
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Sunil J. Wimalawansa | -- | 3349 | 2023-09-23 04:03:13 | | | |
| 2 | Sirius Huang | -402 word(s) | 2947 | 2023-09-26 09:05:44 | | | | |
| 3 | Sunil J. Wimalawansa | -3 word(s) | 2944 | 2023-09-26 17:25:36 | | |
Video Upload Options
Treatment of vitamin D deficiency costs less than 0.01% of one-day hospitalization. Despite cost-benefits, the prevalence of vitamin D deficiency remains high worldwide. This was vivid among those who died from COVID-19—most had vitamin D deficiency. Yet, the lack of direction to use vitamin D as an adjunct therapy from health agencies was astonishing. Data confirmed that keeping an individual’s serum 25(OH)D concentrations above 50 ng/mL (125 nmol/L) (and above 40 ng/mL in the population) reduces risks from community outbreaks and autoimmune disorders. Maintaining such concentrations in 97.5% of people is achievable through daily safe sun exposure (except in countries far from the equator during winter) or taking between 5,000 and 8,000 IU vitamin D supplements daily (average, ~70 to 90 IU/kg body weight). Those with gastrointestinal malabsorption, obesity, or on medications that increase catabolism of vitamin D and a few specific disorders require much higher intake. The text evaluates the doses and administration of vitamin D necessary for better clinical outcomes regarding disease prevention and treatment.
1. The Roles of Vitamin D in Keeping Individuals Healthy
The most active vitamin D metabolite, calcitriol [1,25(OH)2D], is generated intracellularly in peripheral target cells (e.g., immune cells) from the circulatory D3 and 25(OH)D. Sufficient diffusion against a concentration gradient occurs when these precursors in the circulating are above 40 ng/mL (preferably 50 ng/mL) [1][2]. This process allows the intracellular generation of sufficient amounts of calcitriol for signaling and binding to vitamin D (calcitriol) receptors (VDR), initiating genomic activity. Vitamin D sufficiency suppresses pathological processes, including hyper-inflammation, oxidative stress, and hyper-immune reactions, with its genomic and non-genomic actions, including autocrine/intracrine and paracrine signaling mechanisms [3][4]. These actions reduce the risks of cytokine storms and acute respiratory distress syndrome (ARDS) associated with the severe pulmonary system, endothelial instability and thrombosis linked to cardiovascular complications in people with infections and sepsis) such as SARS-CoV-2 [5][6].
1.1. Importance of Cofactors and Micronutrients for The Full Functions of Vitamin D
The complete activity of vitamin D, VDR, and associated enzymatic reactions require the presence of endogenous or supplemented cofactors [7][8]. The latter include magnesium, zinc, vitamins A, B2, C, and K, anti-oxidant trace minerals (zinc and selenium), resveratrol, essential fatty acids such as omega-3, and boron [9][10]. Besides, it is important to note that the immune system and other target cells, during the biological processes, consume vitamin D metabolites and cofactors [11]. This requirement is enhanced due to the multiple immune and metabolic pathways in which vitamin D is intimately involved in vivo. Consequently, it is essential to replace these cofactors, especially during severe illnesses like infections (like COVID-19) and sepsis (a life-threatening organ dysfunction caused by a dysregulated host response to infection) [12]. Although it would help patients, no one seems to be doing this.
Consequently, a continuous supply (preferably daily intake during an illness) of the mentioned micronutrient cofactors is necessary to attain optimal potentials of vitamin D and better clinical outcomes [8][11]. The lack of this is another reason for the few benefits reported in some clinical studies, including RCTs. A nutrient RCT (e.g., vitamin D) cannot be considered well-designed without supplementing the mentioned cofactors, especially in studies involving persons with severe acute illnesses, like SARS-CoV-2 infections (e.g., in ICU setup) and in some longer-term clinical trials. Scientists and healthcare workers are unaware of this fact.
Consequently, in clinical practice and clinical studies, including RCTs, no one is yet utilizing this critical factor, resulting in less-than-optimum clinical outcomes. The lack of incorporation of cofactor therapy is another study design error that negatively affects vitamin D in clinical trial outcomes and patient care in routine clinical practice. At the minimum, study subjects (active and placebo participants) and clinical patients should be provided a multivitamin and essential mineral supplement (e.g., magnesium, zinc, selenium, boron) during an acute illness [7][8][10], enabling them to recover faster.
1.2. Consequences of Hypovitaminosis D and How to Overcome These
Severe vitamin D deficiency impairs immune cell functions and causes immune dysregulation. Consequently, when exposed to a severe infection or sepsis (or an acute illness), they are at a disadvantage for recovery and at higher risk for developing hyperinflammation, oxidative stress, and autoimmunity [13][14][15]—as a result of hyper-reactive but pathological immune response [16]. The failure to correct vitamin D deficiency could lead to cytokine storms with an increased risk of death [17][18], precipitating ARDS [19] and severe asthmatic attacks [20].
