Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Sanda Maria Cretoiu | -- | 2480 | 2023-09-22 12:02:44 | | | |
| 2 | Catherine Yang | Meta information modification | 2480 | 2023-09-25 09:33:04 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Banciu, D.D.; Crețoiu, D.; Crețoiu, S.M.; Banciu, A.; Popa, D.; David, R.; Berghea-Neamtu, C.S.; Cipaian, C.R.; Negrea, M.O.; Gheonea, M.; et al. Telocytes in Modulating Gut Motility Function and Development. Encyclopedia. Available online: https://encyclopedia.pub/entry/49520 (accessed on 08 February 2026).
Banciu DD, Crețoiu D, Crețoiu SM, Banciu A, Popa D, David R, et al. Telocytes in Modulating Gut Motility Function and Development. Encyclopedia. Available at: https://encyclopedia.pub/entry/49520. Accessed February 08, 2026.
Banciu, Daniel Dumitru, Dragoș Crețoiu, Sanda Maria Crețoiu, Adela Banciu, Daniel Popa, Rodica David, Cristian Stefan Berghea-Neamtu, Calin Remus Cipaian, Mihai Octavian Negrea, Mihaela Gheonea, et al. "Telocytes in Modulating Gut Motility Function and Development" Encyclopedia, https://encyclopedia.pub/entry/49520 (accessed February 08, 2026).
Banciu, D.D., Crețoiu, D., Crețoiu, S.M., Banciu, A., Popa, D., David, R., Berghea-Neamtu, C.S., Cipaian, C.R., Negrea, M.O., Gheonea, M., & Neamtu, B. (2023, September 22). Telocytes in Modulating Gut Motility Function and Development. In Encyclopedia. https://encyclopedia.pub/entry/49520
Banciu, Daniel Dumitru, et al. "Telocytes in Modulating Gut Motility Function and Development." Encyclopedia. Web. 22 September, 2023.
Copy Citation
Telocytes (TCs) are interstitial cells with distinct features. They have extensions that react to mechanical stimulation through calcium channels. The ability of TCs to communicate with surrounding cells, especially stem cells (SCs), through gap junctions and extracellular vesicles opens a wide range of questions. Moreover, the hypothesis that TCs are capable of carrying out the cellular niche for stem cell regulation and support suggests that TCs could play an important part in the response to major changes in homeostasis. It also suggests that TCs have a significant functional role in tissues that have an increased turnover.
telocytes
infant colic
inflammatory bowel diseases
Crohn’s disease
ulcerative colitis
irritable bowel syndrome
1. TCs and Inflammatory Bowel Disease (IBD)
TCs represent a distinct category among stromal cells, which include pericytes, myofibroblasts, fibroblasts, vascular endothelial cells, macrophages, and dendritic cells. They have long protrusions (telopodes) consisting of alternating structures, namely podomers (thin segments) and podoms (dilated regions)—both of which contain endoplasmic reticulum, caveolae, and mitochondria [1]. A distinctive combination of CD34 and platelet-derived growth factor receptor α (PDGFRα) correlated with the absence of c-kit (CD117) seems to distinguish stromal telocytes in the GI tract from fibroblasts [2]. Compared to fibroblasts, TCs have distinct patterns of gene expression as well as micro-RNA and proteomic profiles, with important roles in neurotransmission, immunomodulation, extracellular matrix organization, angiogenesis, and regeneration. In addition, recent studies have revealed that both epithelial TCs and subepithelial TCs residing in the GI tract express specific receptors and transcriptional factors [3].
