Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Anna Drabik | -- | 6396 | 2023-09-25 09:46:36 | | | |
| 2 | Lindsay Dong | + 3 word(s) | 6399 | 2023-09-27 02:54:19 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Kret, P.; Bodzon-Kulakowska, A.; Drabik, A.; Ner-Kluza, J.; Suder, P.; Smoluch, M. Mass Spectrometry Imaging of Biomaterials. Encyclopedia. Available online: https://encyclopedia.pub/entry/49587 (accessed on 08 February 2026).
Kret P, Bodzon-Kulakowska A, Drabik A, Ner-Kluza J, Suder P, Smoluch M. Mass Spectrometry Imaging of Biomaterials. Encyclopedia. Available at: https://encyclopedia.pub/entry/49587. Accessed February 08, 2026.
Kret, Paulina, Anna Bodzon-Kulakowska, Anna Drabik, Joanna Ner-Kluza, Piotr Suder, Marek Smoluch. "Mass Spectrometry Imaging of Biomaterials" Encyclopedia, https://encyclopedia.pub/entry/49587 (accessed February 08, 2026).
Kret, P., Bodzon-Kulakowska, A., Drabik, A., Ner-Kluza, J., Suder, P., & Smoluch, M. (2023, September 25). Mass Spectrometry Imaging of Biomaterials. In Encyclopedia. https://encyclopedia.pub/entry/49587
Kret, Paulina, et al. "Mass Spectrometry Imaging of Biomaterials." Encyclopedia. Web. 25 September, 2023.
Copy Citation
The science related to biomaterials and tissue engineering accounts for a growing part of our knowledge. Surface modifications of biomaterials, their performance in vitro, and the interaction between them and surrounding tissues are gaining more and more attention. Mass Spectrometry Imaging (MSI) techniques are able to measure the information about molecular composition simultaneously from biomaterial and adjacent tissue. That is why it can answer the questions connected with biomaterial characteristics and their biological influence.
mass spectrometry imaging
MSI
SIMS
MALDI
DESI
biomaterials
1. Introduction
Mass Spectrometry Imaging (MSI)’s emerging importance is related to its powerful analytical abilities to measure the distribution of molecules on the surface of a variety of materials. That brings a very broad view of the nature of the processes happening on the surface of the material of interest. The importance of these processes is particularly crucial in the case of in vivo performance of biomaterials. Surface properties of biomaterials obviously can be analyzed not only by MSI methods, but many others can also serve in this field. A typical example could be given: atomic force microscopy (AFM) [1][2], Raman spectroscopy [3][4], X-ray photoelectron spectroscopy (XPS) [5][6], energy dispersive X-ray spectroscopy (EDX) [7][8], Fourier transform infrared spectroscopy (FT-IR) [9][10] or scanning electron microscopy (SEM) [11][12][13]. In fact, several of the above-mentioned methods are simultaneously used to obtain reliable results. MSI can visualize detected molecules with high molecular specificity. Additionally, mass spectrometry techniques can provide structural information about analyzed molecules by application of routinely used MS/MS mode. All MSI methods are continuously improved in terms of sensitivity and spatial resolution, as well as sample application, which is extremely important in the case of imaging experiments. At this point, it is worth emphasizing that obtaining high-resolution images requires an interface capable of analyzing the sample with high spatial resolution. The higher the spatial resolution is, the less material ends up in the mass spectrometer. For this reason, the sensitivity of the mass spectrometer must be as high as possible, which will ultimately allow for increased spatial resolution of the analysis and finally receive a more informative image.
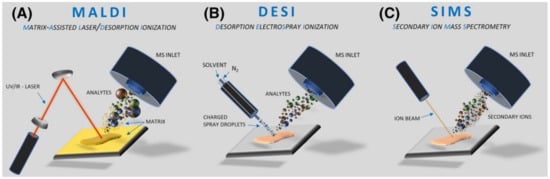
2. Techniques Used for Biomaterial Analysis
2.1. Secondary Ion Mass Spectrometry (SIMS)
SIMS was the first MS-based technique applied for imaging and still is the most popular in that field, particularly in ToF-SIMS mode (Time-of-Flight Secondary Ion Mass Spectrometry). Several reviews have been written describing the instrumentation, applications, and capabilities of this technique [14][15][16]. The principle of SIMS is analyte bombardment with short series of primary ions (like Ga+, Cs+, Au+, and In+), ion clusters or electrons, leading to the ejection from the surface of the secondary ions, molecular fragments, and electrons [17]. Typically, protonated, or deprotonated ions, adducts, or radicals are detected based on their m/z (mass-to-charge) ratio. SIMS requires placing the flat sample in a high vacuum chamber. The sample is mounted on conductive slides, such as silicon, steel, or indium-tin oxide coated glass (ITO) to avoid surface charging which can have a negative impact on spatial and spectrum resolution, and the overall sensitivity of the analysis. The newest generation of SIMS instruments can achieve a mass resolution of up to 50,000 and a spatial resolution of 50 nm [18]. The high spatial resolution of SIMS is related to source construction enabling extremely high focusing of ions heating the analyzed surface. No other MSI-based imaging techniques can offer such good parameters and that is the main advantage of SIMS over other MSI approaches for the analysis of biomaterials. SIMS is the optimal method for elemental and inorganic analyses, as it can desorb covalently or ionically bound material and also penetrate analyte material in the depth of a few layers. In the case of SIMS quite problematic can be high in-source fragmentation, but high demands to detect larger molecules with this technique put a lot of pressure to improve this source in this term. As a consequence, some improvements in SIMS sources are observed [18].
2.2. Matrix-Assisted Laser Desorption/Ionization (MALDI)
MALDI requires the application of a matrix on the surface of the analyte to allow for optimal desorption/ionization process and smooth ions transfer to a mass spectrometer, although matrix-free direct desorption sources exist like DIOS, NIMS, or NAPA [19][20][21]. Sources which do not require matrix application are less popular due to their lower versatility compared to standard sources. MALDI is usually used for large molecules (i.e., proteins), but it works also well for smaller organic compounds and sporadically can be employed for inorganic materials [22]. The principle of MALDI is mixing the sample with the matrix, co-crystallization at ambient conditions, and loading the sample into a vacuum, where the molecules are desorbed and ionized with the laser beam (Figure 1). A modified version of this technique, AP MALDI (Atmospheric Pressure Matrix-assisted laser desorption/ionization) is optional, but markedly less commonly used, mainly due to its lower sensitivity [23]. MALDI imaging usually requires flat and thin samples to ensure equal desorption/ionization conditions within the entire analyzed surface. ITO-coated glass slides for sample deposition are recommended to reduce surface charging. High-quality MALDI images require precise matrix application, recognized as the crucial step for obtaining reliable results. Compared to SIMS, MALDI is a less sensitive method but, as it is a soft ionization technique, mainly protonated molecules with minimal fragmentation are observed, which leads to the generation of easier interpretable mass spectra.
2.3. Desorption Electrospray Ionization (DESI)
SIMS and MALDI play a major role in MSI imaging, but some biomaterials analyses were also performed by alternative approaches. The most useful seems to be desorption electrospray ionization developed in the early 21st century [24]. DESI can be a complementary technique to SIMS and MALDI mainly due to one reason the sample for analysis is deposited at ambient conditions, so it has the potential to analyze non-vacuum compatible samples (i.e., containing water). Theoretically, DESI is shown as a simple and easy-to-use technique, but in fact, for more complex samples it requires a lot of time for source optimization [25]. In general, DESI is related to well-known electrospray (ESI), but in this case, the sample is deposited at a flat surface and hit by a continuous stream of charged droplets of solvent. As a result, a sample is extracted and desorbed (DESI, see Figure 1). The typical spatial resolution for DESI in the range of 100 μm is markedly lower than SIMS or MALDI, although even a 10 μm resolution was also reported [26].

Figure 1. Schemes of major ionization sources applied for biomaterial analyses. Reprinted with permission from John Wiley and Sons [27]. (A) MALDI; (B) DESI; (C) SIMS.