In addition, severe hypovitaminosis D leads to weakened adaptive immunity, which reduces the capacity to generate neutralizing antibodies (including after vaccinations) and impairs the cytotoxic action of immune/killer cells. It also reduces the effectiveness of memory cells and macrophages and causes weaker responses following (any) vaccine. Overall, it causes immune paresis with inadequate antibody responses. Besides, the adverse effects of vaccines are highest among those with vitamin D deficiency. The lower the serum 25(OH)D concentration, the higher the severity of adverse effects. This is due to hypovitaminosis-induced immune dysfunction aggravated by vaccine-induced toxic products, like Spike proteins, in the case of COVID-19 vaccines.
As mentioned, in those with a fragile (weaker) immune system, as in severe hypovitaminosis D, not only SARS-CoV-2 infection but also immunization against it could lead to significant harmful effects [21][22][23][24]. The latter include hyper-immune and autoimmune reactions, generalized hyper-inflammation, and pathological oxidative stress, which increase the risks for systemic complications (blood clots, strokes, etc.) and death—all these complications were reported in severe SARS-CoV-2 infections and following COVID-vaccines, especially in those with vitamin D deficiency. Consequently, in 2020/21, due to the prevailing high incidence of hypovitaminosis among older people and those with comorbidities, COVID-19 primarily affected them, sparing children and the youth [9][25]. However, the vaccine-related adverse effects continue among those with hypovitaminosis D even after 2021 with bivalent COV vaccines.
2. Overcoming Severe Complications from Hypovitaminosis D-derived Weaker Immune System
Cytokine storms are associated with pro-inflammatory and hyper-oxidative stress responses, as seen in severe viral infections. This is one of the prime reasons for intensive care unit (ICU) admissions and deaths, as observed during the COVID-19 pandemic [26][27][28][29]. Children infected with SARS-CoV-2, having less than 12 ng/mL of serum 25(OH)D concentrations (i.e., severe vitamin D deficiency), are at very high risk for developing life-threatening hyper-inflammatory conditions, such as Kawasaki-like disease or multi-system inflammatory syndrome [30][31][32]. These complications and deaths could have been promptly minimized using a single dose of partially hydroxylated vitamin D, calcifediol, a dose between 0.5 and 1 mg [1][2].
Complications such as cytokine storms can be prevented with an appropriate (high) dose and type of vitamin D. Since cholecalciferol (D3) takes several days to get hydroxylated and increase serum 25(OH)D concentrations, calcifediol (between 0.5 and 1.0 as a stat dose: calculated as, 0.014 mg/kg body weight) should be in emergencies and severe disease status, instead of vitamin D3. Due to the impairment of the formation of intracellular calcitriol in the immune cells, hypovitaminosis D also impairs intracrine and paracrine signaling, further weakening the immune system and increasing vulnerability [4][33].
2.1. Different Serum 25(OH)D Concentrations Are Needed for Different Diseases
Many conditions require maintaining serum 25(OH)D concentrations greater than 30 ng/mL for anticipated clinical outcomes. No one has proposed a single optimal serum 25(OH)D concentration covering at least 95% of disorders and providing maximum beneficial clinical outcomes for all body systems [34][35]. While the musculoskeletal system benefits (like preventing rickets and osteomalacia) from low levels like 20 ng/mL, most other body systems require more than 40 ng/mL. Examples include T2D and metabolic syndrome [36][37]. However, alleviating others, such as cancer [38], asthma [20], autoimmunity, infections, and cancer [39][40], etc., requires the maintenance of serum 25(OH)D concentrations greater than 50 ng/mL [41][42].
Over the years, there has been confusion regarding the optimal serum 25(OH)D concentration. Discussion on this is worthless, as different diseases require varying serum 25(OH)D concentrations to obtain the best clinical outcomes and prevent complications [39][43][44]. The arguments and justifications made on whether it is 20 ng/mL (recommended by the Institute of Medicine, USA), 30 ng/ml (the Endocrine Society, USA), or 40 ng/mL (a few other groups) are irrelevant.
The minimum serum 25(OH)D concentrations needed to prevent or lessen the effects of all diseases are illustrated in Figure 1. It indicates the relationships between various disease states and the approximate minimal serum 25(OH)D concentrations needed to improve different conditions [39]. It summarizes the varying steady-state serum 25(OH)D concentrations required to prevent or lessen the effects of common diseases based on many published data.