Telocytes distinguish themselves among the aforementioned cell types in GI layers by participating with interstitial Cajal cells (ICC) in the formation of 2D networks between the mucosal, submucosal, and circular muscle layers or 3D networks in the other muscle layers and the myenteric plexus. To date, TC subtypes have been described according to their location in the GI’s layers and the GI organ [4][5][6] as follows: (i.i) TCs—stem-cell-like (TCs-STML), including (i.ii) those transmitting the pace-maker activity, (i.iii) submucosal (TCs-SM) in the stomach and submucosa/circular layer, and (i.iv) subserosal (TC-SS) in the gut serosa/longitudinal muscle; (ii) septal (TC-SPT) in muscular bundles (along/around muscular fibers); (iii) generating slow-wave GI myenteric activity (TCs-MY) consisting of a network of multipolar pacemaker cells around the myenteric plexus; (iv) modulating the enteric neurotransmission-deep myenteric plexus (TCs-DMP), forming a network of multipolar cells in the proximity of nerve bundles; and (v) intramuscular (TCs-IM) with a bipolar aspect aligned along smooth muscle cells within the circular and longitudinal muscle layers.
Within the mucosal layer, TCs are involved in the transduction of sensory and immune signals, and in the maintenance of mucosal homeostasis [1][2]. Recently, mounting evidence highlights the role of TCs situated in the epithelia and lamina propria. Underlining the intestinal epithelium, TCs act as “nurse” cells for cryptal stem cells with an important role in tissue regeneration and repair [1][5][6][7][8]. The hypothesized mechanisms related to the epithelial cells’ integrity refer to: (i) an on/off programmed function at different moments in time; (ii) a localized response secondary to microbiome spatiality and nutrient gradients; and (iii) epithelial gene expression regulating the villus stem cells and telocytes. An impairment in these mechanisms might lead to inflammation in the mucosa and submucosa. Recent research reports on intestinal inflammatory diseases focus on these issues [8][9]. Epithelial TCs of the gastrointestinal tract markedly expressed Lgr 5+ (a leucine-rich-repeat-containing G-protein-coupled receptor 5) encoded by a gene previously categorized as specific for epithelial cryptal stem cells [8]. In addition, studies have shown that subepithelial TCs express Foxl1 (a winged helix transcription factor) and Gli1 (the hedgehog signaling mediator) as crucial stem cell proliferative triggers (using the Wnt pathway signals) with an essential role in the regeneration of the high-turnover epithelial cell line [5][6].
The stem cell compartment is located at the base of the crypts along with the Paneth cells. Stem cell behavior can be modulated by several signal pathways (Wnt, Hedgehog, Notch, BMP). These pathways seem to be crucial in inflammatory bowel diseases (IBD). Stem cells can differentiate into Paneth cells mainly by the Wnt signaling pathway through the TCF4 transcription factor which triggers the expression of several genes including alpha-defensins [9]. Paneth cells play a defensive role and clean the crypts by releasing antimicrobial molecules such as phospholipase A2, lysozyme, and most importantly, the alpha-defensins HD5 and HD6. Paneth cells play an important role against several virus strains, bacteria (Gram-positive/negative), and fungi [9]. In IBD, the reduction in TCF4 expression, leading to impaired Wnt signaling, is associated with a low level of expression of HD5 and HD6 in both Crohn’s disease (CD) and ulcerative colitis (UC). Conversely, beta-defensins seem to have a higher expression in IBD, particularly HBD1 in CD and HBD2 and HBD3 in UC [9].
TCs are connected with immune cells and endothelial cells by gap junctions and communicate in a paracrine fashion (exo/ectosomes). Hence, TCs seem to be involved in immunomodulatory effects, angiogenesis, and tissue regeneration by inhibiting oxidative stress and, consequently, cellular aging [1][2][7]. Although many research reports have brought forth evidence that the Wnt pathway is strongly connected to other inflammatory pathways and plays an important role in inflammation regulation, it is still unknown how it actually contributes to healing processes [10].
TCs along with fibroblasts organize and control the extracellular matrix (ECM) [1]. They confine the inflammatory cells in their meshes and also seem to play a supportive role in intestinal motility. The loss of TCs in inflammatory bowel diseases is associated with a higher number of fibroblasts and an increased but disorganized ECM output with progressive fibrosis [1][2][7].
