Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Jin, S.; Newton, M.A.A.; Cheng, H.; Zhang, Q.; Gao, W.; Zheng, Y.; Lu, Z.; Dai, Z.; Zhu, J. Types of Skin Wounds and Wound Healing. Encyclopedia. Available online: https://encyclopedia.pub/entry/49636 (accessed on 07 February 2026).
Jin S, Newton MAA, Cheng H, Zhang Q, Gao W, Zheng Y, et al. Types of Skin Wounds and Wound Healing. Encyclopedia. Available at: https://encyclopedia.pub/entry/49636. Accessed February 07, 2026.
Jin, Shanshan, Md All Amin Newton, Hongju Cheng, Qinchen Zhang, Weihong Gao, Yuansheng Zheng, Zan Lu, Zijian Dai, Jie Zhu. "Types of Skin Wounds and Wound Healing" Encyclopedia, https://encyclopedia.pub/entry/49636 (accessed February 07, 2026).
Jin, S., Newton, M.A.A., Cheng, H., Zhang, Q., Gao, W., Zheng, Y., Lu, Z., Dai, Z., & Zhu, J. (2023, September 26). Types of Skin Wounds and Wound Healing. In Encyclopedia. https://encyclopedia.pub/entry/49636
Jin, Shanshan, et al. "Types of Skin Wounds and Wound Healing." Encyclopedia. Web. 26 September, 2023.
Copy Citation
Chronic wounds encompass various types, each exhibiting distinct characteristics that are influenced by changes in the wound microenvironment. Parameters such as temperature, pH value, blood glucose concentration, and pressure undergo fluctuations depending on the type and condition of the wound. Hydrogels, composed of hydrophilic polymers, are promising materials for constructing multifunctional platforms. These hydrogels possess several advantageous properties, including a 3D network structure, high permeability to water and oxygen, and biocompatibility.
wound monitoring
treatment
hydrogel
1. Overview of Skin Trauma
The skin is the primary physical barrier between the human body and the environment into which it comes in contact. It is crucial for thermoregulation and defense against foreign pathogens [1]. In general, the physiological structure of normal skin is divided into the epidermis and dermis. The epidermis layer, which is directly related to the outside world, mainly includes the keratinocyte layer and the germinal layer and has functions such as preventing tissue fluid outflow, anti-friction, and anti-infection. The dermis comprises dense connective tissue with papillary and reticular layers, from superficial to deep. Among them, the papillary layer of the skin is rich in capillaries, lymphatic vessels, nerve endings, tactile bodies, and other receptors, contributing to sensory perception and vascular supply. In contrast, the reticular layer predominantly comprises collagen, elastic, and reticular fibers, providing mechanical strength to the skin [2]. A wound is a defect or damage of the skin caused by external injury-causing factors (such as external force, surgery, thermal injury, chemical substances, etc.) or internal factors of the human body (such as physiological lesions of the body itself), which is usually accompanied by the breakdown of the structural integrity of the skin and the impairment of its functionality.
2. Types of Wound
There are numerous types of skin trauma, and wounds can be classified according to various methods [3]. Wounds can be classified into open wounds (such as abrasions, punctures, and lacerations) and closed wounds (such as crush injuries, contusions, blast injuries, and hematomas), depending on their location and exposure [4]. The wound’s depth can be divided into superficial wounds, partial cortical injury wounds, and entire cortical injury wounds. External wounds involving only the skin’s epidermis entirely heal within ten days. For some wounds that include cortical injury, the healing process is accompanied by scar formation and re-epithelialization, which basically takes 10 to 21 days. However, entire cortical injury wounds exhibiting damage to the dermis and subcutaneous tissue sites take longer to heal. According to the contamination status, wounds can be divided into clean, contaminated, and infected. The classification “clean wound” generally refers to a sterile surgical incision without contamination, such as the incisions made for liver and kidney surgery and thyroid surgery, or a blister that has not yet been contaminated, such as a wound formed by the removal of blister skin via a sterile operation in the complete blister of a second-degree scald. Contaminated wounds refer to injuries contaminated with bacteria that have not yet become infected, involving wounds of the digestive, respiratory, or reproductive systems, including acute trauma wounds. An infected wound is when the surrounding bacteria or pathogenic bacteria in the environment enter the body after the skin is damaged, causing infection. There is then inflammatory secretion at the wound, accompanied by local symptoms of swelling, heat, or pain [5].
