Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Maria Pia Ferraz | -- | 2198 | 2023-09-26 11:43:50 | | | |
| 2 | Jason Zhu | Meta information modification | 2198 | 2023-09-27 03:30:02 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Ferraz, M.P. Microbiome Influence on Atopic Dermatitis. Encyclopedia. Available online: https://encyclopedia.pub/entry/49657 (accessed on 07 February 2026).
Ferraz MP. Microbiome Influence on Atopic Dermatitis. Encyclopedia. Available at: https://encyclopedia.pub/entry/49657. Accessed February 07, 2026.
Ferraz, Maria Pia. "Microbiome Influence on Atopic Dermatitis" Encyclopedia, https://encyclopedia.pub/entry/49657 (accessed February 07, 2026).
Ferraz, M.P. (2023, September 26). Microbiome Influence on Atopic Dermatitis. In Encyclopedia. https://encyclopedia.pub/entry/49657
Ferraz, Maria Pia. "Microbiome Influence on Atopic Dermatitis." Encyclopedia. Web. 26 September, 2023.
Copy Citation
It is acknowledged that humans have a diverse and abundant microbial community known as the human microbiome. Nevertheless, the comprehension of the numerous functions these microorganisms have in human health is still in its early stages. Microorganisms belonging to the human microbiome typically coexist with their host, but in certain situations, they can lead to diseases. They are found in several areas of the human body in healthy individuals. The microbiome is highly diverse, and its composition varies depending on the body site.
human microbiome
skin microbiota
atopic dermatitis
1. Atopic Dermatitis
AD, also known as atopic eczema, is a chronic inflammatory skin disease characterized by dry and red skin, intense itching, and recurrent scaly lesions with a typical distribution [1][2][3][4][5]. The pathogenesis of the disease is not fully understood; however, it appears to result from a complex interaction between defects in the skin barrier function, environmental and infectious agents, and immune system dysregulation [6]. Although it mostly begins in childhood, it is also prevalent in adults, being the leading non-fatal health problem attributed to skin diseases, inflicting a significant psychosocial weight on patients and their families, elevating the likelihood of conditions such as food allergies, asthma, allergic rhinitis, and other immune-related inflammatory ailments, along with mental health issues [7]. Therefore, it carries a relevant weight in healthcare systems [2]. AD is classified as mixed or pure. Mixed AD represents approximately 60% of cases and is associated with respiratory symptoms. On the other hand, pure AD is classified when there is no associated respiratory disease [8].
The symptoms of AD differ depending on the age group. Infants commonly experience AD symptoms on the scalp, face, neck, trunk, and the outer parts of the limbs. In children, the areas affected usually include the inner parts of the limbs such as the elbows, knees, neck, wrists, and ankles. During adolescence and adulthood, the inner parts of the limbs, hands, and feet are frequently more affected. Regardless of age, the itchiness associated with AD typically persists throughout the day and intensifies at night, causing sleep deprivation and significantly reducing the quality of life [7].
In all stages of the disease, the lesions can present as acute, subacute, or chronic eczematous. These stages can occur sequentially, although in most cases, they coexist in different regions of the body or even in the same region, indicating the characteristic episodic evolution of the condition [8].
Due to similarities in some symptoms, it can sometimes be challenging to differentiate AD from other skin conditions (such as seborrheic dermatitis, contact dermatitis, psoriasis, or scabies). Nevertheless, in many instances, the diagnosis is aided by the presence of atopy in the family history and the pattern of lesion distribution [6].