A crucial role of TCs in the GI layers is related to facilitating neurotransmission and regulating gut motility by establishing a functional connection between: (i) the nerve endings in the submucosal plexus and myenteric plexus, (ii) the pacemakers of gut motility and neurotransmission (the ICC), and (iii) smooth muscles (SMC) [1][2][7]. Chronic inflammation and fibrosis in IBD lead to TCs’ sequestration with subsequent dysmotility [2].
Crohn’s disease (CD) and ulcerative colitis (UC) represent the main inflammatory bowel diseases (IBD) with an increasing worldwide incidence. IBD’s clinical presentation is a consequence of the chronic inflammation of the whole intestine with recurrent episodes triggering abdominal pain, bloody stools, and GI dysmotility. In CD, the inflammation affects all the GI layers and involves the whole GI tract, but the terminal ileum and/or the colon are predominantly affected. In UC, the inflammation leads to ulcers affecting both the mucosa and submucosa in the colon and rectum. In IBD, chronic inflammation eventually leads to mucosal loss and severe fibrosis with debilitating dysmotility which is typically more severe in CD. Multiple factors have been incriminated in IBD’s chronic inflammation; however, several independent factors have been found to be associated with its etiology [10]. Genetic susceptibility, epithelial line disruption, microbiome impairment, and subsequent invasion of the submucosa followed by a strong immune response have been described in the literature. The microbiome load concentration ranges from 107–108 organisms/gram in the distal ileum up to 1011–1012 organisms/gram in the luminal content of the colon. As a result, epithelial injury and subsequent pathogen influx trigger an enhanced immune response [9]. Although different phenotypes have been described for CD and UC, it seems to be innate immunity that is predominantly defective rather than adaptative, which relates to the mucosal barrier and its renewal capacity. Both stem cells and TCs play a crucial role in this respect by modulating the local response based on the adjacent microbiome architecture and nutrient gradients.
2. Intestinal Colics as a Simplified Model for Understanding the Role of TCs in Digestive Motility
TCs have been found in lamina propria, i.e., proximal to the lumen of the digestive tract [11]. Important changes in pH in the intestinal lumen have been found to translate to only minor changes in the juxta-mucosal area [12]. Although the changes are reduced in the mucosa by intrinsically changing the pH of the intestinal lumen, the presence of a bolus of materials in the digestive tract can lead to mucosal compression with a decrease in pH. This is done asymmetrically when compared to mechanical compression due to the occurrence of vasodilatory compensatory mechanisms. The outcome is an asymmetry in pH decrease when compared to mechanical compression. The resulting anisotropy allows signaling for the movement of the telocytes in the opposite direction of the fecal bolus movement. The phenomenon described occurs due to the compensating mechanisms that determine the presence of a higher pH gradient in front of the mechanical changes and a lower pH gradient behind (Figure 1).
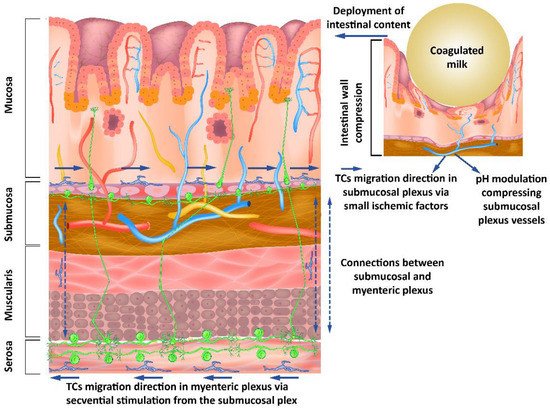
Figure 1. Submucous and myenteric nerve plexuses’ organization showing how modulating the pH as a response to mechanical stimuli from the intestinal lumen influences the neurons through the telocytes’ response to these stimuli.
