Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Bin Lian | -- | 2240 | 2023-09-26 12:45:08 | | | |
| 2 | Lindsay Dong | Meta information modification | 2240 | 2023-09-28 02:49:00 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Li, S.; Lian, B. Calcium Carbonate as a Drugs Controlled Release Carrier. Encyclopedia. Available online: https://encyclopedia.pub/entry/49666 (accessed on 07 February 2026).
Li S, Lian B. Calcium Carbonate as a Drugs Controlled Release Carrier. Encyclopedia. Available at: https://encyclopedia.pub/entry/49666. Accessed February 07, 2026.
Li, Siying, Bin Lian. "Calcium Carbonate as a Drugs Controlled Release Carrier" Encyclopedia, https://encyclopedia.pub/entry/49666 (accessed February 07, 2026).
Li, S., & Lian, B. (2023, September 26). Calcium Carbonate as a Drugs Controlled Release Carrier. In Encyclopedia. https://encyclopedia.pub/entry/49666
Li, Siying and Bin Lian. "Calcium Carbonate as a Drugs Controlled Release Carrier." Encyclopedia. Web. 26 September, 2023.
Copy Citation
A drug carrier usually refers to a tool that can carry the effective ingredients of drugs into the human body. The drug-controlled release system prepared by a new drug carrier can allow the gradual release of the drug in the human body at a stable rate, thus decreasing the frequency of administration and reducing the toxicity and side effects thereof; however, existing drug carriers generally have problems such as low drug loading, poor biocompatibility, stability, and specificity, each of which could be improved. Calcium carbonate can be used as a sustained-release carrier of active substances, with good biocompatibility, biodegradability, low cost, easy preparation, and broad application prospects.
calcium carbonate
drug carrier
controlled release effect
1. Introduction
Traditional drug delivery methods include oral and injection methods; however, these drug delivery methods often have problems such as high drug dosage, frequent use of drugs, strong irritation to the gastrointestinal tract, non-specific drug release, and unsustainable drug delivery. Therefore, enhancing drugs’ sustained and controlled release is critical for current clinical drug therapy. Drug carrier materials usually require drug carriers to be non-toxic, low-cost, capable of loading an appropriate dose of the drug, and able to release the drug at a stable and effective concentration for a certain period and to the correct target site [1]. However, in many drug-controlled release systems, the initial explosive effect of drugs often occurs, causing toxic side effects and harm to the body’s organs. Therefore, investigating suitable drug delivery carriers significantly reduces the biological toxicity and adverse effects of therapeutic drugs [2].
The controlled release carrier of therapeutic drugs refers to a class of carrier preparations that can release the loaded drugs to the target point or target organ in a controlled form at a constant speed (at a low or near-zero-order rate) to play a therapeutic role. Compared with traditional large-size drug carriers (such as collagen, gum Arabic, and alginate), inorganic carrier materials such as calcium carbonate, calcium phosphate, and silicon dioxide are characterized by hollow or porous structure, micro-nano particle size, high hydrophilicity, and specific surface area, which can not only significantly improve the loading rate and utilization rate of ligands or functional, active substances, it can also achieve efficient delivery of water-insoluble drugs [3]. However, the alginic acid gel is fragile, soluble in water, and has poor tensile properties and traditional manufacturing methods cannot guarantee the consistency of its structure [4]. The slow degradation rate of silicon dioxide and refractory properties in the body also limit its use [5]. As a drug carrier, calcium phosphate is more difficult to dissolve. It requires gastric acid dissociation to be absorbed and utilized by the human body, which can easily affect the acidic environment in the stomach. When the level of gastric acid is lacking, low (or non-existent), the dissociation is incomplete, its bioavailability is reduced, and it is more likely to cause gastrointestinal irritation symptoms [6]. Among them, calcium carbonate is widely present in the teeth, shells, pearls, and gallstones of living organisms. It has excellent biocompatibility and bioavailability, conferring outstanding advantages as a drug carrier. Connecting specific calcium carbonate materials with targeting ligands or functional, active substances to prepare targeted carriers with loaded active substances has enormous potential in targeted drug delivery and controlled release [7].