2. Dysbiosis in AD Patients
The exact cause of AD is not completely understood; dysbiosis of the skin microbiota is believed to play a significant role in its development and exacerbation. Therefore, the molecular mechanisms of the underlying dysbiosis of the skin microbiota in atopic dermatitis will be briefly described [9]. Dysbiosis in AD is often associated with a decrease in microbial diversity on the skin, usually referred to as altered microbial diversity. The reduced diversity is thought to be driven by immune dysregulation in AD patients [10]. An overactive immune response can create an inhospitable environment for beneficial bacteria. AD is characterized by an abnormal immune response, including increased levels of pro-inflammatory cytokines such as interleukin-4 (IL-4), interleukin-13 (IL-13), and interleukin-22 (IL-22) [11]. These cytokines contribute to skin inflammation and barrier dysfunction. In response to inflammation, the skin may produce antimicrobial peptides (AMPs) such as defensins and cathelicidins, which can disrupt the balance of skin bacteria [12]. These peptides are part of the skin’s innate immune defense system, but can also harm beneficial bacteria. An altered lipid composition is also important to AD development. The skin’s lipid barrier, composed of lipids such as ceramides, plays a critical role in maintaining the skin’s integrity and preventing water loss. In AD, there is a disruption in the composition of these lipids that affects the growth and survival of certain skin microorganisms that metabolize specific lipid substrates, leading to imbalances in the skin microbiota [13]. Staphylococcus aureus is often found in higher abundance on the skin of AD patients compared to healthy individuals. This bacterium can produce toxins and virulence factors that exacerbate skin inflammation. Staphylococcus aureus can outcompete other commensal bacteria in AD patients, further reducing microbial diversity [14]. A compromised skin barrier, also called an impaired skin barrier, is a hallmark of AD. It allows for increased transepidermal water loss and the penetration of allergens, irritants, and microbes into the skin. The damaged skin barrier can create an environment that is conducive to the growth and colonization of pathogenic bacteria while hindering the growth of beneficial microbes [14]. Genetic factors also play a role in AD susceptibility. Mutations in genes related to skin barrier function and immune regulation, such as filaggrin (FLG) and various cytokine genes, can increase the risk of AD and contribute to dysbiosis [15].
In summary, dysbiosis of the skin microbiota in atopic dermatitis is a complex interplay of altered microbial composition, immune dysregulation, changes in skin lipids, and impaired skin barrier function. These molecular mechanisms collectively contribute to the chronic inflammation and skin manifestations seen in AD. Understanding these mechanisms is crucial for developing targeted treatments to restore the balance of the skin microbiota and alleviate symptoms in AD patients.
The mechanisms of the skin microbiota’s influence on AD pathogenesis are depicted in Figure 1.
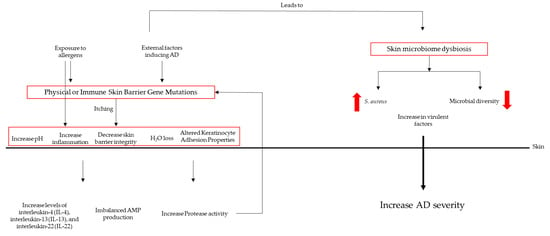
Figure 1. Mechanisms of skin microbiota’s influence on AD pathogenesis.
3. Microbial Colonization
Profound changes in the skin microbiota occur in some patients with AD, and the pathogenic importance of microbial organisms is recognized [16]. The diversity of the microbiome decreases in inflamed atopic skin, with a reduction in the genera Streptococcus, Corynebacterium, and Cutibacterium, as well as the phylum Proteobacteria, in favor of an increase in the genus Staphylococcus, particularly S. aureus [17].
In AD, there is a notable loss of strictly anaerobic bacteria, altering the skin’s microbiome from anaerobic to aerobic metabolism. Healthy skin is normally devoid of oxygen, but dry and scaly skin with compromised epidermal barrier function can increase oxygenation and decrease the abundance of strictly anaerobic bacteria such as Lactobacillus spp. or Finegoldia spp. In the absence of oxygen, skin bacteria metabolize organic substances through fermentation, such as the amino acid serine derived from the breakdown of filagrine, producing lactic acid, propionic acid, and other short-chain fatty acids. These metabolic products reduce the skin’s pH to below 5.5, preserving the skin’s protective acidic environment. Additionally, Gram-positive anaerobic cocci such as Finegoldia, Anaerococcus, and Peptoniphilus stimulate a rapid antimicrobial peptide (AMP) response in human keratinocytes, which could be an important signaling mechanism for keratinocytes when the skin is damaged. When these organisms are partially or completely absent, the signaling mechanisms in keratinocytes and other protective functions may be compromised, increasing the likelihood of S. aureus colonization [18].
Colonization by S. aureus on AD skin has been directly linked to disease severity, but, as mentioned earlier, the role of other constituents within the skin’s bacterial community may be just as significant [2]. Dysbiosis related to AD is often characterized by colonization by S. aureus and the simultaneous loss of other potentially beneficial species, although the loss of anaerobes in AD does not seem to be driven by the presence of S. aureus [18].