TCs have the ability to signal via TGF-β1 [13] with morphological alterations. The ability of TCs to migrate predominantly into the nearby mucosal layer may be correlated with their ability to influence synaptogenesis through TGF-β1 [14]. Moreover, it has been shown that TGF-β1 increases neuronal excitability [15]. The ability of TGF-β to influence astrocyte-mediated synaptogenesis [16], which can act as a scavenger mechanism positively influencing neuronal reorganization, or as a neuroprotector for the preservation of neuronal architecture, creates the premise of modulation between TCs and neurons in the intestinal tract. Lesions that lead to deprivation of stimuli may determine a decrease in TGF-β and TGF-β1 due to oligodendrocytes, but not to astrocytes and microglia [17]. Due to the ICC-LC age-dependent response to obstruction in cavity organs [18], it is important to note that the response is maximal in the first year of life if quantified by the number of these cells. The first year of life has, with regard to the aforementioned aspects, a significant advantage given by the uniformity of the contents of the intestinal tract as achieved by breastfeeding. Maternal milk has a genetic determinism with a high degree of uniformity and relatively small changes depending on external factors, compared to adult diets.
Interrelations between neurons, their supporting cells, and TCs suggest a distal-to-proximal organization of telomeres in the submucosal space, with a similar orientation of neurons through modulatory mechanisms of synaptogenesis. In the present model, the existence of the myenteric nervous plexus near the submucosal nervous plexus, with diffuse connections between them, creates an opportunity for organizing the myenteric plexus in the opposite direction due to the synaptic plasticity considerations [19].
Our hypothesis explores the differences between the peak of the pH changes and the peak of the mechanical changes in pH anisotropy (Figure 2) as a representation of a theoretical model that can be evaluated from the perspective of the presented literature. Consistent changes in the fecal bolus may lead to a decrease in intestinal mucosal compression, resulting in a decrease in pH changes, as well as a decrease in anisotropy. This is explained by the existence of pH-dependent compensatory vascular mechanisms. Thus, a significant decrease in pH increases the importance of compensatory mechanisms, but a low pH decrease implies a reduced weight of these mechanisms. On the one hand, the pH drop peak is close to the peak of the mechanical changes, and on the other hand, the pH decrease and increase slopes flatten with decreasing fecal bowl consistency. A decrease in fecal bowel consistency will lead to a decrease in the anisotropy of pH changes mediated by the decrease in mechanical compression and pH-dependent vascular compensatory mechanisms.
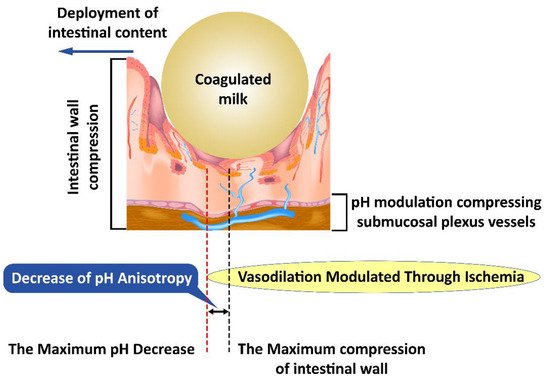
Figure 2. Mechanical compression induces anisotropy of pH changes; first submucosal vessels are affected and subsequently the pH-dependent vascular compensatory mechanisms.
Changes induced by pH anisotropy are sufficiently sensitive to be modulated by bacterial flora in the intestinal lumen. This may explain the need to have a uniformly distributed flora along the digestive tract, although conditions vary slightly from one area to another. The decrease in colonic microbiotic dysplasia [20] presents itself as a specific marker and creates the opportunity to specifically identify the need for bacterial strains that have not developed sufficiently with possible therapeutic implications. Intestinal colic, however, is not an aggressive pathology with a specific treatment [21]. This opportunity seems to be confirmed by the existence of pH-based bacterial screening mechanisms [22] as well as by the existence of distinct pH receptors for Helicobacter pylori [23], which may be possible for other intestinal bacteria. The bacterial selection along the intestinal tract leads to striking a balance between the various bacterial clones, the environmental conditions, and the fecal bowl consistency (Figure 2).