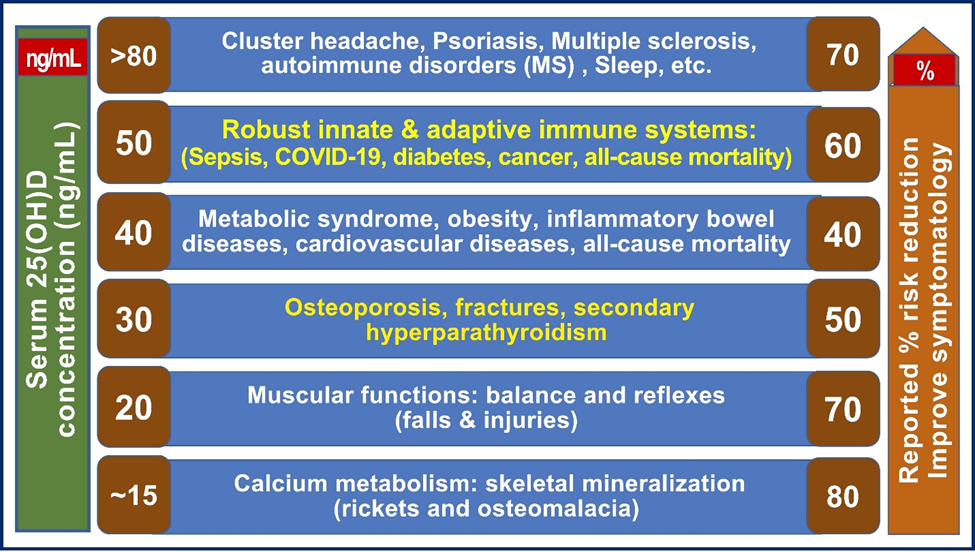
Figure 1. Different diseases (and tissues) require separate steady-state serum 25(OH)D concentrations to achieve improvement (illustrated as a percentage on the right side). The need for varied serum 25(OH)D concentrations to subdue various disease statuses is illustrated (modified from Wimalawansa, S.J. Steroid Biochemistry [39].
2.2. Dose of Vitamin D Needed to Maintain Optimum Serum 25(OH)D Concentration
Governments and scientific societies’ recommended doses of vitamin D and serum 25(OH)D concentrations are grossly outdated. They are insufficient to reduce incidences and severity of cancer, infections, sepsis, or all-cause mortality [1][2]. Recommended doses of vitamin D need to be multiplied by ten and serum 25(OH)D levels by 2.5 times to make it practical and effective [42]. To agree on a universally agreeable minimum serum, 25(OH)D (and the range) must cover the most common disorders, at least over 95 to 98%, not just one condition or a body system, as the Institute of Medicine (IoM/NAS) [45][46] and National Institutes of Health [47] suggested (and continuing) incorrectly. Especially considering vitamin D is economical to use and a widely available generic nutrient that does not need a prescription and has no adverse effects on recommended doses.
Dark-skinned people in central Africa living traditional lifestyles have a mean serum 25(OH)D concentration of 47 ng/mL (119 nmol/L) (range, 30 to 70 ng/mL) [48][49]. However, with imbalanced macro-nutrient diets, micro-nutrient deficiencies, unwholesome modern dietary constituents and practices (e.g., consisting of processed food, fast food, trans fat, and preservatives, some of which also increase the catabolism of micronutrients), environmental pollution, and passive indoor lifestyles, many people requiring higher intakes of vitamin D than mentioned-above than those recommended by governments and health-related societies and agencies.
Generally, adults require between six to ten-fold of standard recommendations to maintain a higher serum 25(OH)D concentration, such as between 50 and 80 ng/mL, to obtain full benefits from vitamin D-related. That would cover approximately 99.7% of all disorders affecting humans. Achieving the mentioned therapeutic blood concentrations requires a daily vitamin D intake between 5,000 and 8,000 IU for healthy [1][2] non-obese adults of 70 kg or 50,000 IU weekly (or once in ten days), with a tolerable upper limit of 15,000 IU/day [5,13,104].
3. Dose-Responses and Vitamin D Requirements for Adults
3.1. Vitamin D Dose-Responses
Administration of high oral doses of nutrient vitamin D in D-deficient persons leads to a meaningful, measurable change in the serum 25(OH)D concentrations within three to four days [42][50][51], causing intended beneficial outcomes. The lower the serum 25(OH)D concentration, the higher the percentage increase (∆) in the circulation and the higher the likelihood of demonstrating better clinical outcomes. However, such a dose-clinical response relationship does not exist in those who are vitamin D sufficient. Figure 2 illustrates a typical dose-clinical response curve for nutrients such as vitamin D.