3. Mass Spectrometry Imaging of Biomaterials in the Vascular System: Models and Therapies
3.1. Model Improvement for the Vascular System Diseases
The implantation of the typical bare metal stents (BMS) or bioresorbable vascular scaffolds (BVS) is surgically and therapeutically well recognized [28]. The third type: drug-eluting stents (DES) seems to be the most promising in some circumstances [29]. However, the appropriate and highly reliable models of such a stent’s interaction with tissues are necessary, as there is still a wide room for improvement here. DES links a scaffold and drug release properties. The use of DES is limited to some types of illnesses and there are still important issues with their application, like in the case of diabetic patients [30] or other patient groups, responding by severe calcifications in the implanted area [31]. Based on the reliable investigation models, at least some of the questions of their application could be solved soon. To date, the most prevalent ones use porcine coronary arteries of young animals. Unfortunately, such models have a limited predictive value for DES application, as young animals’ arteries do not mimic atherosclerotic tissue well. Nevertheless, this porcine model is still recognized as a “gold standard” in the evaluation of stenting safety [32]. Even involving animals with atherosclerotic plaque, determined as better for DES testing [33], is associated with some problems related to repeatability, animal availability, overall costs of the experiments, and other issues. Trying to resolve some of these problems, Razzi and co-workers designed an excellent, ex vivo model of atherosclerotic plaque for drug delivery studies [34]. They simulated atherosclerotic plaque in a model of an extracted coronary artery, mounting the lipid-free and lipid-filled hydrogels between the internal artery wall and implanted stent. To obtain reasonable results such a model needs extensive supervision with the aid of analytical techniques. Here, MALDI-MSI was selected as a convenient and fast screening method to check the transport efficiency of everolimus, a drug routinely used in DES implanted into patients undergoing percutaneous coronary interventions [35]. The authors have proven that their approach is advantageous over the techniques used up to date, especially in combination with mass spectrometry imaging.
To successfully finalize an exemplary approach shortly described here, extensive optimization of the MSI methods is necessary. This part of the analytical methodologies is usually underestimated by the scientific community; however, technical articles showing the optimization towards special tissues or models should be appreciated, as the presented results spare hundreds of hours of work for analytical systems worldwide. Method development is especially important in MSI techniques, as optimization steps are particularly time-consuming and demanding, due to their multidimensionality. Excellent work has been conducted by Huang et al., where commercially available DES: Cypher® and NEVO™, both from Cordis Corporation, were tested [36]. They focused on the visualization of another typical immunosuppressant drug: sirolimus [37] distribution in stent polymers and its release to the surrounding tissue. Interestingly, instead of the typically applied “one factor at a time” optimization method, routinely used in the available literature [38][39] they used factorial analysis, which allows for changing all necessary factors simultaneously to receive the best possible settings of the analytical system. Moreover, they focused on the 3D stent analysis, which is atypical for the MALDI-MSI approach.
In fact, up to date, the methodology of vascular stent surface analysis was dominated by ToF-SIMS imaging, usually combined with Raman Spectroscopy [40][41] or with X-ray photoelectron spectroscopy (XPS) [42][43]. A nice example of the approach using ToF-SIMS is a 3D analysis of polymeric coatings used in DES is discussed below. In opposition to MALDI-MSI, “3D” here does not mean imaging of three-dimensionally stretched objects, but rather in-depth analysis of polymer coating with an excellent spatial resolution. It was shown that using special conditions and SIMS sources, even 6.5 μm, the entire depth of the polymer film over the stent was susceptible to the analysis, while the typical depth does not exceed the limit of 1.5–2 μm [43][44].
3.2. Interactions of the Implanted Materials with Living Tissues Monitored during the Disease Progress
There is a limited quantity of research articles considering the direct interactions between the stents or vascular grafts and the tissue in situ, investigated by the MSI, probably due to limitations of the samples, post-mortem materials availability, or other difficulties. However, at least a few articles touching this problem were found. The work of Fröhlich et al. presents the proteins and lipids adsorption on the biodegradable vascular implants studied by MALDI-MSI [45].
In the first mentioned article, an in vivo rat model was used. The primary goal of the analyses was to check protein and lipid deposition onto two polymers typically used for graft manufacturing: expanded polytetrafluorethylene (ePTFE) and thermoplastic polyurethane (TPU). In general, it is known that the profile of lipid and protein deposition in grafts could be related to the risks of post-surgery complications and other long-term risk factors for patients. Such investigations were conducted previously with the aid of other, time-consuming methodologies [46][47].
Polyethylene terephtalate InterGard® vascular graft (Gore Medical, Newark, NJ, USA) was removed from the patient’s body two years after implantation. The reason for removal was an infection, recognized as typical for this material [48] and, as a result, occlusion of the vessel. The imaging method was desorption electrospray [24], a technique useful especially for lipids imaging in biological tissues [49] if the spatial resolution of the image is not the crucial factor. This opportunity to analyze a unique sample has revealed the real substances deposition in the graft after two years of interaction with the human body.
3.3. Characterization of the Drug-Polymer Profile and Drug Elution in DES
Implants equipped with a drug delivery system, in the case of vascular system diseases, are designed to avoid restenosis (subsequent narrowing of the vessel) after placement of an arterial stent. The release rate of the pharmaceutical agent can be controlled by the formulation of the drug/polymer coating and the process of dispersion of the drug in the polymer matrix. The ToF-SIMS technique is applied in this field to study these processes.
Sirolimus (rapamycin) is one of the major anti-restenotic drugs, usually encapsulated in Poly (lactic-co-glycolic acid) (PLGA). Three-dimensional (3D) characterization of a drug-eluting stent coating composed of 0 to 50% (w/w) sirolimus in a PLGA matrix was shown by Mahoney et al. They proved that is possible to use dual beam cluster SIMS (SF5+ ions for sputtering and Bi3+ ions for analysis) for 3D study of polymer drug delivery systems at low temperatures. Coatings in the range of 0–50% (w/w) sirolimus in PLGA were prepared on two substrates: MP35N metal alloy coupons (metal samples) and BMS. In the obtained SIMS cluster depth profiles, temperature has a significant influence. The best profiles were obtained at −100 °C, which allowed us to study the film at the entire depth reaching up to 6.5 μm. All coatings indicated a drug-enriched surface area, a drug-depleted region, and an area of constant bulk composition. The thickness of the drug’s top layer depended on the drug content. Assembling the raw data into 3D images showed that samples with 5% of the sirolimus were lateral scale homogeneous, with higher values: 25% and 50% showed domain formation in the surface, subsurface, and bulk regions [44].
Another group of researchers examined four formulations containing from 5 to 50 (w/w) rapamycin (Rap) in PLGA, both with and without a PLGA topcoat (“coating”) at a thickness of 7 μm on coupons and on stents. In this study, applying the ToF-SIMS (SF5+ sputter source, Bi3+ acquisition source) confirms the presence of both rapamycin and PLGA through the detection of intact molecular signals and molecular fragments. Formulations containing 5% rapamycin/95% PLGA, 25% rapamycin/75% PLGA, 50% rapamycin/50% PLGA, and 25% rapamycin/75% PLGA with a PLGA cap coat were used. For the sample, 5% Rap/95% PLGA, the signal is maintained in the ToF-SIMS depth profile through the entire thickness of the coating. However, for the samples with higher drug concentrations, there is a problem with maintaining the signal along with the depth.
4. Mass Spectrometry Imaging of Bone Implant Biomaterials
People worldwide are living longer, and according to WHO, by 2050 the world’s population of people aged 60 years and older will double. The number of humans aged more than 80 years is expected to triple by that time. Aging consequences such as the increase in a wide variety of molecular and cellular damage lead to a decline in physical and mental capabilities, a growing risk of disease, and eventually death. Osteoporosis is an age-related disease, characterized by a reduction in bone density due to loss of bone mineral content, and changes in bone microstructure. These alterations result in instability of bone that can lead to more frequent fractures and consequently to prolonged or incomplete fracture healing. Osteoporotic fractures are a significant health problem worldwide with almost 9 million cases every year, especially at sites like vertebrae, proximal femurs, proximal humeri distal radii, and femur [50].