The theoretical model proposed to explain intestinal colic allows us to understand the interaction between TCs, surrounding tissues, and microbes. In addition, it opens a window toward understanding the pathologies that affect the normal functionality of TCs and their modulating role in morphogenesis.
Due to the limited spatial changes in the reduction in the number of bacterial clones in intestinal colic, we can extrapolate the information that TCs communicate via extracellular vectors, and consequently, they are modulated by the different locally available membrane lipids. The effect of intestinal bacteria is outlined not only as an intestinal bolus with a spatially-appropriate mechanical consistency varying with location along the intestinal tract, but also as a lipid source that can alter membrane fluidity and the processes that depend on this fluidity. For example, TCs could modify the response to channel-mediated mechanical stimulation [24], while also modifying TCS-specific exocytosis and endocytosis.
An explanation of the pattern of intestinal colic is outlined as a punctual decrease in the rate of migration of TCs in the submucosa, which can lead to a consecutive decrease in the myenteric plexus. This theoretical model would explain a punctual decrease in the rate of fecal bolus movement, and consequently a slight distension and colic-specific pain. The occurence of punctual localizations would explain the relatively consistent association with the time of food intake and would provide insight into the disappearance of local dysmotility and associated symptoms by the action of the unaffected proximal muscles (Figure 3).
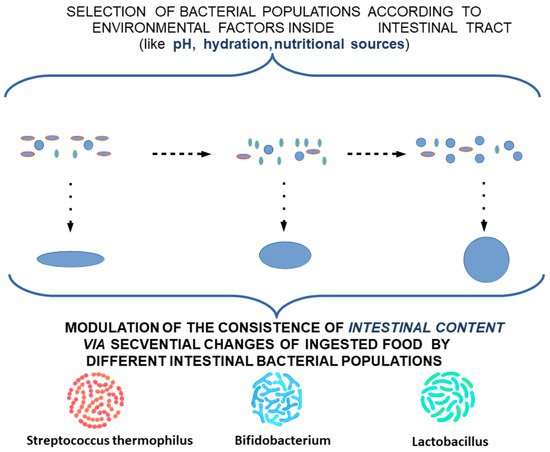
Figure 3. Modulation of the consistency of intestinal content by different intestinal bacterial populations.
This can explain the need for the adaptation of intestinal flora with the transition from an exclusive diet to a rich diet, which is also associated with changes in the maturation of intestinal structures according to new environmental conditions. To alleviate the stress factors affecting the child and the family, and to ease the transition of intestinal flora and intestinal tract adaptation to these changes, a potential treatment using a combination of different lactobacillus subclones with a high clonal diversity—highlighting missing subclones according to age and food supplementation—can be proposed. Due to the variation along the digestive tract in the optimum conditions for each cell subclone, extracellular lipid feeding can be supplemented as a feasible measure to oppose the low viability of cell clones induced by food intake (Figure 4 and Figure 5).
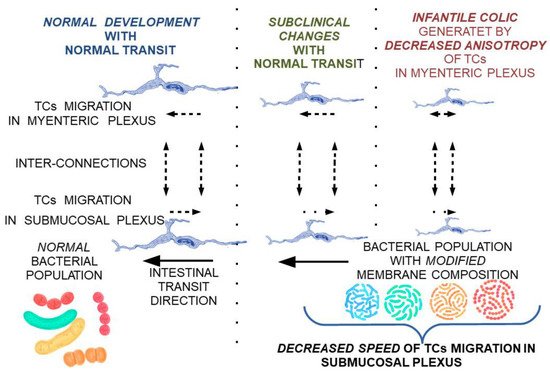
Figure 4. Theoretical model on the reversible progression of maturation changes of the submucosal and myenteric nerve plexus under the influence of TCs modulated by membrane fluidity changes secondary to bacterial populations with a membrane lipid composition not adapted to local conditions.