2. Synthesis and Structural Characteristics of Calcium Carbonate Carrier Materials
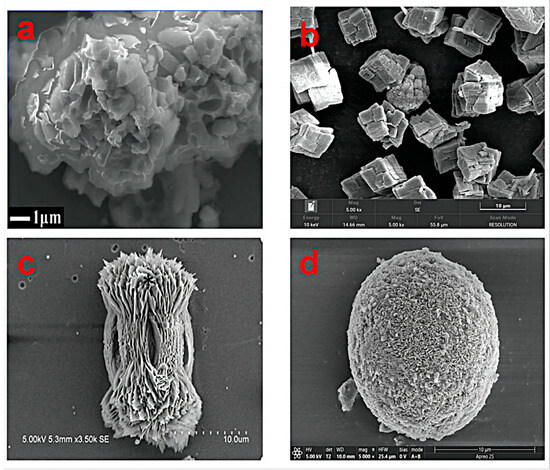
The common forms of calcium carbonate as one of the common inorganic substances on the earth include amorphous calcium carbonate, calcite, aragonite and vaterite (Figure 1). It exists in chalk, limestone, marble, and other rocks and is the main component of animal bones or shells. In addition, there are several forms of calcium carbonate in the form of hydrate, of which calcite is the most thermodynamic stable crystal phase (other forms are thermodynamically [8]).

Figure 1. Morphologies of several typical calcium carbonates ((a) amorphous calcium carbonate; (b) calcite; (c) aragonite; (d) vaterite).
Although calcium carbonate materials are widely distributed, the components of carbonate rocks formed in different geological deposition environments and geological ages vary greatly, especially the post-deposition alteration, which can affect the cementation, secondary pore formation and morphological changes in carbonate particles. In addition, naturally formed carbonate rocks will also contain some amounts of associated minerals—such as silicates, phosphates, glauconite and hydrogenic rock minerals, and it is difficult to ensure a low cost of production and the quality of carrier materials with such raw materials for making drug carriers. Therefore, it is necessary to investigate the effective synthetic methods of calcium carbonate for drug carrier materials to reduce the cost and improve the loading efficiency and controlled release performance of calcium carbonate carriers.
Calcium carbonate is a white fine crystalline powder that is odorless, low-density, and acid-soluble. Crystalline calcium carbonate can also be divided into a rhombic crystal system and a hexagonal crystal system (e.g., anhydrous calcium carbonate is an orthorhombic crystal, while hexahydrate calcium carbonate is a monoclinic crystal), which typically appears as prismatic or rhombic in shape. Aragonite commonly occurs in prismatic, tabular, bipyramidal, and rhombic forms [9]. Aragonite belongs to the orthorhombic system, the crystal form thereof is columnar or spear-like, and there are often pseudo-hexagonal triple crystals. Vaterite-type calcium carbonate is a polycrystal formed by the aggregation of nanoscale spherical particles belonging to the hexagonal crystal system. The CO32− triangular plane is oblique to the third axis, resulting in the carbonate group of aragonites being parallel to the C-axis, while the carbonate group of calcites is perpendicular to the C-axis, which also leads to the instability and looseness of vaterite [10]. Vaterite has a polycrystalline form, so it is difficult to study its crystal structure and characteristics [3].
As an essential phase in carbonate, vaterite has been found in many geological environments, such as marine and freshwater crustaceans, bird eggs, salmon inner ears, meteorites, rocks in caves, etc. Vaterite is generally considered to be the most thermodynamically unstable. A phase change will occur when it exists in an aqueous solution for 20 to 25 h at room temperature [11], and it will generally be converted into the stable phase of calcite [12]. However, compared with vaterite found in the natural environment, vaterite induced by microorganisms under experimental conditions seems more stable.
At present, the commonly used methods for preparing vaterite mainly include the carbonization method and double decomposition method [3]. The carbonization method refers to the Ca (OH)2-H2O-CO2 reaction system, that is, the alkaline solution containing calcium salt is used as the calcium source to prepare vaterite by introducing CO2 into the solution. The double decomposition method refers to the Ca2+-H2O-CO32− reaction system, that is, the calcium salt solution and carbonate solution were mixed under certain conditions to prepare vaterite. Several studies have shown that vaterite’s crystal form and morphology can be controlled by using these two methods. Due to the fact that the carbonization method utilizes the reaction between CO32− obtained from CO2 dissolution and Ca2+ to generate calcium carbonate, the rate of calcium carbonate generation depends on the rate of dissolution of CO2. Under normal temperature and pressure, the dissolution rate of CO2 is generally low, so the yield of vaterite prepared by the carbonization method is low. The double decomposition method entails direct mixing of the two salt solutions, and CO32− reacts rapidly with Ca2+, producing a high yield, endowing the method with good prospects for large-scale application; however, it should be pointed out that the use of this method often requires the addition of surfactants to stabilize the crystal form and morphology of the vaterite.
3. Calcium Carbonate as a Controlled-Release Drug Carrier
The history of research on calcium carbonate as a drug carrier can be traced back to the 1960s [13]. At that time, researchers [14] used the double oil in a water emulsion system as a biomimetic environment to prepare calcium carbonate particles. They proposed that calcium carbonate can be used as a drug carrier according to the synthesized particle morphology. Since then, the research on calcium carbonate as a drug carrier has gradually gained widespread attention. Currently, the application of calcium carbonate as a drug carrier is mainly used in oral drugs, cancer treatment, topical drugs, bone repair materials, gene delivery and other drug carriers.
