1K
Revisions:
2 times
(View History)
Update Date:
28 Sep 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