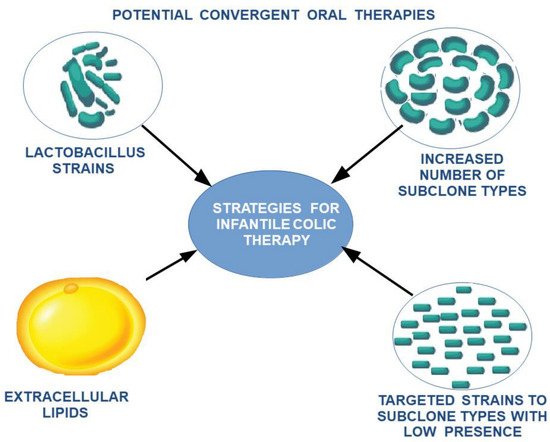
Figure 5. The proposed therapeutic strategy for the reduction in intestinal colic.
References
- Vannucchi, M.G. The Telocytes: Ten Years after Their Introduction in the Scientific Literature. An Update on Their Morphology, Distribution, and Potential Roles in the Gut. Int. J. Mol. Sci. 2020, 21, 4478.
- Milia, A.F.; Ruffo, M.; Manetti, M.; Rosa, I.; Conte, D.; Fazi, M.; Messerini, L.; Ibba-Manneschi, L. Telocytes in Crohn’s Disease. J. Cell. Mol. Med. 2013, 17, 1525–1536.
- Zheng, Y.; Cretoiu, D.; Yan, G.; Cretoiu, S.M.; Popescu, L.M.; Fang, H.; Wang, X. Protein profiling of human lung telocytes and microvascular endothelial cells using iTRAQ quantitative proteomics. J. Cell. Mol. Med. 2014, 18, 1035–1059.
- Foong, D.; Zhou, J.; Zarrouk, A.; Ho, V.; O’Connor, M.D. Understanding the Biology of Human Interstitial Cells of Cajal in Gastrointestinal Motility. Int. J. Mol. Sci. 2020, 21, 4540.
- Kaestner, K.H. The Intestinal Stem Cell Niche: A Central Role for Foxl1-Expressing Subepithelial Telocytes. Cell Mol. Gastroenterol. Hepatol. 2019, 8, 111–117.
- Shoshkes-Carmel, M.; Wang, Y.J.; Wangensteen, K.J.; Tóth, B.; Kondo, A.; Massasa, E.E.; Itzkovitz, S.; Kaestner, K.H. Subepithelial telo-cytes are an important source of Wnts that supports intestinal crypts. Nature 2018, 557, 242–246, Erratum in Nature 2018, 560, E29.
- Wang, X.; Cretoiu, D. (Eds.) Telocytes; Springer: Singapore, 2016; Volume 913.
- Bahar, H.K.; Massalha, H.; Zwick, R.K.; Moor, A.E.; Castillo-Azofeifa, D.; Rozenberg, M.; Farack, L.; Egozi, A.; Miller, D.R.; Aver-bukh, I.; et al. Lgr5+ telocytes are a signaling source at the intestinal villus tip. Nat. Commun 2020, 11, 1936.
- Gersemann, M.; Wehkamp, J.; Fellermann, K.; Stange, E.F. Crohn’s disease-Defect in innate defence. World. J. Gastroenterol. 2008, 14, 5499–5503.
- Koch, S.; Capaldo, C.T.; Hilgarth, R.S.; Fournier, B.; Parkos, C.A.; Nusrat, A. Protein kinase CK2 is a critical regulator of epithelial homeostasis in chronic intestinal inflammation. Mucosal Immunol. 2013, 6, 136–145.
- Vannucchi, M.G.; Traini, C.; Manetti, M.; Ibba-Manneschi, L.; Faussone-Pellegrini, M.S. Telocytes express PDGFRα in the human gastrointestinal tract. J. Cell. Mol. Med. 2013, 17, 1099–1108.