Bone marrow exists in two different states; the osteogenic form, also termed red bone marrow, and the yellow bone marrow, which develops during bone maturation. Whereas red bone marrow mostly contains hematopoietic cells like blood cells, platelets, mesenchymal stem cells, fibroblasts, adipocytes, osteoblasts, osteoclasts, macrophages, or endothelial cells, the yellow bone marrow is mainly composed of bone marrow adipocytes. The percentage of yellow bone marrow increases up to 70–80% during bone maturation [51]. Bone is a highly vascular, mineralized connective tissue extraordinary for its strength and regenerative capacity. The composition of bone tissue is of great significance for the understanding of fracture healing, bone implant interactions, and mineralization disturbances such as osteoporosis, and Turner’s syndrome. The application of ToF-SIMS in osteoporotic bone research allows simultaneous visualization of mineralized and non-mineralized bone tissue, as well as implanted biomaterials and bone implant interphases [52]. ToF-SIMS enables the simultaneous identification of both organic and inorganic substances within the same sample in a single analysis process in 2D and 3D [51]. With ToF-SIMS the ions distribution in bone cross-sections is mapped semi-quantitatively with a lateral resolution of up to 1 μm [53]. ToF-SIMS was successfully applied for tracking pharmaceuticals in a biomaterial from in vitro to in vivo experiments, as well as for the investigation and imaging of osteoporotic bone in an animal model [54].
Obtained ion maps are able to reveal the distribution of the organic compounds, as well as the localization of mineralized parts of the bone; however, the quantification of concentrations is challenging, as the ionization process and hence the ion yields strongly depend on the chemical environment of the elements and molecules at the surface. Therefore, appropriate standards with almost identical chemical composition as the analyzed material are required, and the relative fragment mass intensity of the analyte of interest should follow a linear relationship with its concentration in the standard [53].
In osteoporosis therapy, several drugs are administered systemically to prevent fractures by slowing down the bone resorption process or stimulating bone formation. Numerous drugs, such as antibiotics, osteoanabolic substances, or anti-resorptive drugs can be released into a specific bone defect and locally promote tissue restoration and bone formation, including bioactive metal ions like Sr2+, Mg2+, Cu2+, and Co2+. Those metal ions can reduce bone resorption and simultaneously stimulate osteoblastic bone formation, which promotes bone healing and fracture repair [55]. Bone processes are essential for maintaining healthy bones. Studies have shown that systemic osteoporosis therapy with orally administered strontium ions (Sr2+) in the form of strontium ranelate has also improved the osseointegration of bone implants.
To meet the specific requirements of osteoporotic bone fracture healing, when seeking new implant materials, fracture stabilization plays an important role. Material benefits, such as ease in surgical handling, high biocompatibility, resistance to abrasion, small coefficient of friction, and self-lubricating properties are also important [56]. Studies on the drug release and distribution process of therapeutically active Sr2+-ions in healthy and osteoporotic bone were performed using ToF-SIMS analysis of metal ions diffusion coefficient [57].
Since Sr2+ ions are an effective therapeutic agent for the healing of osteoporotic bone fractures, they are often used in the form of strontium-modified bone cement. Additional studies using ToF-SIMS led to the development of the experimental protocol for transport studies in bovine bone marrow [51]. Additionally, orbitrap secondary ion mass spectrometry (OrbiSIMS) was applied for definite signal identification of lipids and fatty acid species in rat bone marrow [58]. Comprehensive 2D and 3D mass spectrometric imaging analyses, depth profiling, as well as OrbiSIMS spectrometric analysis, discovered faster Sr2+ diffusion in rat bone marrow areas correlated with lower intensity of lipid and fatty acid signals than in areas with higher lipid/fatty acid content [51].
5. Evaluation of Biomaterial Influence on the Adjacent Tissue by Mass Spectrometry Imaging Techniques
The MSI techniques are able to investigate the interaction between the biomaterial and the tissue since they can elucidate the distribution of different molecules inside and around the artificial material. It means that they are able to provide information about how the used material behaves in vivo and what is the reaction of surrounding tissues and the host immune system.
Klerk et al. conducted one of the first studies in this field [59]. In this work, they studied hydrogel drug delivery carriers composed of elongated polymer chains cross-linked by the quadruple hydrogen bonding ureidopyrimidinone group [60]. The biomaterial was implanted under the renal capsule of rats, and the kidneys were collected 15 days after implantation. The kidney tissue slices were covered with gold and measured in the positive and negative secondary ionization modes. PCA analysis was performed to indicate polymer-specific signals and biologically relevant peaks. Cellular infiltration was indicated into the polymer, and it was direct evidence of the presence of active cells inside the hydrogel. To distinguish between different types of macrophages that may play a vital role in this process, the macrophages were in vitro polarized by six different polarization agents for 6 days. After culturing, the cells were washed with sucrose (300 mM) to remove salts, cytospined on indium tin oxide (ITO) coated glass slides, covered with gold, and measured. The gold-covered and non-covered samples were compared to eliminate the Au adducts.
Hydrogels belong to the class of materials that could be either synthetic (made from molecules such as poly(ethylene glycol)) or based on native proteins, such as collagen or fibrin, and may be used as 3D scaffolds for tissue engineering [61]. It was shown that injectable collagen hydrogels might help restore the myocardium’s mechanical properties and reduce scar size after acute myocardial infarction [62].
6. Mass Spectrometry Imaging of Biomaterials Used for In Vitro Cell Cultures
Properly designed peptides may be used to create a matrix that forces special kinds of cells to migrate in a given and desired direction. Moreover, specific sequences of amino acids grafted to synthetic or natural materials may promote cell survival, attachment, and proliferation and may influence the expression of specific receptors [63][64]. The MSI approach is very useful in characterizing such materials—since it does not demand any kind of labeling prior to the analysis. Moreover, in the case of MALDI analysis, intact chemical species on the analyzed surface may be identified.
Many cells use the chemical gradient to migrate in the desired direction. Among them, Schwann cells are in the spotlight because they are responsible for neuroregeneration after peripheral nerve injury. Motta et al. used a confined channel vapor deposition to create a concentration gradient of five different peptides on the microscope glass coverslips [65]. The selected sequences (RGD, PDSRG, YIGSR, IIKDI, IKVAV) were proven to be involved in peripheral nerve regeneration [63][66][67][68]. Their study assessed Schwann cell adhesion, proliferation, morphology, and migration parameters, relative to the peptide sequence, peptide concentration, and gradient slope. Multiple methods have been used to study generated surfaces (among them, contact angle measurement and X-ray Photoelectron Spectroscopy).
In this case, it was not a classical MSI approach since the original surface was not prepared on the ITO glass. The procedure demanded the liberation of peptides from the glass by applying NH4F and covering the surface with a CHCA matrix using a thin-layer chromatography (TLC) sprayer. The obtained results provided evidence that the peptide gradient was preserved despite such a complicated preparation procedure.
In conclusion, the study proved that YIGSR showed a selective hypotactic effect on Schwann cells. More generally, such matrices with specific profiles of bioactive peptides may be used as therapeutic devices that facilitate functional recovery of the peripheral nerves [69]. Moreover, MALDI MSI analysis may be used here to confirm the correct surface preparation.
A slightly different approach for gradient preparation was proposed by Ender et al. [70]. They utilize a unique feature of amyloid-peptides functionalized with RGD motif to form a concentration gradient. Amyloid peptides, formerly associated with pathological conditions, are increasingly exploited as biomaterials. Those peptides are able to self-assemble into highly ordered fibrils with characteristic amyloid β-sheet structures. During this transformation, they gain special physical prosperities such as long-term stability, mechanical stiffness, and strong adhesion to various substrates [71][72][73]. In the mentioned study RGD motif was connected via a photocleavable linker (PLC) to the bioactive self-assembling peptide CKFKFQF [74]. In the physiological environment, the modified peptides (RGD-PCL-CKFKFQF) were self-assembled into the amyloid-like structure, and a gradient was created in a dose-dependent manner by surface exposure to UV light. Light irritation causes the RGD epitopes to cleave off and release, but the fibril morphology is maintained, which was confirmed by thioflavin T (ThT) assay, Fourier transform infrared spectroscopy (FT-IR), and TEM measurements.