Wound healing can be categorized into the healing of acute and chronic wounds, based on their healing time. Acute wounds are caused by sudden trauma and typically heal within a relatively short period. Conversely, chronic wounds are characterized by a delayed healing process that does not follow the standard and orderly repair sequence, resulting in the incomplete restoration of normal tissue. Various factors contribute to the development of chronic wounds, including conditions such as diabetic ulcers, venous ulcers, arterial ulcers, traumatic ulcers, and pressure ulcers. These wounds pose significant challenges due to their impaired healing potential and require specialized management strategies to promote successful wound closure [6]; chronic wounds significantly burden patients and the healthcare system [7]. Chronic wounds can be divided into infection wounds, burn injuries, diabetic ulcers, and pressure ulcers. They share standard features such as the upregulation of protease levels, elevated proinflammatory cytokines, excessive reactive oxygen species (ROS) levels, the persistence of senescent fibroblasts, prolonged infection, and stem cell dysfunction or insufficiency [8]. However, the characteristics of different types of chronic wounds and the underlying pathological mechanisms are diverse.
2.1. Infected Wounds
In chronic wound repair, a bacterial infection often occurs at the wound site. Once the wound is infected, bacteria will trigger persistent inflammation in the infected area, affecting the healing process or even causing the injury to fail to heal [9]. The clinical manifestations of infected wounds are erythema, edema, warmth, and the aggravation of pain. There is increased exudation or drainage from the wound and a growing stench. If the patient develops systemic symptoms, such as fever, chills, and leukocytosis, the infection will progress to bacteremia or sepsis [10]. Therefore, controlling wound infection is considered one of the most crucial challenges in bioengineering applications.
2.2. Burn Wounds
Burns are one of the most common and devastating forms of wounds, and the evaluation of burn patients involves two key parameters: wound depth and total burn area. First-degree burns affect the superficial layer of the epidermis, while superficial second-degree burns involve the epidermis and dermis. Deep second-degree burns extend through the entire epidermis and dermis. Third-degree burns are the most severe, affecting the epidermis, dermis, and subcutaneous tissue. The classification of burns into these degrees aids in assessing the severity of the injury and guiding appropriate treatment strategies for optimal healing and recovery [11]. Second-degree and third-degree burns impair many vital functions of the epidermis and dermis. Severe burns, characterized by extensive tissue damage, can be life-threatening due to factors such as severe infection, hyperinflammation, reduced angiogenesis, insufficient production of the extracellular matrix, and inadequate stimulation of vascular growth factors (GFs). When the skin is affected by heat, rapid and dangerous fluid loss occurs in the body, along with condensation and the loss of proteins, including immunoglobulins, potentially leading to irreversible tissue damage and raising susceptibility to infection. In addition, cell membrane dysfunction can cause severe changes in the distribution of water and sodium in the body, while the loss of extracellular fluid and sodium consumption can further reduce blood volume and alter the electrolyte balance, leading to the death of burn patients [12][13][14].