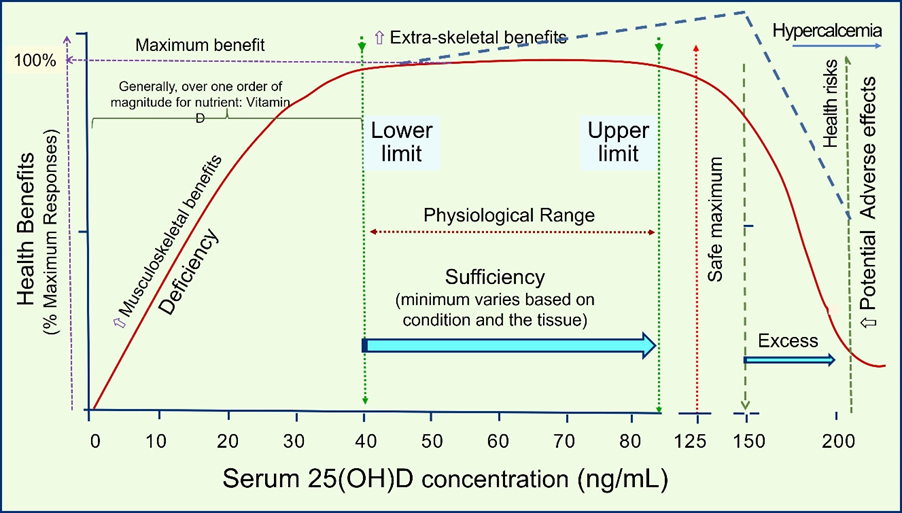
Figure 2. Illustration of the dose—25(OH)D concentrations achieved in the circulation vs. responses (clinical health benefits and potential risks). The figure also provides the basic pharmacodynamics of a typical nutrient, taking vitamin D as an example. When tissue sufficiency occurred, generally, there would not be additional benefits by raising the circulatory concentration by increasing the intake. However, there are exceptions in a small percentage; pharmacological doses are needed under medical guidance in less than 0.01% of the population to overcome resistance to achieve the desired clinical goals (indicated in the dashed blue line, as described in the previous section) [42].
The data strongly suggests that most (about 80%) health benefits are seen when serum 25(OH)D concentrations are maintained at more than 40 ng/mL (100 nmol/L) [52], with further improvements seen when levels kept over 50 ng/mL (over 99.7%) [42][2]. This can be cost-effectively achieved by providing safe sun exposure guidance and appropriate intakes of vitamin D supplements. The emphasis is on sensible, safe, balanced vitamin D (and other micronutrient intake) that provides cost–benefits to the public [53][54][55].
3.2. Minimum and The Range of Serum 25(OH)D (ng/mL) Necessary to Minimize Diseases and Obtain Maximum Benefits
Considering broader biological and physiological benefits, changing disease patterns (increasing incidences of metabolic diseases, obesity, diabetes, sepsis, and viral infections), the behavior of people (sun avoidance), and additional broader risk factors (pollution, harmful diets, medications, etc.) [56], and passive lifestyles acquired in this Millenium [57], the published evidence justifies the range of serum 25(OH)D (50 to 80 ng/mL). Based on the data, it is reasonable to contemplate that the minimum serum 25(OH)D concentration needed for a healthy life for all ages of humans is 50 ng/mL. The latter is essential because age does not change the threshold of the immune system; thus, vitamin D adequacy helps everyone.
Overcoming different diseases and disorders requires varied concentrations of serum 25(OH)D (see section 1.4). In addition, the evidence also strongly suggests that there are tissue-specific differences in serum 25(OH)D concentration thresholds to elicit its full biological effects. In 2012, the Endocrine Society suggested a minimum serum 25(OH)D concentration needed for humans is—30 ng/mL [58]. However, it would only protect about a quarter of common disorders (primarily calcium homeostasis and musculoskeletal). In contrast, based on the data reported over the past decade, researchers suggest that the minimum serum 25(OH)D concentration needed is—50 ng/mL—with a range of 50 to 80 ng/mL. This would cover 99.7% of health conditions with no adverse effects. One minimum serum 25(OH)D concentration that covered virtually all diseases [42][59].
3.3. A Small Number of People Need Much Higher Serum 25(OH)D Concentration to Overcome Specific Disorders
In contrast, if one considers 80 ng/mL as the minimum level (as some suggested), it will cover 99.9% of health conditions. However, with additional negligible benefits (0.2%), it is likely to increase adverse effects; thus, it is not recommended or justified in routine clinical practice. Data supports the idea that less than 0.1% of the population requires very high doses of vitamin D to obtain a high response rate [41][42]. Such disorders include preventing intractable migraine headaches, asthma, psoriasis, specific autoimmune diseases (e.g., multiple sclerosis) reactions, tissue/organ graft rejection, and vitamin D-resistant syndromes [41][42].
The mentioned disorders respond to higher doses and maintain more elevated serum 25(OH)D concentrations than those noted above. Such persons must be treated by specialists experienced with using higher amounts of vitamin D. These high-dose vitamin D regimens [60], like the Coimbra protocol [61], should not be undertaken by primary care physicians, internal medicine, general and nurse practitioners, immunologists, endocrinologists, etc., without expertise (and immediate availability) in handling them properly. These patients should be managed under close medical supervision by expert clinicians (or centers) to maximize benefits and minimize adverse effects, benefiting them. Per medical ethics and common sense, healthcare workers must balance safety (vs. harm) and cost-effectiveness. Such an approach with the correct dose for a given person (i.e., highly selected individualized therapy) with specific conditions can provide them with significantly beneficial clinical outcomes while avoiding adverse effects.