The skin of AD harbors a microbial growth environment that is very different from that of normal skin, and this may be crucial in explaining the dysbiosis observed in AD. A dysfunctional physical barrier of the skin leads to an increased pH on the skin surface, which favors the growth of S. aureus [2]. Besides the frequent presence of S. aureus on the skin of people with AD, there are other factors that strengthen the idea that the microbiota has a significant impact on the development of the disease [1].
4. The Role of S. aureus in Atopic Dermatitis
AD has a known relation with altered skin microbiota, with a high prevalence of colonization by S. aureus and secondary infections that were recognized long before the application of DNA sequencing approaches [17]. S. aureus colonizes approximately 9 out of 10 patients with AD [6], and it can initiate or exacerbate skin diseases either through barrier defects or altered immunity [1].
S. aureus is detectable in more than 90% of AD skin, although it is less frequently detected in healthy skin [19]. AD has been associated with a notable rise in the prevalence of S. aureus and a decline in anaerobic species [18], and it can act as a persistent allergen, stimulating the production of IgE antibodies, or as an irritant with inflammatory potential when colonizing atopic skin [16]. Beside the awareness of S. aureus’s capacity to induce inflammation and the occurrence of dysbiosis in different skin conditions, it remains uncertain whether these alterations are a result of the disease itself or if S. aureus plays a role in initiating the disease [1].
In AD, S. aureus dominates the microbial landscape and negatively correlates with various skin commensals such as Staphylococcus epidermidis and Corynebacterium spp., thus potentially eliminating the regulatory or protective potential of these microorganisms [18]. The quantity of Staphylococcus spp., particularly S. aureus and S. epidermidis, is higher during the eruption period (episodic exacerbation) of AD compared to the post-eruption period. However, individuals with more-severe exacerbations are colonized with dominant strains of S. aureus. The correlation of S. aureus with AD results in the exacerbation of the pathology [1]. It is important to note that S. epidermidis may limit the growth of S. aureus, and the severity of the disease is inversely correlated with the abundance of S. epidermidis versus the abundance of S. aureus [18].
There is a sophisticated relationship between the host and S. aureus, where host factors, including the hostile environment created by the physical, chemical, and antimicrobial properties of healthy skin, may be altered in AD skin, facilitating colonization. Specific pathogen factors include highly evolved mechanisms that facilitate adhesion, epidermal invasion, and pro-inflammatory mechanisms, promoting or exacerbating the inflammatory component of AD [17].
The prevalence of S. aureus carriers in patients with AD is about 70% on lesional skin compared to about 39% on non-lesional or healthy skin in the same patient [17]. There is a higher abundance of Staphylococcus spp. at 2 months in newborns who do not develop AD by 1 year of age compared to those who do develop AD by 1 year. This suggests that exposure to Staphylococcus spp. at an early age is beneficial for proper immune system education [1].
S. aureus plays a highly influential role in the pathogenesis of AD, is associated with severe disease flares, and significantly influences the disease phenotype [17].
5. Gut Microbiota in AD Patient
In a typical healthy gut, Firmicutes, Bacteroidetes, Actinobacteria, and Proteobacteria are present. These categories are quite consistent overall, but the specific types of bacteria within them can vary from person to person.
Xue et al. discovered that certain groups of bacteria, such as Tenericutes, Mollicutes, Clostridia, Bifidobacteriaceae, Bifidobacteriales, Bifidobacterium, Christensenellaceae R7, Bacilli, and Anaerostipes, were linked to a reduced risk of developing atopic dermatitis (AD) [20]. On the other hand, bacteria groups such as the Eubacterium hallii group, Clostridiaceae_1, Bacteroidaceae, Bacteroides, Anaerotruncus, an unknown genus, and Lachnospiraceae UCG 001 appear to be potential factors that could increase the risk of AD [20].
Park et al. noticed differences in the gut bacteria of children with atopic dermatitis (AD). Children with transient AD had lower levels of Streptococcus bacteria, but higher levels of Akkermansia. Conversely, children with persistent AD had the opposite pattern [21]. Moreover, in children with AD, the Clostridium genus became more abundant. There were also changes in the functional genes of their gut bacteria related to energy metabolism and short-chain fatty acid (SCFA) production, with a decrease in these functions. This conclusion was supported by another study by Lee, who used metagenomic analysis and arrived at a similar finding [22].
Yap et al. showed that, in children with early-stage AD, higher levels of Enterococcus and Shigella were present. Interestingly, these higher levels of these bacteria have been linked to increased production of serotonin, which, in turn, worsens skin pigmentation. Furthermore, the abundance of Bifidobacterium, a beneficial type of bacteria, tends to decrease in these children [23].