2.3. Diabetic Wounds
Diabetes mellitus, a prevalent metabolic disorder, has become a significant global health concern. Diabetic wounds exhibit distinct characteristics that are caused by hyperglycemia, chronic inflammation, hypoxia, inadequate vascularization, cellular infiltration, and fragile granulation tissue. These factors collectively impair the normal skin regeneration process, resulting in challenges for physiological wound healing. Consequently, treating diabetic wounds poses considerable difficulties in achieving successful closure and restoration. The intricate interplay of these pathophysiological factors underscores the need for specialized approaches to address the unique healing complexities associated with diabetic wounds. As a result, diabetic wounds take longer to heal than ordinary chronic wounds, and severe diabetic wounds, such as diabetic foot ulcers, can require amputation. Moreover, diabetic wounds often exhibit prolonged healing periods, with some cases persisting for an average duration of 12 to 13 months. Additionally, there is a general trend of diabetic wounds having a recurrence rate of 60 to 70%. However, it is essential to note that individual cases may vary regarding healing time and recurrence risk [15]. Treating diabetic wounds constitutes at least 12–15% of the total expenditure for diabetes treatment, contributing to 40% of the national healthcare costs. The complex pathogenesis, pathogen invasion, and high incidence increase the cost and difficulty of treatment and seriously affect the comfort and health of patients [12][16][17][18]. Treating diabetic wounds is particularly difficult since the wound requires the highly ordered and continuous residency and recruitment of cells, GFs, and cytokines to facilitate healing [19].
2.4. Pressure Ulcers
Pressure ulcers, also known as pressure sores, usually occur in areas where the bones protrude, such as the sacrum (the base of the spine), buttocks, and heels [20]. Pressure ulcers are caused by prolonged pressure, friction, or shear forces that impair the blood supply to the affected area and cause tissue malnutrition. This type of pressure ulcer is common in patients with reduced mobility, paralysis, coma, or the long-term bedridden. In such patients, it is not possible to release pressure areas adequately. The prolonged exposure of an area of the body to pressure interrupts local blood circulation and triggers a series of biochemical changes that may lead to tissue damage and ulceration [10]. According to the severity of the wound, pressure ulcers can be divided into four stages. The first stage is congestion and redness, mainly in the form of local skin swellings, pain, and numbness. The second stage is the inflammatory infiltration stage; the skin will turn purple, and the affected area has induration and is accompanied by pain. Stage three is the superficial ulcer stage, where the affected area will be ulcerated, and the subcutaneous tissue will be exposed. Stage 4 is the necrotic ulcer stage; patients will develop symptoms of necrosis in the affected area, and this may even cause sepsis. Pressure ulcers that cannot be staged in this way are typically characterized by a full-thickness loss of tissue, a covering of the decaying flesh at the base of the ulcer (yellow, tan, gray, green, or brown), or an eschar attachment to the wound bed (carbon, brown, or black) [21]. The patients themselves experience malnutrition, long-term bed rest, and even paraplegia, along with some nerve loss, leading to difficult wound healing. Some patients are also in difficult economic situations; therefore, it is difficult to heal the wounds of such patients with pressure ulcers. In the early stages, systematic and standardized treatment plans should be formulated, including strengthening the nutrition of patients, with sequential treatment and the treatment of wounds.
3. The Process of Wound Healing
When skin tissue is injured, the body’s immune system immediately initiates a cascade of chemical signals between the different tissue cells, including immune function cells. At the same time, human biological signal-triggering molecules will immediately start the subsequent wound repair process [22]. As shown in Figure 1, the healing process of chronic wounds is mainly divided into four stages, namely, the hemostasis stage, the inflammation stage, the tissue proliferation stage, and the tissue remodeling stage [23].
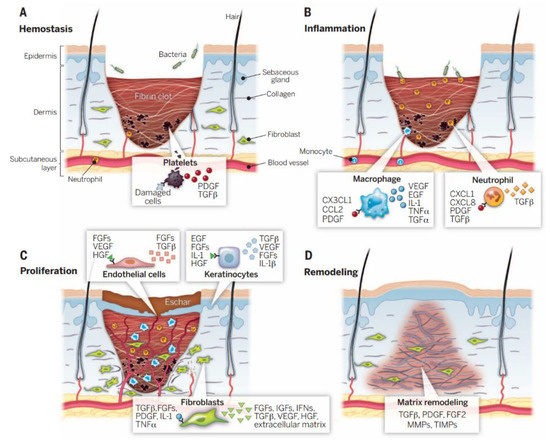
Figure 1. Wound healing is a complex process that can be broadly divided into four sequential stages: (A) hemostatic stage, (B) inflammatory stage, (C) proliferative stage, and (D) remodeling stage [24].