(1) Oral drugs: calcium carbonate can be used as a slow-release agent to control the rate of release of the drug, thus enhancing the efficacy of oral drugs. In addition, calcium carbonate can also act as a protective agent to protect the drug from damage by gastric acid. CaCO3-based composite nanocarriers have the potential for pulmonary aerosol delivery of peptides and proteins and oral delivery of insulin.
(2) Cancer therapy: taking advantage of the sensitivity of drug-loaded calcium carbonate to acidic conditions, the drug can be released slowly after being transported to specific organs or tissues with pH slightly acidic in the body, which can improve the drug utilization rate [15]. Li et al. [16] prepared hollow calcium carbonate microspheres with sodium dodecyl sulphate as the surfactant and CaCl2 and Na2CO3 as the substrate and loaded ibuprofen. The results showed that the microspheres had both a good loading rate and rate of drug release; because calcium carbonate is very stable in alkaline body fluid (pH is about 7.4), the loaded drug will barely be released, which can greatly reduce the damage to normal cells. In the acidic environment of cancer cells, calcium carbonate loaded with anticancer drugs such as doxorubicin hydrochloride (DOX) can be released slowly.
(3) Topical drugs: calcium carbonate mainly acts as an adsorbent and protective agent in topical drugs, enhancing the permeability and stability of the drugs. As mentioned, calcium carbonate can also be used as a slow-release agent to control the rate of release of the drug, thus enhancing the efficacy of the drug. Volodkin et al. [17] prepared porous calcium carbonate microspheres for loading lactalbumin and applied them in clinical research. The results showed that the aragonite-type calcium carbonate has good drug-loading capacity. The use of high-molecular-weight polymer improves the encapsulation efficiency because the polymer is only adsorbed on the surface of porous particles and does not come into contact with materials previously adsorbed in the pores. The preparation can be used to encapsulate biomaterials, such as proteins, enzymes, and combinations of various bioactive macromolecules.
(4) Special biotherapeutic materials: Kim’s research team [18] coated calcium carbonate nano-materials into a flexible biocompatible nano-carrier based on Plannik and used it as an ultrasound contrast agent (UECA). The results showed that this nanoscale UECA showed high colloidal stability in the medium containing serum and greatly improved the intensity of ultrasound imaging contrast signal at tumor sites within 1 h: This indicates that nanoscale UECA can be used as an effective diagnostic method for ultrasound imaging of tumors in vivo. The CaCO3 nanoparticles in the nano-carrier prepared by the research group are not completely crystalline but form a mixture in a relatively unstable state. After intravenous injection of the nano-UECA into tumor-bearing mice, UECA can preferentially target the tumor site within 1 h without targeting ligands and then be rapidly cleared from the body.
4. Summary
In summary, as an inorganic controlled release carrier, calcium carbonate shows excellent characteristics in drug delivery applications. It can potentially provide new strategies for the long-term treatment of many malignant diseases, especially cancer. However, to make this emerging inorganic controlled release carrier more widely used in clinical drug therapy, the following issues need to be addressed:
- (1)
-
The application research of calcium carbonate prepared in the laboratory in drug carrier needs to be expanded, such as the loading and slow-release ability and stability of enzymes, proteins, and other active substances, the safety of calcium carbonate carriers from different sources and types in the human body, and how to ensure the new metabolism to ensure that the drug carrier can be rapidly discharged from the body after playing its role, which warrants further research;
- (2)
-
The conditions in the preparation process, such as temperature, pH value and calcium ion concentration, will be further explored to endow the calcium carbonate microspheres with more effective morphological characteristics and microsphere size distributions, the better to load drugs and improve their utilization;
- (3)
-
The optimal conditions of the release process, such as drug loading, loading time, release rate, and environmental factors, should be investigated to achieve the goal of accurate, targeted, stable release and delivery of drugs.
Although significant progress has been made in synthesising these calcium carbonate biomaterials, the true biomedical challenge requires further breakthroughs in synthesising complex multi-component carriers with high repeatability and homogeneity to achieve effective treatment at the single-cell level. The application of microfluidic technology is one of the feasible methods to overcome these limitations, opening up new avenues for the synthesis of advanced nanostructured materials [19]. The emerging interdisciplinary technology based on microfluidic technology is currently at the forefront of laboratory methods and a unique method for producing nanometers and microparticles. Microfluid technology has various advanced characteristics, such as high repeatability, small differences between batches, precise control of particle characteristics, and ease of scaling up. Although predicting the future development of CaCO3 nanoparticle and microparticle synthesis may be challenging, the overall progress trend driven by state-of-the-art science and technology is evident. In addition, artificial intelligence methods are the next development direction for solving practical problems in science and technology, including the development of new materials. These technologies go far beyond the scope of computer science and provide new insights for professional fields in chemical applications. One highly attractive application is the automated material synthesis laboratory driven by artificial intelligence. The introduction of advanced laboratory automation and artificial intelligence methods into Microfluidics technology will determine the future of new material synthesis and known reaction optimization.