MALDI—MSI was used for relative label-free quantitation of precursor (RGD-PCL-CKFKFQF—m/z 1613.7) and fragment ions (RGDG—m/z 404), based on the corresponding ions intensities in mass spectra. In this case, a separate sample was prepared where the peptide solution was spray-coated with an airbrush on the ITO glass surface. Then the RDG gradient was fabricated by UV-irradiation, and the CHCA matrix was used for the final analysis, which confirmed the gradual change in the signal 1613.7 m/z from a non-irridated surface relative to the irritated part.
The analysis of the kinetics of photocleavage on the surface in the dry state was also possible due to the MALDI-MSI usage. The peptide fibril-coated ITO glass was irradiated with UV light for longer times (0, 1, 2, 3, 4, 5, 6, 8, and 10 min). Then the signal intensity of 1613.7 m/z from the irritated surface relative to the non-irritated surface was used as a quantitative measure for the remaining precursor, and the kinetics of the reaction could be established.
The MSI approach combined with in vitro studies, can also be used to analyze organic nanoparticles designed as potential drug carriers. ToF-SIMS is a method of choice in such studies, due to its high spatial resolution. Once again, as with all MSI techniques, it is label-free, so it does not need any kind of modification for the original nanoparticle.
In their study, Kokesch-Himmelreich et al. [75] focused on the analysis of polyelectrolyte complex nanoparticles made of polyethylenimine and cellulose sulfate (PEI/CS) in human bone marrow-derived stromal cells (hBMSC). The cells were cultured with those nanoparticles on silicon wafers to enable SIMS analysis. At the end of the procedure, the cells were washed with phosphate buffer saline (PBS) without Ca2+ and Mg2+, incubated with 4% paraformaldehyde for 10 min, and stored at 4 °C in PBS. Before the measurements, all samples were washed twice with pure water and measured using Bi3+ cluster in positive ion mode. To indicate specific masses for the PEI/CS nanoparticles, PCA analysis of the spectra of pure silicon wafer, PEI/CS nanoparticles alone, and chemically fixed control hBMSC was performed. The analysis indicated the m/z values that showed higher intensities in the nanoparticle mass spectra than in the cell-derived mass spectra. These values were used to show the distribution of nanoparticles in the cells cultured in their presence. Interestingly, only cellulose sulfate-related peaks (CS) and not nitrogen-related peaks (PEI) were indicated and could be used for the imaging experiments. These signals have relatively low intensities compared to the cell-derived signals, so a longer analyzing time is required to obtain reasonable images with high lateral resolution and good contrast.
7. Studying the Implant Formulation and Active Pharmaceutical Ingredient Release from Biomaterials Such as LAP and Microspheres with the Aid of Mass Spectrometry Imaging
Drug release profiles from polymeric implants depend both on polymer erosion and diffusion process. For this reason, the characterization of implant formulations is very important for understanding the active pharmaceutical ingredient (API) release mechanism and for all interactions with the polymeric matrix. The release profile of the drug might be limited by the formulation of the pharmaceutic or polymer coating and the dispersion process. Another aspect is the surface chemistry. The use of 3D chemical images allows the study of both surface and subsurface distribution of drug molecules and allows visualization of drug distribution as a function of elution time.
Long acting parenterals (LAPs) implants are based on a drug delivery technology that enables the delivery of therapeutically effective doses of a drug over a wide range of time from weeks to years. Pierson et al. used DESI coupled with an ion mobility analyzer to study the spatial distribution of entecavir on the surface of cylindrical biodegradable poly(D,L-lactide) PLA implants without any special sample preparation. The DESI-MSI results indicate that there are differences in drug release between the two solutions. A more uniform release of entecavir from the implant was obtained using a low pH PBS solution, non-uniform release of entecavir was observed with methanol solution (MeOH: H2O (50:50, v/v)), and a higher relative concentration in the core of the implant. In addition, a decrease in entecavir concentration was observed from the surface of the implant to a diameter of approximately 1.5 mm from the center of the implant, while maintaining the maximum relative amount of entecavir at the center. This drug distribution shows that entecavir is mainly released from the outer surface of the implant. It is worth mentioning that the DESI-MSI images strictly depend on the intensity of the ion signal in the MS spectrum and two main aspects: the relative concentration of the drug in pixels, and the relative distance between the DESI capillary and the sample surface. Normalization as a function of ion intensity to the total ion chromatogram (TIC) of each pixel allowed for better relative comparisons of differences in drug distribution [76].
Polymer microspheres enable the controlled release of various active substances The distribution of the drug in the microsphere has a very large impact on the release profile; therefore, the analysis of the microsphere volume is important for the pharmaceutical characterization of the system. The next stage of the research is to show the spatial location of the protein, surfactant, and polymer substrate using imaging of the surface of microparticles utilizing the AFM technique in combination with ToF-SIMS. Lysozyme released from PLGA microspheres was used as a protein-based model. The microspheres were prepared using a water-in-oil-in-water double emulsion method containing 1.5%, 3%, 5%, and 10% lysozyme. ToF-SIMS spectra were used for imaging the distribution of the PLGA, PVA, and lysozyme from the surface of the microspheres based on reference ions (lysozyme (CNO−), PLGA (C3H3O2−, C3H5O2−, C3H3O3−, C3H5O3−), PVA (C2H3O2−)). The use of imaging techniques shows that the lysozyme on the surface of the microparticles was localized in different ways: most of the surface proteins appear to be densely clustered, which may be due to incomplete encapsulation of the protein-rich water droplets in the process of microparticle formation. There is also protein dispersed in the surfactant film itself, which may be due to an ionic interaction between the protein and the PVA. ToF-SIMS and Raman spectroscopy analysis has shown the presence of smaller pores (2–5 μm) containing protein dispersed in microparticles.
8. Analysis of Designed Nanofibers by Mass Spectrometry Imaging
Long fibers with small diameters (50–500 nm) are called nanofibers. They can have many applications in the fields of biology, biomedical engineering, or cancer treatment. The main polymers used are polyurethane, polybenzimidazoles, polycarbonate, polyacrylonitrile, poly(vinyl alcohol), poly(lactic acid), poly(ethylene-co-vinyl acetate), poly(ethylene oxide), collagen, polyaniline, and poly(ethylene glycol); among them, silk, chitosan, and collagen, as well as poly(lactic-co-glycolic acid) (PLGA).
Among other applications, nanofibers can be used in the processes of capturing circulating tumor cells, which is possible due to the ability of nanofibers to fuse with cell matrices. Such a process is called electrospinning, and raw polymers and surface-modified polymers are used. Using the electrospinning process Yu et al. prepared PLGA nanofibrous arrays on glass slides [77]. They studied PLGA surface structures to improve the isolation of cancer cells from blood samples. During this process, special circulating tumor cells (CTCs) were used, which are important during cancer diagnosis, individual cancer therapy and cancer development. The authors modified special chips embedding the PLGA nanofiber arrays using sequential coating for the surface with many compounds, including biotin-(PEG)7-amine. They used the ToF-SIMS to study the surface modification of PEGylated biotin nanofibers [77].
The possibility of studying the spatial distribution of different components using the ToF-SIMS was also presented by Scoutaris et al. [78]. In their study, they dealt with the polymer/API (active pharmaceutical ingredients) system, where they used polymer compounds (PVP and PLGA) as drug carriers and hydrochlorothiazide (HCT) and felodipine. They used inkjet printing of formulations of HCT/PLGA, HCT/PVP, and felodipine/PVP. Those formulations were printed as micro-dot arrays and analyzed by ToF-SIMS. There was heterogeneity in HCT/PLGA formulation, so multivariate curve resolution for the ToF-SIMS hyperspectral image dataset was applied. After that, chemical components: HCT, PLGA, substrate material, and many contaminants were identified. In order to mitigate the heterogeneity of the drug and other chemical components observed with the HCT/PLGA spots, PLGA was replaced with a PVP polymer. Images of secondary ions characteristic of both the hydrochlorothiazide and the polymer matrix obtained for the printed HCT/PVP spots showed a uniform surface appearance, confirming a homogeneous distribution.