For those taking higher vitamin D doses (e.g., above 7000 IU/day or 50,000 IU/week), additional precautionary steps need to be followed to prevent potential soft tissue calcification. These include avoiding calcium supplements and high calcium-containing food and taking vitamin K2 (MK-7: Menaquinone-7, present in fermented food), 100 micrograms/day or 800 micrograms, once a week, etc. The latter diverts ionized calcium from the blood to skeletal tissues and, thus, prevents soft tissue calcification.
3.4. What is Required Now?
Population serum 25(OH)D concentrations above 40 ng/mL can control several acute and chronic conditions [62]. Therefore, it is logical to aim to maintain the population serum 25(OH)D concentrations above 40 ng/mL, which covers approximately 85% of disorders [42]. In comparison, keeping individuals’ serum 25(OH)D concentrations above 50 ng/mL covers over 99.7% of conditions, including cancer, infections, and all-cause mortality, and keeps people healthy and minimizes absenteeism [2][1].
Doubling the current prevailing population serum 25(OH)D concentration of approximately 20 ng/mL is needed to benefit the population and reduce morbidities and all-cause mortality [63][64]. This is achievable by increasing the current government recommendation of vitamin D for adults from 400 to 800 IU to 4,000 to 8000 IU (i.e., an increase of tenfold). This would mitigate the ongoing low-grade inflammation and chronic diseases in the people. It would also open doors to achieving broader benefits from vitamin D, such as wider control of inflammation and oxidative stress [13]. As an indirect benefit, such an approach will also reduce myocardial infarctions and strokes, with huge cost-benefits. It would also enhance beneficial cellular effects, such as membrane stabilization, protection from DNA damage and repair, improving the efficiency of mitochondria functions (e.g., energy metabolism), and minimizing infectious outbreaks and sepsis.
4. Conclusions
Overall evidence suggests that vitamin D deficiency, as determined by maintaining serum 25(OH)D concentrations of more than 40 ng/mL, is associated with increased risks of illnesses and disorders and higher all-cause mortality, even among otherwise healthy individuals [65]. The proper functioning of the vitamin D endocrine, paracrine, and autocrine systems is essential for many physiological activities and maintaining good health. As discussed, data addressed critical functions of vitamin D that extend beyond its calcium and phosphate homeostasis and prevention and treatment of rickets, osteomalacia, and bone loss.
The dosages of vitamin D prescribed for non-obese deficient persons of average weight of 70 kg should be between 4000 and 7000 IU/day, 20,000 IU twice a week, or 50,000 IU once a week or once in 10 days [41][42]. Such doses would allow approximately 9.5% of people to maintain their serum 25(OH)D concentrations above 40 ng/mL [5,30]. However, intermittent doses at intervals longer than once a month are unphysiological and thus ineffective. Studies have shown that daily vitamin D supplements are more beneficial than supplementation administered less frequently.
References
- Wimalawansa, S. Overcoming Infections Including COVID-19, by Maintaining Circulating 25(OH)D Concentrations Above 50 ng/mL. Pathol. Lab. Med. Int. 2022, ume 14, 37–60.
- Wimalawansa, S.J. Rapidly Increasing Serum 25(OH)D Boosts the Immune System, against Infections—Sepsis and COVID-19. Nutrients 2022, 14, 2997.
- Chauss, D.; Freiwald, T.; McGregor, R.; Yan, B.; Wang, L.; Nova-Lamperti, E.; Kumar, D.; Zhang, Z.; Teague, H.; West, E.E.; et al. Autocrine vitamin D signaling switches off pro-inflammatory programs of TH1 cells. Nat. Immunol. 2022, 23, 62–74.
- McGregor, E.; Kazemian, M.; Afzali, B.; Freiwald, T.; Yan, B.; Wang, L.; Nova-Lamperti, E.; Zhang, Z.; Teague, H.; West, E.E.; et al. An autocrine Vitamin D-driven Th1 shutdown program can be exploited for COVID-19. bioRxiv 2020.
- Xu, J.; Sriramula, S.; Xia, H.; Moreno-Walton, L.; Culicchia, F.; Domenig, O.; Poglitsch, M.; Lazartigues, E. Clinical Relevance and Role of Neuronal AT 1 Receptors in ADAM17-Mediated ACE2 Shedding in Neurogenic Hypertension. Circ. Res. 2017, 121, 43–55.
- Xu, J.; Yang, J.; Chen, J.; Luo, Q.; Zhang, Q.; Zhang, H. Vitamin D alleviates lipopolysaccharide-induced acute lung injury via regulation of the renin-angiotensin system. Mol. Med. Rep. 2017, 16, 7432–7438.
- Tsoukalas, D.; Sarandi, E. Micronutrient deficiencies in patients with COVID-19: How metabolomics can contribute to their prevention and replenishment. BMJ Nutr. Prev. Health 2020, 3, 419–420.
- Dai, Q.; Zhu, X.; Manson, J.E.; Song, Y.; Li, X.; Franke, A.A.; Costello, R.B.; Rosanoff, A.; Nian, H.; Fan, L.; et al. Magnesium status and supplementation influence vitamin D status and metabolism: Results from a randomized trial. Am. J. Clin. Nutr. 2018, 108, 1249–1258.