During the hemostasis phase, tissue coagulation is triggered immediately after skin trauma. Platelets in the blood components come into contact with exposed collagens and other elements of the body’s natural extracellular matrix. This contact quickly triggers the release of platelet coagulation factors, after which many endothelial cells gather. The dynamic balance between platelet-induced coagulation and fibrinolysis collaborates to regulate the hemostatic responses, vasoconstriction, and the exudation of blood and tissue fluid [25][26]. During the inflammatory phase, white blood cells will enter the wound area from the capillaries around the wound tissue and absorb a large amount of tissue-inflammatory substances. The inflammatory cells will then release a large amount of growth factors. The signal factors will immediately stimulate macrophages and other immune cells and will continue to migrate to the wound to phagocytose cell debris. The injury will appear red, swollen, hot, and painful, in a pathological phenomenon [3]. In the stage of tissue proliferation, along with the migration of fibroblasts, new ECM tissue structures are continuously synthesized at the wound site, and a large amount of ECM accumulation will further promote cell migration [27]. Finally, during the stage of tissue remodeling, the newly generated collagen matrix becomes more directional, and new epithelial tissue and scar tissue gradually form [28].
References
- Chong, E.J.; Phan, T.T.; Lim, I.J.; Zhang, Y.Z.; Bay, B.H.; Ramakrishna, S.; Lim, C.T. Evaluation of electrospun PCL/gelatin nanofibrous scaffold for wound healing and layered dermal reconstitution. Acta Biomater. 2007, 3, 321–330.
- Boer-Auer, A.; Schacht, V. Histopathology of the skin-clinically relevant and innovative. Hautarzt 2018, 69, 526–527.
- Dabiri, G.; Damstetter, E.; Phillips, T. Choosing a Wound Dressing Based on Common Wound Characteristics. Adv. Wound Care 2016, 5, 32–41.
- Nethi, S.K.; Das, S.; Patra, C.R.; Mukherjee, S. Recent advances in inorganic nanomaterials for wound-healing applications. Biomater. Sci. 2019, 7, 2652–2674.
- Ambekar, R.S.; Kandasubramanian, B. Advancements in nanofibers for wound dressing: A review. Eur. Polym. J. 2019, 117, 304–336.
- Xiao, J.S.; Chen, S.Y.; Yi, J.; Zhang, H.; Ameer, G.A. A Cooperative Copper Metal-Organic Framework-Hydrogel System Improves Wound Healing in Diabetes. Adv. Funct. Mater. 2017, 27, 1604872.
- Han, G.; Ceilley, R. Chronic Wound Healing: A Review of Current Management and Treatments. Adv. Ther. 2017, 34, 599–610.
- Frykberg, R.G.; Banks, J. Challenges in the Treatment of Chronic Wounds. Adv. Wound Care 2015, 4, 560–582.
- Cai, W.J.; Han, P. Progress in the treatment of chronic wounds with hydrogel dressing. Int. J. Orthop. 2020, 41, 195–198. (In Chinese)
- Fonder, M.A.; Lazarus, G.S.; Cowan, D.A.; Aronson-Cook, B.; Kohli, A.R.; Mamelak, A.J. Treating the chronic wound: A practical approach to the care of nonhealing wounds and wound care dressings. J. Am. Acad. Dermatol. 2008, 58, 185–206.
- Shu, W.T.; Wang, Y.N.; Zhang, X.; Li, C.; Le, H.; Chang, F. Functional Hydrogel Dressings for Treatment of Burn Wounds. Front. Bioeng. Biotechnol. 2021, 9, 788461.
- Mogosanu, G.D.; Grumezescu, A.M. Natural and synthetic polymers for wounds and burns dressing. Int. J. Pharm. 2014, 463, 127–136.
- Mohamad, N.; Mohd Amin, M.C.I.; Pandey, M.; Ahmad, N.; Rajab, N.F. Bacterial cellulose/acrylic acid hydrogel synthesized via electron beam irradiation: Accelerated burn wound healing in an animal model. Carbohydr. Polym. 2014, 114, 312–320.