9. Dialyzer Polymer Membranes and Modified Surfaces Analyzed by Mass Spectrometry Imaging
9.1. Dialyzer Polymer Membranes
Dialyzer is a model of an artificial kidney. It is used during dialysis treatment for patients with kidney diseases. The task of the dialyzer is to filtrate a patient’s blood via hundreds of hollow fiber membranes, from which it is made. Although this is a very important and useful treatment, it still needs improvement. One of those is the type of membrane that must be biocompatible and permeable. A high number of those features possess a polysulfone (PSf) membrane. Another type is an asymmetry cellulose triacetate (ATA) membrane, which has an enhanced asymmetric structure in terms of cross-sectional pore size.
Polysulfone membranes can be modified by adding hydrophilic compounds, such as polyvinylpyrrolidone (PVP). Biocompatibility is very important in the dialysis process, and it is influenced by polymer distribution. That is why compound identification and localization have a huge impact on membrane characterization. Biocompatible membranes cause the least inflammatory response in patients exposed. Polysulfone-based biomaterials are considered a gold standard for the production of biocompatible hemodialyzers. Firstly, MALDI imaging was used for investigating the chemical surface structure, by analyzing the distribution of the polymeric compounds on both sides of the membrane. The study showed that PVP was condensed in patches, and PS exhibited homogenous distribution at the abluminal (external) side. On the luminal side of the membrane, imaging showed that PVP was more homogenous than PS. Thus in this case, analysis of biocomponent membranes has shown differences in the composition of abluminal (external) and luminal (internal) membrane surfaces and polymer distribution [79].
9.2. General Modified Surface Analysis
Modifying surfaces are more frequently used, especially those possessing carbohydrates (glycans) as a modification. Glycomics-based modifications have a lot of biomedical applications, like functional biomaterials or glycan-based biosensors. Carbohydrates are taking part in many biological processes. As it was mentioned, ToF-SIMS is a surface-sensitive technique, suitable for probing molecular composition at the surface of biomaterials. This feature was used by the Bolles et al. group with printed glycan microarrays surface, which was analyzed by ToF-SIMS imaging and Surface Plasmon Resonance imaging (SPRi) [80]. They used a microarray platform with located carbohydrate modifications. The use of those two techniques allowed us to examine glycoarray surface chemistries and bioactivity. ToF-SIMS was used to analyze individual spots on the arrays.
The functionalization of nanoparticles enables the adjustment of interactions between them and the surrounding environment, which leads to obtaining nanostructured materials with a wide range of applications and, at the same time, gaining unique properties. Nanoparticles conjugated to biomolecules that can be used for biomimetics, targeted delivery or biosensing, are a key aspect in the design of new biomaterials. Shell cross-linked nanoparticles (SCK) are a group of polymeric nanostructured materials containing a hydrophobic core domain and a hydrophilic shell layer. Because they create stable nanoscale biocompatible scaffolds, they are a good starting point for further research and modifications [81]. In subsequent studies, biosynthetic hybrid multilayer structures composed of biotinylated nanoparticles with a cross-linked coating (SCK) on the streptavidin/biotin self-assembled monolayers (SAM) surface were built. At each stage of assembly, the chemistry and structure of the construct were monitored using a combination of ToF-SIMS (in positive and negative ion modes) and other imaging techniques. Prior to biotinylation, the ToF-SIMS images showed a uniform distribution of low ion intensities derived from SC5H11+, characteristic of self-assembled monolayers (SAM).
The key aspect of good biointegration of an implant is the surface properties. Many biomaterials are porous, presenting analytical challenges compared to solid materials. The aim of the surface modification to receive a highly porous, fully fluorinated polymer substrate, so-called expanded poly(tetrafluoroethylene) (ePTFE), is to improve the biocompatibility and bioavailability of its surface which may lead to better biointegration. A number of specific modifications were used for this purpose. Graft copolymerization (induced by gamma irradiation) was performed using monoacryloxyethyl phosphate (MAEP) and methacryloxyethyl phosphate (MOEP) monomers in different solvent systems (methanol, water, methyl ethyl ketone and their mixtures). Detailed analysis of the grafted ePTFE membranes was obtained using various imaging techniques. Based on the received results, it was found that the copolymer penetration depth depends on the monomer, its concentration, the solvent used and the method of sample preparation. ToF-SIMS imaging was used to explore a 200 μm2 surface with a spatial resolution of ~120 nm. High-intensity ions were chosen for surface mapping. The F− ion was used to represent the presence of the fluoropolymer substrate, while the PO3− fragment was used to represent the grafted copolymer. By ToF-SIMS imaging, it was confirmed that the presence of graft copolymer was observed for all grafting modifications, especially in the fibrous regions of the membrane.
10. Conclusions
The growing number of applications in recent years has proved the great potential of MSI for the analysis of biomaterials. These methods offer unprecedented sensitivity, high specificity, and structural information capabilities, usually unattainable with other surface imaging techniques. Thus, along with other non-mass spectrometry-based analytical methods, scientists have a powerful tool to study biomaterials. As mentioned here, the great advantage of the MSI methods is their ability to indicate the chemical composition of the analyzed sample and to measure many different molecules in a single experiment simultaneously without any labeling. This enables us to learn and understand many biological issues.
MSI techniques are beneficial when the investigation of selected substances is important. It is clearly shown in the analysis of different biomaterials designed to elute active substances, such as drug-eluting stents, metal ion-eluting bone implants, or long-acting parenteral implants (LAP). Here, the analysis of elution of active substances from the material and its distribution in the surrounding tissue is important. Such research allows us to create kinetic models for the observed phenomena which may in the future allow us to obtain better materials and reduce the number of animal experiments [57]. Knowledge obtained in that way is crucial because in the future this kind of biomaterials may deliver drugs directly to where they should be active, replace inconvenient drug dosage, or reduce the amount of active substances that have to be administrated. It is worth mentioning that this kind of formulation may be preferred for the treatment of chronic diseases.
Another kind of study is devoted to analyzing the interaction of biomaterial with the surrounding tissues. Such investigations may indicate proteins and lipids that interact with the designed biomaterial. Once again obtained data may help to create biomaterials with better parameters adapted to their functions, since, in some circumstances, strong integration is welcome, and in others, not (for example, in the case of dialyzer membranes).
References
- Sambani, K.; Kontomaris, S.V.; Yova, D. Atomic Force Microscopy Imaging of Elastin Nanofibers Self-Assembly. Materials 2023, 16, 4313.
- Chelu, M.; Popa, M.; Ozon, E.A.; Pandele Cusu, J.; Anastasescu, M.; Surdu, V.A.; Calderon Moreno, J.; Musuc, A.M. High-Content Aloe Vera Based Hydrogels: Physicochemical and Pharmaceutical Properties. Polymers 2023, 15, 1312.
- Al-Harbi, N.; Hussein, M.A.; Al-Hadeethi, Y.; Felimban, R.I.; Tayeb, H.H.; Bedaiwi, N.M.H.; Alosaimi, A.M.; Bekyarova, E.; Chen, M. Bioactive Hybrid Membrane-Based Cellulose Acetate/Bioactive Glass/Hydroxyapatite/Carbon Nanotubes Nanocomposite for Dental Applications. J. Mech. Behav. Biomed. Mater. 2023, 141, 105795.
- Dłucik, R.; Orzechowska-Wylęgała, B.; Dłucik, D.; Puzzolo, D.; Santoro, G.; Micali, A.; Testagrossa, B.; Acri, G. Comparison of Clinical Efficacy of Three Different Dentin Matrix Biomaterials Obtained from Different Devices. Expert. Rev. Med. Devices 2023, 20, 313–327.
- Salamanca, E.; Choy, C.S.; Aung, L.M.; Tsao, T.-C.; Wang, P.-H.; Lin, W.-A.; Wu, Y.-F.; Chang, W.-J. 3D-Printed PLA Scaffold with Fibronectin Enhances in Vitro Osteogenesis. Polymers 2023, 15, 2619.