- Wimalawansa, S.J. Reducing Risks from COVID-19: Cost-Effective Ways of Strengthening Individual’s and the Population Immunity with Vitamin D. J. Endocrinol. Sci. 2020, 2, 5–13.
- van Ballegooijen, A.J.; Pilz, S.; Tomaschitz, A.; Grubler, M.R.; Verheyen, N. The synergistic interplay between vitamins D and K for bone and cardiovascular Health: A narrative review. Int. J. Endocrinol. 2017, 2017, 7454376.
- Wimalawansa, S.J. Fighting against COVID-19: Boosting the immunity with micronutrients, stress reduction, physical activity, and vitamin D. Nutr. Food Sci. J. 2020, 3, 126.
- Pagano, G.; Manfredi, C.; Pallardo, F.V.; Lyakhovich, A.; Tiano, L.; Trifuoggi, M. Potential roles of mitochondrial cofactors in the adjuvant mitigation of proinflammatory acute infections, as in the case of sepsis and COVID-19 pneumonia. Inflamm Res 2021, 70, 159-170, doi:10.1007/s00011-020-01423-0.
- Colotta, F.; Jansson, B.; Bonelli, F. Modulation of inflammatory and immune responses by vitamin D. J. Autoimmun. 2017, 85, 78–97.
- Wimalawansa, S.J. Vitamin D Deficiency: Effects on Oxidative Stress, Epigenetics, Gene Regulation, and Aging. Biology 2019, 8, 30.
- Jamilian, M.; Amirani, E.; Asemi, Z. The effects of vitamin D and probiotic co-supplementation on glucose homeostasis, inflammation, oxidative stress and pregnancy outcomes in gestational diabetes: A randomized, double-blind, placebo-controlled trial. Clin. Nutr. 2018, 38, 2098–2105.
- Sims, J.T.; Krishnan, V.; Chang, C.-Y.; Engle, S.M.; Casalini, G.; Rodgers, G.H.; Bivi, N.; Nickoloff, B.J.; Konrad, R.J.; de Bono, S.; et al. Characterization of the cytokine storm reflects hyperinflammatory endothelial dysfunction in COVID-19. J. Allergy Clin. Immunol. 2020, 147, 107–111.
- Hojyo, S.; Uchida, M.; Tanaka, K.; Hasebe, R.; Tanaka, Y.; Murakami, M.; Hirano, T. How COVID-19 induces cytokine storm with high mortality. Inflamm. Regen. 2020, 40, 37.
- Iannaccone, G.; Scacciavillani, R.; Del Buono, M.G.; Camilli, M.; Ronco, C.; Lavie, C.J.; Abbate, A.; Crea, F.; Massetti, M.; Aspromonte, N. Weathering the Cytokine Storm in COVID-19: Therapeutic Implications. Cardiorenal Med. 2020, 10, 277–287.
- Caccamo, D.; Ricca, S.; Currò, M.; Ientile, R. Health Risks of Hypovitaminosis D: A Review of New Molecular Insights. Int. J. Mol. Sci. 2018, 19, 892.
- Székely, J.I.; Pataki, Á. Effects of vitamin D on immune disorders with special regard to asthma, COPD and autoimmune diseases: A short review. Expert Rev. Respir. Med. 2012, 6, 683–704.
- Baggs, J.; Gee, J.; Lewis, E.; Fowler, G.; Benson, P.; Lieu, T.; Naleway, A.; Klein, N.P.; Baxter, R.; Belongia, E.; et al. The Vaccine Safety Datalink: A Model for Monitoring Immunization Safety. Pediatrics 2011, 127, S45–S53.
- Kadkhoda, K. Post-adenoviral-based COVID-19 vaccines thrombosis: A proposed mechanism. J. Thromb. Haemost. 2021, 19, 1831–1832.
- Long, B.; Bridwell, R.; Gottlieb, M. Thrombosis with thrombocytopenia syndrome associated with COVID-19 vaccines. Am. J. Emerg. Med. 2021, 49, 58–61.
- Swan, D.A.; Bracis, C.; Janes, H.; Moore, M.; Matrajt, L.; Reeves, D.B.; Burns, E.; Donnell, D.; Cohen, M.S.; Schiffer, J.T.; et al. COVID-19 vaccines that reduce symptoms but do not block infection need higher coverage and faster rollout to achieve population impact. Sci. Rep. 2021, 11, 1553.
- Wimalawansa, S.J. Commonsense Approaches to Minimizing Risks from COVID-19. Open J. Pulmonol. Respir. Med. 2020, 2, 28–37.
- Argano, C.; Bocchio, R.M.; Natoli, G.; Scibetta, S.; Monaco, M.L.; Corrao, S. Protective Effect of Vitamin D Supplementation on COVID-19-Related Intensive Care Hospitalization and Mortality: Definitive Evidence from Meta-Analysis and Trial Sequential Analysis. Pharmaceuticals 2023, 16, 130.