References
- Wu, M.; Wu, M.; Awangjimi; Tao, Y.S. Research progress of clinical application of different nano-drug carriers. Chin. Pharm. Sci. 2022, 31, 128–131.
- Li, K.Z.; Liu, Y.H.; Dai, Y.H.; Wang, D.K. Research progress of gold nanoparticles as anti-tumor drug carriers. Chin. Pharm. Sci. 2022, 20, 139–155.
- Wang, Y.X.; Xu, Y.; Wang, D.P.; Li, X.K. Properties and applications of vaterite. Anhui Univ. Nat. Sci. 2017, 37, 76–80.
- Yin, F.M.; Zhang, K.; Xue, W.T. Application and research progress of alginic acid. Cereals Oils 2022, 35, 12–15.
- Li, C.; Wan, J.; Wang, Y. Recent progress in drug delivery. Acta Pharm. Sin. B 2019, 9, 1145–1162.
- Munir, M.U.; Salman, S.; Javed, I.; Bukhari, S.; Ahmad, N.; Shad, N.A.; Aziz, F. Nano-hydroxyapatite as a delivery system: Overview and advancements. Artif. Cells Nanomed. Biotechnol. 2021, 49, 717–727.
- Qiu, L.; Wang, C.; Cui, P.F.; Zhou, S.H.; Hu, H.A.Z.; Jiang, P.J.; Wang, J.H. Application of calcium carbonate based nano drug delivery system in cancer therapy. Jiangsu Polytech. Univ. 2023, 35, 78–85.
- Rautaray, D.; Sainkar, R.; Sastry, M. Thermally evaporated aerosol ot thin films as templates for the room temperature synthesis of aragonite crystals. Chem. Mater. 2003, 15, 2809–2814.
- Wang, J.; Zhang, T.; Qiu, Y.Q.; Li, L.J.; Ye, J.J.; Cui, W.Y. The first principles of the crystal structure and active sites of calcite. Chin. J. Eng. Des. 2017, 39, 487–493.
- Shaikh, A.W. A new crystal growth form of vaterite, CaCO3. J. Appl. Crystallogr. 1990, 23, 263–269.
- Ogino, T.; Suzuki, T.; Sawada, K. The formation and transformation mechanism of calciumcarbonate in water. Geochim. Cosmochim. Acta 1987, 51, 2757–2767.
- Lv, J.J.; Ma, F.; Li, F.C.; Chen, J.N. Vaterite induced by Lysinibacillus sp. GW-2 strainand its stability. Struct. Biol. 2017, 200, 97–105.
- Fadia, P.; Tyagi, S.; Bhagat, S.; Nair, A.; Panchal, P.; Dave, H.; Dang, S.; Singh, S. Calcium carbonate nano- and microparticles: Synthesis methods and biological applications. 3 Biotech 2021, 11, 457.
- Takayuki, H.; Shuichi, H.; Isao, K. Biomimetic synthesis of calcium carbonate particles in a pseudovesicular double emulsion. Langmuir 1997, 13, 6650–6653.
- Trushina, D.B.; Borodina, T.N.; Belyako, S.; Antipina, M.N. Calcium carbonatevaterite particles for drug delivery: Advances and challenges. Mater. Today Adv. 2022, 14, 100214.
- Li, L.; Zhu, Y.J.; Cao, S.W.; Ma, M.Y. Preparation and drug release properties of nanostructured CaCO3 porous hollow microspheres. Inorg. Mater. 2009, 24, 166–170.
- Volodkin, D.V.; Larionova, N.I.; Sukhorukov, G.B. Protein encapsulationvia porous CaCO3 microparticles templating. Biomacromolecules 2004, 5, 1962–1972.
- Kim, M.; Lee, J.H.; Kim, S.E.; Tae, G. Nanosized ultrasound enhanced-contrast agent for in vivo tumor imaging via intravenous injection. ACS Appl. Mater. Interfaces 2016, 8, 8409–8418.
- Lengert, E.V.; Trushina, D.B.; Soldatov, M.; Ermakov, A.V. Microfluidic synthesis and analysis of bioinspired structures based on CaCO3 for potential applications as drug delivery carriers. Pharmaceutics 2022, 14, 139.
More
Information
Subjects:
Materials Science, Biomaterials
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
687
Revisions:
2 times
(View History)
Update Date:
28 Sep 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