- Bhattacharjee, A.; Goodall, E.; Pereira, B.L.; Soares, P.; Popat, K.C. Zinc (Zn) Doping by Hydrothermal and Alkaline Heat-Treatment Methods on Titania Nanotube Arrays for Enhanced Antibacterial Activity. Nanomaterials 2023, 13, 1606.
- Valinezhad, N.; Talebi, A.F.; Alamdari, S. Biosynthesize, Physicochemical Characterization and Biological Investigations of Chitosan-Ferula Gummosa Essential Oil (CS-FEO) Nanocomposite. Int. J. Biol. Macromol. 2023, 241, 124503.
- Tosco, V.; Vitiello, F.; Monterubbianesi, R.; Gatto, M.L.; Orilisi, G.; Mengucci, P.; Putignano, A.; Orsini, G. Assessment of the Remineralizing Potential of Biomimetic Materials on Early Artificial Caries Lesions after 28 Days: An in Vitro Study. Bioengineering 2023, 10, 462.
- Pourmadadi, M.; Tajiki, A.; Abdouss, M. A Green Approach for Preparation of Polyacrylic Acid/Starch Incorporated with Titanium Dioxide Nanocomposite as a Biocompatible Platform for Curcumin Delivery to Breast Cancer Cells. Int. J. Biol. Macromol. 2023, 242, 124785.
- Jakubowski, M.; Domke, A.; Ratajczak, M.; Szczuka, J.; Buchwald, T.; Ławniczak, Ł.; Homa, J.; Voelkel, A.; Sandomierski, M. Chitosan Hydrogel Modified with Lanthanum as a Drug Delivery System for Epigallocatechin Gallate: Investigation of Hydrogel—Drug Interaction by FT-IR and Raman Spectroscopy. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2023, 297, 122748.
- Suni, A.O.; Lassila, L.V.J.; Tuokko, J.K.; Garoushi, S.; Vallittu, P.K. Adhesion of Individually Formed Fiber Post Adhesively Luted with Flowable Short Fiber Composite. Biomater. Investig. Dent. 2023, 10, 2209593.
- Alazab, M.H.; Abouelgeit, S.A.; Aboushelib, M.N. Histomorphometric Evaluation of 3D Printed Graphene Oxide-Enriched Poly(ε-Caprolactone) Scaffolds for Bone Regeneration. Heliyon 2023, 9, e15844.
- Li, L.; Lu, P.; Liu, Y.; Yang, J.; Li, S. Three-Dimensional-Bioprinted Bioactive Glass/Cellulose Composite Scaffolds with Porous Structure towards Bone Tissue Engineering. Polymers 2023, 15, 2226.
- Paine, M.R.L.; Kooijman, P.C.; Fisher, G.L.; Heeren, R.M.A.; Fernández, F.M.; Ellis, S.R. Visualizing Molecular Distributions for Biomaterials Applications with Mass Spectrometry Imaging: A Review. J. Mater. Chem. B 2017, 5, 7444–7460.
- Belu, A.M.; Graham, D.J.; Castner, D.G. Time-of-Flight Secondary Ion Mass Spectrometry: Techniques and Applications for the Characterization of Biomaterial Surfaces. Biomaterials 2003, 24, 3635–3653.
- Kingshott, P.; Andersson, G.; McArthur, S.L.; Griesser, H.J. Surface Modification and Chemical Surface Analysis of Biomaterials. Curr. Opin. Chem. Biol. 2011, 15, 667–676.
- Vickerman, J.C. Impact of Mass Spectrometry in Surface Analysis. Analyst 1994, 119, 513–523.
- Brunet, M.A.; Kraft, M.L. Toward Understanding the Subcellular Distributions of Cholesterol and Sphingolipids Using High-Resolution NanoSIMS Imaging. Acc. Chem. Res. 2023, 56, 752–762.
- Liu, Q.; Guo, Z.; He, L. Mass Spectrometry Imaging of Small Molecules Using Desorption/Ionization on Silicon. Anal. Chem. 2007, 79, 3535–3541.
- Northen, T.R.; Yanes, O.; Northen, M.T.; Marrinucci, D.; Uritboonthai, W.; Apon, J.; Golledge, S.L.; Nordström, A.; Siuzdak, G. Clathrate Nanostructures for Mass Spectrometry. Nature 2007, 449, 1033–1036.
- Stopka, S.A.; Rong, C.; Korte, A.R.; Yadavilli, S.; Nazarian, J.; Razunguzwa, T.T.; Morris, N.J.; Vertes, A. Molecular Imaging of Biological Samples on Nanophotonic Laser Desorption Ionization Platforms. Angew. Chem. Int. Ed. Engl. 2016, 55, 4482–4486.
- Sun, R.; Zhang, Y.; Tang, W.; Li, B. Submicron 3,4-Dihydroxybenzoic Acid-TiO2 Composite Particles for Enhanced MALDI MS Imaging of Secondary Metabolites in the Root of Differently Aged Baical Skullcap. Analyst 2022, 147, 3017–3024.
- Laiko, V.V.; Baldwin, M.A.; Burlingame, A.L. Atmospheric Pressure Matrix-Assisted Laser Desorption/Ionization Mass Spectrometry. Anal. Chem. 2000, 72, 652–657.
- Takáts, Z.; Wiseman, J.M.; Gologan, B.; Cooks, R.G. Mass Spectrometry Sampling under Ambient Conditions with Desorption Electrospray Ionization. Science 2004, 306, 471–473.
- Bodzon-Kulakowska, A.; Drabik, A.; Ner, J.; Kotlinska, J.H.; Suder, P. Desorption Electrospray Ionisation (DESI) for Beginners—How to Adjust Settings for Tissue Imaging. Rapid Commun. Mass. Spectrom. 2014, 28, 1–9.
- Yin, R.; Burnum-Johnson, K.E.; Sun, X.; Dey, S.K.; Laskin, J. High Spatial Resolution Imaging of Biological Tissues Using Nanospray Desorption Electrospray Ionization Mass Spectrometry. Nat. Protoc. 2019, 14, 3445–3470.
- Holzlechner, M.; Eugenin, E.; Prideaux, B. Mass Spectrometry Imaging to Detect Lipid Biomarkers and Disease Signatures in Cancer. Cancer Rep. 2019, 2, e1229.
- Scafa Udriște, A.; Niculescu, A.-G.; Grumezescu, A.M.; Bădilă, E. Cardiovascular Stents: A Review of Past, Current, and Emerging Devices. Materials 2021, 14, 2498.
- Nicolas, J.; Pivato, C.A.; Chiarito, M.; Beerkens, F.; Cao, D.; Mehran, R. Evolution of Drug-Eluting Coronary Stents: A Back-and-Forth Journey from the Bench to Bedside. Cardiovasc. Res. 2023, 119, 631–646.
- Chiarito, M.; Mehran, R. Drug-Eluting Stents in Diabetic Patients: Are We Still Treading Water? Catheter. Cardiovasc. Interv. 2020, 96, 253–254.
- Torii, S.; Jinnouchi, H.; Sakamoto, A.; Mori, H.; Park, J.; Amoa, F.C.; Sawan, M.; Sato, Y.; Cornelissen, A.; Kuntz, S.H.; et al. Vascular Responses to Coronary Calcification Following Implantation of Newer-Generation Drug-Eluting Stents in Humans: Impact on Healing. Eur. Heart J. 2020, 41, 786–796.
- Iqbal, J.; Chamberlain, J.; Francis, S.E.; Gunn, J. Role of Animal Models in Coronary Stenting. Ann. Biomed. Eng. 2016, 44, 453–465.
- Tellez, A.; Seifert, P.S.; Donskoy, E.; Sushkova, N.; Pennington, D.E.; Milewski, K.; Krueger, C.G.; Kaluza, G.L.; Eppihimer, M.J.; Huibregtse, B.A.; et al. Experimental Evaluation of Efficacy and Healing Response of Everolimus-Eluting Stents in the Familial Hypercholesterolemic Swine Model: A Comparative Study of Bioabsorbable versus Durable Polymer Stent Platforms. Coron. Artery Dis. 2014, 25, 198–207.