Several other studies are being conducted to clarify the relation between the gut microbiota and AD, enhancing the knowledge about the microbiota profiles in AD patients.
Several studies are confirming the idea that the immune responses in AD are influenced by the gut microbiota [24]. Figure 2 summarizes this information and focuses on three key aspects: (1) the anti-inflammatory effects of short-chain fatty acids (SCFAs), (2) the impact of tryptophan metabolites on the aryl hydrocarbon receptor (AHR) pathway, and (3) the role of toll-like receptor signaling, which can be influenced by the gut microbiota and is highly relevant to AD. Briefly, the gut microbiota, in particular Bifidobacteria, produces short-chain fatty acids, which activate SCFA-sensing G-protein-coupled receptors and inhibit histone deacetylases. These phenomena reduce inflammatory responses and promote TH1/TH2 equilibrium. This equilibrium is also helped with the production of D-tryptophan, which, in turn, is a microbial metabolite, which can also activate aryl hydrocarbon receptors, inhibiting inflammatory responses and promoting the skin barrier. TH1/TH2 balance is also promoted by molecular patterns associated with the presence of pathogens produced by the gut microbiota.
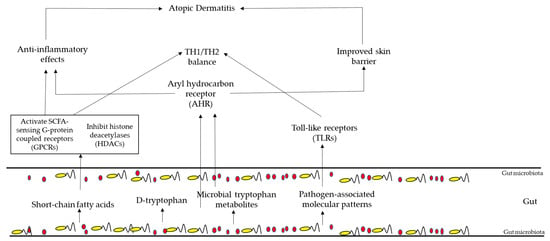
Figure 2. AD pathogenesis regulation by the gut microbiota.
References
- Byrd, A.L.; Belkaid, Y.; Segre, J.A. The human skin microbiome. Nat. Rev. Microbiol. 2018, 16, 143–155.
- Williams, M.R.; Gallo, R.L. The role of the skin microbiome in atopic dermatitis. Curr. Allergy Asthma Rep. 2015, 15, 65.
- Ziehfreund, S.; Tizek, L.; Hangel, N.; Fritzsche, M.C.; Weidinger, S.; Smith, C.; Bryce, P.J.; Greco, D.; van den Bogaard, E.H.; Flohr, C.; et al. Requirements and expectations of high-quality biomarkers for atopic dermatitis and psoriasis in 2021-a two-round Delphi survey among international experts. J. Eur. Acad. Dermatol. Venereol. 2022, 36, 1467–1476.
- Harder, I.; Stolzl, D.; Sander, N.; Hartmann, J.; Rodriguez, E.; Mazur, C.; Kerzel, S.; Kabesch, M.; Kuster, D.; Schmitt, J.; et al. Effects of Early Emollient Use in Children at High Risk of Atopic Dermatitis: A German Pilot Study. Acta Derm. Venereol. 2023, 103, adv5671.
- Paller, A.S.; Weidinger, S.; Capozza, K.; Pink, A.E.; Tang, M.; Guillaume, X.; Praestgaard, A.; Leclerc, M.; Chuang, C.C.; Thomas, R.B.; et al. Similarities and Differences in the Perception of Atopic Dermatitis Burden Between Patients, Caregivers, and Independent Physicians (AD-GAP Survey). Dermatol. Ther. 2023, 13, 961–980.
- Kapur, S.; Watson, W.; Carr, S. Atopic dermatitis. Allergy Asthma Clin. Immunol. 2018, 14, 52.
- Weidinger, S.; Novak, N. Atopic dermatitis. Lancet 2016, 387, 1109–1122.
- Tokura, Y.; Hayano, S. Subtypes of atopic dermatitis: From phenotype to endotype. Allergol. Int. 2022, 71, 14–24.
- Blicharz, L.; Rudnicka, L.; Czuwara, J.; Waskiel-Burnat, A.; Goldust, M.; Olszewska, M.; Samochocki, Z. The Influence of Microbiome Dysbiosis and Bacterial Biofilms on Epidermal Barrier Function in Atopic Dermatitis-An Update. Int. J. Mol. Sci. 2021, 22, 8403.
- Lunjani, N.; Ahearn-Ford, S.; Dube, F.S.; Hlela, C.; O’Mahony, L. Mechanisms of microbe-immune system dialogue within the skin. Genes. Immun. 2021, 22, 276–288.