- Davies, G.; Mazess, R.B.; Benskin, L.L. Letter to the editor in response to the article: “Vitamin D concentrations and COVID-19 infection in UK biobank” (Hastie et al.). Diabetes Metab. Syndr. Clin. Res. Rev. 2021, 15, 643–644.
- Hastie, C.E.; Pell, J.P.; Sattar, N. Vitamin D and COVID-19 infection and mortality in UK Biobank. Eur. J. Nutr. 2020, 60, 545–548.
- Raisi-Estabragh, Z.; McCracken, C.; Bethell, M.S.; Cooper, J.; Cooper, C.; Caulfield, M.J.; Munroe, P.B.; Harvey, N.C.; E Petersen, S. Greater risk of severe COVID-19 in Black, Asian and Minority Ethnic populations is not explained by cardiometabolic, socioeconomic or behavioural factors, or by 25(OH)-vitamin D status: Study of 1326 cases from the UK Biobank. J. Public Health 2020, 42, 451–460.
- Wallis, G.; Siracusa, F.; Blank, M.; Painter, H.; Sanchez, J.; Salinas, K.; Mamuyac, C.; Marudamuthu, C.; Wrigley, F.; Corrah, T.; et al. Experience of a novel community testing programme for COVID-19 in London: Lessons learnt. Clin. Med. 2020, 20, e165–e169.
- Walter, L.A.; McGregor, A.J. Sex- and Gender-specific Observations and Implications for COVID-19. West J. Emerg. Med. 2020, 21, 507–509.
- Stagi, S.; Rigante, D.; Lepri, G.; Cerinic, M.M.; Falcini, F. Severe vitamin D deficiency in patients with Kawasaki disease: A potential role in the risk to develop heart vascular abnormalities? Clin. Rheumatol. 2016, 35, 1865–1872.
- McGregor, T.B.; Sener, A.; Yetzer, K.; Gillrie, C.; Paraskevas, S. The impact of COVID-19 on the Canadian Kidney Paired Donation program: An opportunity for universal implementation of kidney shipping. Can. J. Surg. 2020, 63, E451–E453.
- Wyon, M.A.; Koutedakis, Y.; Wolman, R.; Nevill, A.M.; Allen, N. The influence of winter vitamin D supplementation on muscle function and injury occurrence in elite ballet dancers: A controlled study. J. Sci. Med. Sport 2014, 17, 8–12.
- Khan, S.R.; Whiteman, D.C.; Kimlin, M.G.; Janda, M.; Clarke, M.W.; Lucas, R.M.; Neale, R.E. Effect of solar ultraviolet radiation exposure on serum 25(OH)D concentration: A pilot randomised controlled trial. Photochem. Photobiol. Sci. 2018, 17, 570–577.
- Pérez-López, F.R. Vitamin D and its implications for musculoskeletal health in women: An update. Maturitas 2007, 58, 117–137.
- Nasri, H.; Behradmanesh, S.; Ahmadi, A.; Rafieian-Kopaei, M. Impact of oral vitamin D (cholecalciferol) replacement therapy on blood pressure in type 2 diabetes patients; a randomized, double-blind, placebo controlled clinical trial. J. Nephropathol. 2014, 3, 29–33.
- Lappe, J.; Watson, P.; Travers-Gustafson, D.; Recker, R.; Garland, C.; Gorham, E.; Baggerly, K.; McDonnell, S.L. Effect of Vitamin D and Calcium Supplementation on Cancer Incidence in Older Women: A Randomized Clinical Trial. JAMA 2017, 317, 1234–1243.
- Wimalawansa, S.J. Non-musculoskeletal benefits of vitamin D. J. Steroid Biochem. Mol. Biol. 2018, 175, 60–81.
- Cauley, J.A.; LaCroix, A.Z.; Wu, L.; Horwitz, M.; Danielson, M.E.; Bauer, D.C.; Lee, J.S.; Jackson, R.D.; Robbins, J.A.; Wu, C.; et al. Serum 25 HydroxyVitamin D Concentrations and the Risk of Hip Fractures: The Women's Health Initiative. Ann. Intern. Med. 2008, 149, 242–250.
- Wimalawansa, S.J. Controlling chronic diseases and acute infections with vitamin D sufficiency. Nutrients 2023, 15, doi:10.3390/nu15163623.
- Wimalawansa, S.J. Physiological Basis for Using Vitamin D to Improve Health. Biomedicines 2023, 11, 1542.
- Wimalawansa, S.J. Vitamin D in the New Millennium. Curr. Osteoporos. Rep. 2012, 10, 4–15.
- Armas, L.A.G.; Hollis, B.W.; Heaney, R.P. Vitamin D2 Is Much Less Effective than Vitamin D3 in Humans. J. Clin. Endocrinol. Metab. 2004, 89, 5387–5391.