- Razzi, F.; Lovrak, M.; Gruzdyte, D.; Den Hartog, Y.; Duncker, D.J.; van Esch, J.H.; van Steijn, V.; van Beusekom, H.M.M. An Implantable Artificial Atherosclerotic Plaque as a Novel Approach for Drug Transport Studies on Drug-Eluting Stents. Adv. Healthc. Mater. 2022, 11, e2101570.
- Meng, M.; Gao, B.; Wang, X.; Bai, Z.; Sa, R.; Ge, B. Long-Term Clinical Outcomes of Everolimus-Eluting Stent versus Paclitaxel-Eluting Stent in Patients Undergoing Percutaneous Coronary Interventions: A Meta-Analysis. BMC Cardiovasc. Disord. 2016, 16, 34.
- Huang, J.-T.; Hannah-Qiuhua, L.; Szyszka, R.; Veselov, V.; Reed, G.; Wang, X.; Price, S.; Alquier, L.; Vas, G. Molecular Imaging of Drug-Eluting Coronary Stents: Method Development, Optimization and Selected Applications. J. Mass. Spectrom. 2012, 47, 155–162.
- Pilgrim, T.; Rothenbühler, M.; Siontis, G.C.; Kandzari, D.E.; Iglesias, J.F.; Asami, M.; Lefèvre, T.; Piccolo, R.; Koolen, J.; Saito, S.; et al. Biodegradable Polymer Sirolimus-Eluting Stents vs Durable Polymer Everolimus-Eluting Stents in Patients Undergoing Percutaneous Coronary Intervention: A Meta-Analysis of Individual Patient Data from 5 Randomized Trials. Am. Heart J. 2021, 235, 140–148.
- Mielczarek, P.; Suder, P.; Kret, P.; Słowik, T.; Gibuła-Tarłowska, E.; Kotlińska, J.H.; Kotsan, I.; Bodzon-Kulakowska, A. Matrix-Assisted Laser Desorption/Ionization Mass Spectrometry Imaging Sample Preparation Using Wet-Interface Matrix Deposition for Lipid Analysis. Rapid Commun. Mass. Spectrom. 2023, 37, e9531.
- Mielczarek, P.; Suder, P.; Kotsan, I.; Bodzon-Kulakowska, A. The Influence of Matrix Concentration and Solvent Composition on the Results of MALDI MSI, with the Aid of Wet-Interface Matrix Deposition. J. Mass. Spectrom. 2023, 58, e4916.
- Belu, A.; Mahoney, C.; Wormuth, K. Chemical Imaging of Drug Eluting Coatings: Combining Surface Analysis and Confocal Raman Microscopy. J. Control. Release 2008, 126, 111–121.
- Balss, K.M.; Long, F.H.; Veselov, V.; Orana, A.; Akerman-Revis, E.; Papandreou, G.; Maryanoff, C.A. Multivariate Analysis Applied to the Study of Spatial Distributions Found in Drug-Eluting Stent Coatings by Confocal Raman Microscopy. Anal. Chem. 2008, 80, 4853–4859.
- Fisher, G.L.; Belu, A.M.; Mahoney, C.M.; Wormuth, K.; Sanada, N. Three-Dimensional Time-of-Flight Secondary Ion Mass Spectrometry Imaging of a Pharmaceutical in a Coronary Stent Coating as a Function of Elution Time. Anal. Chem. 2009, 81, 9930–9940.
- Sosnik, A.; Sodhi, R.N.S.; Brodersen, P.M.; Sefton, M.V. Surface Study of Collagen/Poloxamine Hydrogels by a “deep Freezing” ToF-SIMS Approach. Biomaterials 2006, 27, 2340–2348.
- Mahoney, C.M.; Fahey, A.J.; Belu, A.M. Three-Dimensional Compositional Analysis of Drug Eluting Stent Coatings Using Cluster Secondary Ion Mass Spectrometry. Anal. Chem. 2008, 80, 624–632.
- Fröhlich, S.M.; Eilenberg, M.; Svirkova, A.; Grasl, C.; Liska, R.; Bergmeister, H.; Marchetti-Deschmann, M. Mass Spectrometric Imaging of in Vivo Protein and Lipid Adsorption on Biodegradable Vascular Replacement Systems. Analyst 2015, 140, 6089–6099.
- Guidoin, R.; Maurel, S.; Chakfé, N.; How, T.; Zhang, Z.; Therrien, M.; Formichi, M.; Gosselin, C. Expanded Polytetrafluoroethylene Arterial Prostheses in Humans: Chemical Analysis of 79 Explanted Specimens. Biomaterials 1993, 14, 694–704.
- Mantovani, D.; Vermette, P.; Guidoin, R.; Laroche, G. Lipid Uptake in Synthetic Vascular Prostheses Explanted from Humans. Biomaterials 1999, 20, 1023–1032.
- Liedenbaum, M.H.; Verdam, F.J.; Spelt, D.; de Groot, H.G.W.; van der Waal, J.; van der Laan, L. The Outcome of the Axillofemoral Bypass: A Retrospective Analysis of 45 Patients. World J. Surg. 2009, 33, 2490–2496.
- Bodzon-Kulakowska, A.; Cichon, T.; Golec, A.; Drabik, A.; Ner, J.; Suder, P. DESI-MS as a Tool for Direct Lipid Analysis in Cultured Cells. Cytotechnology 2015, 67, 1085–1091.
- Kauschke, V.; Schneider, M.; Jauch, A.; Schumacher, M.; Kampschulte, M.; Rohnke, M.; Henss, A.; Bamberg, C.; Trinkaus, K.; Gelinsky, M.; et al. Effects of a Pasty Bone Cement Containing Brain-Derived Neurotrophic Factor-Functionalized Mesoporous Bioactive Glass Particles on Metaphyseal Healing in a New Murine Osteoporotic Fracture Model. Int. J. Mol. Sci. 2018, 19, 3531.
- Kern, C.; Jamous, R.; El Khassawna, T.; Rohnke, M. Characterisation of Sr2+ Mobility in Osteoporotic Rat Bone Marrow by Cryo-ToF-SIMS and Cryo-OrbiSIMS. Analyst 2022, 147, 4141–4157.
- Malmberg, P.; Nygren, H. Methods for the Analysis of the Composition of Bone Tissue, with a Focus on Imaging Mass Spectrometry (TOF-SIMS). Proteomics 2008, 8, 3755–3762.
- Henss, A.; Rohnke, M.; Knaack, S.; Kleine-Boymann, M.; Leichtweiss, T.; Schmitz, P.; El Khassawna, T.; Gelinsky, M.; Heiss, C.; Janek, J. Quantification of Calcium Content in Bone by Using ToF-SIMS—A First Approach. Biointerphases 2013, 8, 31.
- Kern, C.; Ray, S.; Gelinsky, M.; Bellew, A.T.; Pirkl, A.; Rohnke, M. New Insights into ToF-SIMS Imaging in Osteoporotic Bone Research. Biointerphases 2020, 15, 031005.
- Rentsch, B.; Bernhardt, A.; Henß, A.; Ray, S.; Rentsch, C.; Schamel, M.; Gbureck, U.; Gelinsky, M.; Rammelt, S.; Lode, A. Trivalent Chromium Incorporated in a Crystalline Calcium Phosphate Matrix Accelerates Materials Degradation and Bone Formation in Vivo. Acta Biomater. 2018, 69, 332–341.
- Fröhlich, S.M.; Archodoulaki, V.-M.; Allmaier, G.; Marchetti-Deschmann, M. MALDI-TOF Mass Spectrometry Imaging Reveals Molecular Level Changes in Ultrahigh Molecular Weight Polyethylene Joint Implants in Correlation with Lipid Adsorption. Anal. Chem. 2014, 86, 9723–9732.
- Rohnke, M.; Pfitzenreuter, S.; Mogwitz, B.; Henß, A.; Thomas, J.; Bieberstein, D.; Gemming, T.; Otto, S.K.; Ray, S.; Schumacher, M.; et al. Strontium Release from Sr2+-Loaded Bone Cements and Dispersion in Healthy and Osteoporotic Rat Bone. J. Control. Release 2017, 262, 159–169.