- Kim, K.; Kim, H.; Sung, G.Y. An Interleukin-4 and Interleukin-13 Induced Atopic Dermatitis Human Skin Equivalent Model by a Skin-On-A-Chip. Int. J. Mol. Sci. 2022, 23, 2116.
- Yamasaki, K.; Gallo, R.L. Antimicrobial peptides in human skin disease. Eur. J. Dermatol. 2008, 18, 11–21.
- Li, S.; Villarreal, M.; Stewart, S.; Choi, J.; Ganguli-Indra, G.; Babineau, D.C.; Philpot, C.; David, G.; Yoshida, T.; Boguniewicz, M.; et al. Altered composition of epidermal lipids correlates with Staphylococcus aureus colonization status in atopic dermatitis. Br. J. Dermatol. 2017, 177, e125–e127.
- Demessant-Flavigny, A.L.; Connetable, S.; Kerob, D.; Moreau, M.; Aguilar, L.; Wollenberg, A. Skin microbiome dysbiosis and the role of Staphylococcus aureus in atopic dermatitis in adults and children: A narrative review. J. Eur. Acad. Dermatol. Venereol. 2023, 37 (Suppl. S5), 3–17.
- Tamai, M.; Yamazaki, Y.; Ito, T.; Nakagawa, S.; Nakamura, Y. Pathogenic role of the staphylococcal accessory gene regulator quorum sensing system in atopic dermatitis. Front. Cell Infect. Mi 2023, 13, 435.
- Ring, J.; Ring, J.; Przybilla, B.; Ruzicka, T. Handbook of Atopic Eczema, 2nd ed.; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 2006.
- Paller, A.S.; Kong, H.H.; Seed, P.; Naik, S.; Scharschmidt, T.C.; Gallo, R.L.; Luger, T.; Irvine, A.D. The microbiome in patients with atopic dermatitis. J. Allergy Clin. Immunol. 2019, 143, 26–35.
- Fyhrquist, N.; Muirhead, G.; Prast-Nielsen, S.; Jeanmougin, M.; Olah, P.; Skoog, T.; Jules-Clement, G.; Feld, M.; Barrientos-Somarribas, M.; Sinkko, H.; et al. Microbe-host interplay in atopic dermatitis and psoriasis. Nat. Commun. 2019, 10, 4703.
- D’Auria, E.; Banderali, G.; Barberi, S.; Gualandri, L.; Pietra, B.; Riva, E.; Cerri, A. Atopic dermatitis: Recent insight on pathogenesis and novel therapeutic target. Asian Pac. J. Allergy Immunol. 2016, 34, 98–108.
- Xue, Y.; Zhang, L.; Chen, Y.; Wang, H.; Xie, J. Gut microbiota and atopic dermatitis: A two-sample Mendelian randomization study. Front. Med. 2023, 10, 1174331.
- Park, Y.M.; Lee, S.Y.; Kang, M.J.; Kim, B.S.; Lee, M.J.; Jung, S.S.; Yoon, J.S.; Cho, H.J.; Lee, E.; Yang, S.I.; et al. Imbalance of Gut Streptococcus, Clostridium, and Akkermansia Determines the Natural Course of Atopic Dermatitis in Infant. Allergy Asthma Immunol. Res. 2020, 12, 322–337.
- Lee, M.J.; Kang, M.J.; Lee, S.Y.; Lee, E.; Kim, K.; Won, S.; Suh, D.I.; Kim, K.W.; Sheen, Y.H.; Ahn, K.; et al. Perturbations of gut microbiome genes in infants with atopic dermatitis according to feeding type. J. Allergy Clin. Immunol. 2018, 141, 1310–1319.
- Yap, G.C.; Loo, E.X.; Aw, M.; Lu, Q.; Shek, L.P.; Lee, B.W. Molecular analysis of infant fecal microbiota in an Asian at-risk cohort-correlates with infant and childhood eczema. BMC Res. Notes 2014, 7, 166.
- McKenzie, C.; Tan, J.; Macia, L.; Mackay, C.R. The nutrition-gut microbiome-physiology axis and allergic diseases. Immunol. Rev. 2017, 278, 277–295.
More
Information
Subjects:
Dermatology
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
546
Revisions:
2 times
(View History)
Update Date:
27 Sep 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