- Rosen, C.J.; Gallagher, J.C. The 2011 IOM report on vitamin D and calcium requirements for north america: clinical implications for providers treating patients with low bone mineral density. J Clin Densitom 2011, 14, 79-84, doi:10.1016/j.jocd.2011.03.004.
- Heaney, R.P.; Holick, M.F. Why the IOM recommendations for vitamin D are deficient. J Bone Miner Res 2011, 26, 455-457, doi:10.1002/jbmr.328.
- NIH. Dietary supplement fact sheet: vitamin D. Available online: http://ods.od.nih.gov/factsheets/vitamind.asp
- Luxwolda, M.F.; Kuipers, R.S.; Kema, I.P.; Dijck-Brouwer, D.J.; Muskiet, F.A. Traditionally living populations in East Africa have a mean serum 25-hydroxyvitamin D concentration of 115 nmol/l. Br. J. Nutr. 2012, 108, 1557–1561.
- Luxwolda, M.F.; Kuipers, R.S.; Kema, I.P.; van der Veer, E.; Dijck-Brouwer, D.A.J.; Muskiet, F.A.J. Vitamin D status indicators in indigenous populations in East Africa. Eur. J. Nutr. 2013, 52, 1115–1125.
- Heaney, R.P. Guidelines for optimizing design and analysis of clinical studies of nutrient effects. Nutr. Rev. 2014, 72, 48–54.
- Heaney, R.P.; Vieth, R.; Hollis, B.W. Vitamin D Efficacy and Safety. Arch. Intern. Med. 2011, 171, 266.
- Grant, W.B.; Whiting, S.J.; Schwalfenberg, G.K.; Genuis, S.J.; Kimball, S.M. Estimated economic benefit of increasing 25-hydroxyvitamin D concentrations of Canadians to or above 100 nmol/L. Derm. Endocrinol. 2016, 8, e1248324.
- Cangoz, S.; Chang, Y.-Y.; Chempakaseril, S.J.; Guduru, R.C.; Huynh, L.M.; John, J.S.; John, S.T.; Joseph, M.E.; Judge, R.; Kimmey, R.; et al. Vitamin D and type 2 diabetes mellitus. J. Clin. Pharm. Ther. 2013, 38, 81–84.
- Pilz, S.; Gaksch, M.; Hartaigh, B.; Tomaschitz, A.; März, W. Vitamin D in preventive medicine. Anticancer Res. 2015, 35, 1161–1170.
- Weinert, L.S.; Silveiro, S.P. Maternal–Fetal Impact of Vitamin D Deficiency: A Critical Review. Matern. Child Health J. 2015, 19, 94–101.
- Ekwaru, J.P.; Zwicker, J.D.; Holick, M.F.; Giovannucci, E.; Veugelers, P.J. The Importance of Body Weight for the Dose Response Relationship of Oral Vitamin D Supplementation and Serum 25-Hydroxyvitamin D in Healthy Volunteers. PLoS ONE 2014, 9, e111265.
- Adler, N.E.; Boyce, T.; Chesney, M.A.; Cohen, S.; Folkman, S.; Kahn, R.L.; Syme, S.L. Socioeconomic status and health: The challenge of the gradient. Am. Psychol. 1994, 49, 15–24.
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M.; Endocrine, S. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2011, 96, 1911-1930, doi:10.1210/jc.2011-0385.
- Wimalawansa, S.J. Infections and autoimmunity-The immune system and vitamin D: A Systematic Review. Nutrients 2023, 15, doi:10.3390/nu15173842.
- McCullough, P.J.; Lehrer, D.S.; Amend, J. Daily oral dosing of vitamin D3 using 5000 TO 50,000 international units a day in long-term hospitalized patients: Insights from a seven year experience. J Steroid Biochem Mol Biol 2019, 189, 228-239, doi:10.1016/j.jsbmb.2018.12.010.
- Amon, U.; Yaguboglu, R.; Ennis, M.; Holick, M.F.; Amon, J. Safety data in patients with autoimmune diseases during treatment with high doses of vitamin D3 according to the "Coimbra Protocol". Nutrients 2022, 14, 1-20, doi:10.3390/nu14081575.
- Shen, Y.M. Role of nutritional vitamin D in chronic kidney disease-mineral and bone disorder: A narrative review. Medicine 2023, 102, e33477.
- Grant, W.B. An estimate of the global reduction in mortality rates through doubling vitamin D levels. Eur. J. Clin. Nutr. 2011, 65, 1016–1026.
- Dudenkov, D.V.; Mara, K.C.; Petterson, T.M.; Maxson, J.A.; Thacher, T.D. Serum 25-Hydroxyvitamin D Values and Risk of All-Cause and Cause-Specific Mortality: A Population-Based Cohort Study. Mayo Clin. Proc. 2018, 93, 721–730.
- A Hamed, E.; Abu Faddan, N.H.; Elhafeez, H.A.A.; Sayed, D. Parathormone-25(OH)-vitamin D axis and bone status in children and adolescents with type 1 diabetes mellitus. Pediatr. Diabetes 2011, 12, 536–546.