- Schaepe, K.; Werner, J.; Glenske, K.; Bartges, T.; Henss, A.; Rohnke, M.; Wenisch, S.; Janek, J. ToF-SIMS Study of Differentiation of Human Bone-Derived Stromal Cells: New Insights into Osteoporosis. Anal. Bioanal. Chem. 2017, 409, 4425–4435.
- Klerk, L.A.; Dankers, P.Y.W.; Popa, E.R.; Bosman, A.W.; Sanders, M.E.; Reedquist, K.A.; Heeren, R.M.A. TOF-Secondary Ion Mass Spectrometry Imaging of Polymeric Scaffolds with Surrounding Tissue after in Vivo Implantation. Anal. Chem. 2010, 82, 4337–4343.
- Sijbesma, R.P.; Beijer, F.H.; Brunsveld, L.; Folmer, B.J.; Hirschberg, J.H.; Lange, R.F.; Lowe, J.K.; Meijer, E.W. Reversible Polymers Formed from Self-Complementary Monomers Using Quadruple Hydrogen Bonding. Science 1997, 278, 1601–1604.
- Antoine, E.E.; Vlachos, P.P.; Rylander, M.N. Review of Collagen I Hydrogels for Bioengineered Tissue Microenvironments: Characterization of Mechanics, Structure, and Transport. Tissue Eng. Part B Rev. 2014, 20, 683–696.
- McLaughlin, S.; McNeill, B.; Podrebarac, J.; Hosoyama, K.; Sedlakova, V.; Cron, G.; Smyth, D.; Seymour, R.; Goel, K.; Liang, W.; et al. Injectable Human Recombinant Collagen Matrices Limit Adverse Remodeling and Improve Cardiac Function after Myocardial Infarction. Nat. Commun. 2019, 10, 4866.
- Huber, M.; Heiduschka, P.; Kienle, S.; Pavlidis, C.; Mack, J.; Walk, T.; Jung, G.; Thanos, S. Modification of Glassy Carbon Surfaces with Synthetic Laminin-Derived Peptides for Nerve Cell Attachment and Neurite Growth. J. Biomed. Mater. Res. 1998, 41, 278–288.
- Zheng, J.; Kontoveros, D.; Lin, F.; Hua, G.; Reneker, D.H.; Becker, M.L.; Willits, R.K. Enhanced Schwann Cell Attachment and Alignment Using One-Pot “Dual Click” GRGDS and YIGSR Derivatized Nanofibers. Biomacromolecules 2015, 16, 357–363.
- Motta, C.M.M.; Endres, K.J.; Wesdemiotis, C.; Willits, R.K.; Becker, M.L. Enhancing Schwann Cell Migration Using Concentration Gradients of Laminin-Derived Peptides. Biomaterials 2019, 218, 119335.
- Chalazonitis, A.; Tennyson, V.M.; Kibbey, M.C.; Rothman, T.P.; Gershon, M.D. The Alpha1 Subunit of Laminin-1 Promotes the Development of Neurons by Interacting with LBP110 Expressed by Neural Crest-Derived Cells Immunoselected from the Fetal Mouse Gut. J. Neurobiol. 1997, 33, 118–138.
- Stauffer, W.R.; Cui, X.T. Polypyrrole Doped with 2 Peptide Sequences from Laminin. Biomaterials 2006, 27, 2405–2413.
- Zhu, L.; Wang, K.; Ma, T.; Huang, L.; Xia, B.; Zhu, S.; Yang, Y.; Liu, Z.; Quan, X.; Luo, K.; et al. Noncovalent Bonding of RGD and YIGSR to an Electrospun Poly(ε-Caprolactone) Conduit through Peptide Self-Assembly to Synergistically Promote Sciatic Nerve Regeneration in Rats. Adv. Healthc. Mater. 2017, 6, 1600860.
- Shin, H.; Jo, S.; Mikos, A.G. Biomimetic Materials for Tissue Engineering. Biomaterials 2003, 24, 4353–4364.
- Ender, A.M.; Kaygisiz, K.; Räder, H.-J.; Mayer, F.J.; Synatschke, C.V.; Weil, T. Cell-Instructive Surface Gradients of Photoresponsive Amyloid-like Fibrils. ACS Biomater. Sci. Eng. 2021, 7, 4798–4808.
- Adamcik, J.; Ruggeri, F.S.; Berryman, J.T.; Zhang, A.; Knowles, T.P.J.; Mezzenga, R. Evolution of Conformation, Nanomechanics, and Infrared Nanospectroscopy of Single Amyloid Fibrils Converting into Microcrystals. Adv. Sci. 2021, 8, 2002182.
- Knowles, T.P.J.; Mezzenga, R. Amyloid Fibrils as Building Blocks for Natural and Artificial Functional Materials. Adv. Mater. 2016, 28, 6546–6561.
- Wei, G.; Su, Z.; Reynolds, N.P.; Arosio, P.; Hamley, I.W.; Gazit, E.; Mezzenga, R. Self-Assembling Peptide and Protein Amyloids: From Structure to Tailored Function in Nanotechnology. Chem. Soc. Rev. 2017, 46, 4661–4708.
- Schilling, C.; Mack, T.; Lickfett, S.; Sieste, S.; Ruggeri, F.S.; Sneideris, T.; Dutta, A.; Bereau, T.; Naraghi, R.; Sinske, D.; et al. Sequence-Optimized Peptide Nanofibers as Growth Stimulators for Regeneration of Peripheral Neurons. Adv. Funct. Mater. 2019, 29, 1809112.
- Kokesch-Himmelreich, J.; Woltmann, B.; Torger, B.; Rohnke, M.; Arnhold, S.; Hempel, U.; Müller, M.; Janek, J. Detection of Organic Nanoparticles in Human Bone Marrow-Derived Stromal Cells Using ToF–SIMS and PCA. Anal. Bioanal. Chem. 2015, 407, 4555–4565.
- Pierson, E.E.; Midey, A.J.; Forrest, W.P.; Shah, V.; Olivos, H.J.; Shrestha, B.; Teller, R.; Forster, S.; Bensussan, A.; Helmy, R. Direct Drug Analysis in Polymeric Implants Using Desorption Electrospray Ionization—Mass Spectrometry Imaging (DESI-MSI). Pharm. Res. 2020, 37, 107.
- Yu, C.-C.; Chen, Y.-W.; Yeh, P.-Y.; Hsiao, Y.-S.; Lin, W.-T.; Kuo, C.-W.; Chueh, D.-Y.; You, Y.-W.; Shyue, J.-J.; Chang, Y.-C.; et al. Random and Aligned Electrospun PLGA Nanofibers Embedded in Microfluidic Chips for Cancer Cell Isolation and Integration with Air Foam Technology for Cell Release. J. Nanobiotechnol. 2019, 17, 31.
- Scoutaris, N.; Hook, A.L.; Gellert, P.R.; Roberts, C.J.; Alexander, M.R.; Scurr, D.J. ToF-SIMS Analysis of Chemical Heterogenities in Inkjet Micro-Array Printed Drug/Polymer Formulations. J. Mater. Sci. Mater. Med. 2012, 23, 385–391.
- Krueger, K.; Terne, C.; Werner, C.; Freudenberg, U.; Jankowski, V.; Zidek, W.; Jankowski, J. Characterization of Polymer Membranes by MALDI Mass-Spectrometric Imaging Techniques. Anal. Chem. 2013, 85, 4998–5004.
- Bolles, K.M.; Cheng, F.; Burk-Rafel, J.; Dubey, M.; Ratner, D.M. Imaging Analysis of Carbohydrate-Modified Surfaces Using ToF-SIMS and SPRi. Materials 2010, 3, 3948–3964.
- Qi, K.; Ma, Q.; Remsen, E.E.; Clark, C.G.; Wooley, K.L. Determination of the Bioavailability of Biotin Conjugated onto Shell Cross-Linked (SCK) Nanoparticles. J. Am. Chem. Soc. 2004, 126, 6599–6607.
More
Information
Subjects:
Materials Science, Biomaterials
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
400
Revisions:
2 times
(View History)
Update Date:
27 Sep 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