- Seow, W.Y.; Salgado, G.; Lane, E.B.; Hauser, C.A. Transparent crosslinked ultrashort peptide hydrogel dressing with high shape-fidelity accelerates healing of full-thickness excision wounds. Sci. Rep. 2016, 6, 32670.
- Richmond, N.A.; Maderal, A.D.; Vivas, A.C. Evidence-based management of common chronic lower extremity ulcers. Dermatol. Ther. 2013, 26, 187–196.
- Barshes, N.R.; Sigireddi, M.; Wrobel, J.S.; Mahankali, A.; Robbins, J.M.; Kougias, P.; Armstrong, D.G. The system of care for the diabetic foot: Objectives, outcomes, and opportunities. Diabet Foot Ankle 2013, 4, 21847.
- Fan, L.P.; Wang, H.S.; Zhang, K.H.; Cai, Z.; He, C.; Sheng, X.; Mo, X. Vitamin C-reinforcing silk fibroin nanofibrous matrices for skin care application. RSC Adv. 2012, 2, 4110–4119.
- Schaper, N.C.; Van Netten, J.J.; Apelqvist, J.; Lipsky, B.A.; Bakker, K.; International Working Group on the Diabetic, F. Prevention and management of foot problems in diabetes: A Summary Guidance for Daily Practice 2015, based on the IWGDF Guidance Documents. Diabetes Metab. Res. Rev. 2016, 32 (Suppl. 1), 7–15.
- Wang, H.N.; Xu, Z.J.; Zhao, M.; Liu, G.; Wu, J. Advances of hydrogel dressings in diabetic wounds. Biomater. Sci. 2021, 9, 1530–1546.
- Bansal, C.; Scott, R.; Stewart, D.; Cockerell, C.J. Decubitus ulcers: A review of the literature. Int. J. Dermatol. 2005, 44, 805–810.
- Dumville, J.C.; Stubbs, N.; Keogh, S.J.; Walker, R.M.; Liu, Z. Hydrogel dressings for treating pressure ulcers. Cochrane Database Syst. Rev. 2015, 2, CD011226.
- Velnar, T.; Bailey, T.; Smrkolj, V. The wound healing process: An overview of the cellular and molecular mechanisms. J. Int. Med. Res. 2009, 37, 1528–1542.
- Liang, Y.; Zhao, X.; Hu, T.; Chen, B.; Yin, Z.; Ma, P.X.; Guo, B. Adhesive Hemostatic Conducting Injectable Composite Hydrogels with Sustained Drug Release and Photothermal Antibacterial Activity to Promote Full-Thickness Skin Regeneration During Wound Healing. Small 2019, 15, e1900046.
- Sun, B.K.; Siprashvili, Z.; Khavari, P.A. Advances in skin grafting and treatment of cutaneous wounds. Science 2014, 346, 941–945.
- Hickman, D.A.; Pawlowski, C.L.; Sekhon, U.D.S.; Marks, J.; Gupta, A.S. Biomaterials and Advanced Technologies for Hemostatic Management of Bleeding. Adv. Mater. 2018, 30, 1700859.
- Liang, Y.P.; He, J.H.; Guo, B.L. Functional Hydrogels as Wound Dressing to Enhance Wound Healing. ACS Nano 2021, 15, 12687–12722.
- De Luca, I.; Pedram, P.; Moeini, A.; Cerruti, P.; Peluso, G.; Di Salle, A.; Germann, N. Nanotechnology Development for Formulating Essential Oils in Wound Dressing Materials to Promote the Wound-Healing Process: A Review. Appl. Sci. 2021, 11, 1713.
- Ishida, Y.; Kondo, T.; Takayasu, T.; Iwakura, Y.; Mukaida, N. The essential involvement of cross-talk between IFN-gamma and TGF-beta in the skin wound-healing process. J. Immunol. 2004, 172, 1848–1855.
More
Information
Subjects:
Materials Science, Composites
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
734
Revisions:
2 times
(View History)
Update Date:
27 Sep 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