- Barmpatsalou, V.; Dubbelboer, I.R.; Rodler, A.; Jacobson, M.; Karlsson, E.; Pedersen, B.L.; Bergström, C. Physiological properties, composition and structural profiling of porcine gastrointestinal mucus. Eur. J. Pharm. Biopharm. 2021, 169, 156–167.
- Song, D.; Tang, L.; Huang, J.; Wang, L.; Zeng, T.; Wang, X. Roles of transforming growth factor-β and phosphatidylinositol 3-kinase isoforms in integrin β1-mediated bio-behaviors of mouse lung telocytes. J. Transl. Med. 2019, 17, 431.
- Araujo, A.P.; Diniz, L.P.; Eller, C.M.; de Matos, B.G.; Martinez, R.; Gomes, F.C. Effects of Transforming Growth Factor Beta 1 in Cerebellar Development: Role in Synapse Formation. Front. Cell. Neurosci. 2016, 10, 104.
- Zhang, X.; Zheng, H.; Zhu, H.Y.; Hu, S.; Wang, S.; Jiang, X.; Xu, G.Y. Acute Effects of Transforming Growth Factor-β1 on Neuronal Excitability and Involvement in the Pain of Rats with Chronic Pancreatitis. J. Neurogastroenterol. Motil. 2016, 22, 333–343.
- Diniz, L.P.; Matias, I.; Siqueira, M.; Stipursky, J.; Gomes, F.C.A. Astrocytes and the TGF-Β1 Pathway in the Healthy and Diseased Brain: A Double-Edged Sword. Mol. Neurobiol. 2019, 56, 4653–4679.
- Matejuk, A.; Vandenbark, A.A.; Offner, H. Cross-Talk of the CNS with Immune Cells and Functions in Health and Disease. Front. Neurol. 2021, 12, 672455.
- Sanders, K.M.; Ward, S.M.; Koh, S.D. Interstitial cells: Regulators of smooth muscle function. Physiol. Rev. 2014, 94, 859–907.
- Citri, A.; Malenka, R.C. Synaptic plasticity: Multiple forms, functions, and mechanisms. Neuropsychopharmacology 2008, 33, 18–41.
- Chernikova, D.A.; Madan, J.C.; Housman, M.L.; Zain-Ul-Abideen, M.; Lundgren, S.N.; Morrison, H.G.; Sogin, M.L.; Williams, S.M.; Moore, J.H.; Karagas, M.R.; et al. The premature infant gut microbiome during the first 6 weeks of life differs based on gestational maturity at birth. Pediatr. Res. 2018, 84, 71–79.
- Skonieczna-Żydecka, K.; Janda, K.; Kaczmarczyk, M.; Marlicz, W.; Łoniewski, I.; Łoniewska, B. The Effect of Probiotics on Symptoms, Gut Microbiota and Inflammatory Markers in Infantile Colic: A Systematic Review, Meta-Analysis and Meta-Regression of Randomized Controlled Trials. J. Clin. Med. 2020, 9, 999.
- Krulwich, T.A.; Sachs, G.; Padan, E. Molecular aspects of bacterial pH sensing and homeostasis. Nature reviews. Microbiology 2011, 9, 330–343.
- Goers Sweeney, E.; Henderson, J.N.; Goers, J.; Wreden, C.; Hicks, K.G.; Foster, J.K.; Parthasarathy, R.; Remington, S.J.; Guillemin, K. Structure and proposed mechanism for the pH-sensing Helicobacter pylori chemoreceptor TlpB. Structure 2012, 20, 1177–1188.
- Campeanu, R.A.; Radu, B.M.; Cretoiu, S.M.; Banciu, D.D.; Banciu, A.; Cretoiu, D.; Popescu, L.M. Near-infrared low-level laser stimulation of telocytes from human myometrium. Lasers Med. Sci. 2014, 29, 1867–1874.
More
Information
Subjects:
Cell Biology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
702
Revisions:
2 times
(View History)
Update Date:
25 Sep 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




