
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Thomas Joyce | -- | 3257 | 2023-09-21 22:10:00 | | | |
| 2 | Jessie Wu | -40 word(s) | 3217 | 2023-09-22 02:55:28 | | | | |
| 3 | Jessie Wu | Meta information modification | 3217 | 2023-09-22 02:57:20 | | |
Video Upload Options
Biomarkers for resistance in Glioblastoma multiforme (GBM) are lacking, and progress in the clinic has been slow to arrive. CD133 (prominin-1) is a membrane-bound glycoprotein on the surface of cancer stem cells (CSCs) that has been associated with poor prognosis, therapy resistance, and tumor recurrence in GBM. Due to its connection to CSCs, to which tumor resistance and recurrence have been partially attributed in GBM, there is a growing field of research revolving around the potential role of CD133 in each of these processes. However, despite encouraging results in vitro and in vivo, the biological interplay of CD133 with these components is still unclear, causing a lack of clinical application. In parallel, omic data from biospecimens that include CD133 are beginning to emerge, increasing the importance of understanding CD133 for the effective use of these highly dimensional data sets. Given the significant mechanistic overlap, prioritization of the most robust findings is necessary to optimize the transition of CD133 to clinical applications using patient-derived biospecimens.
1. Introduction
2. CD133 and Glioblastoma Multiforme Prognosis
3. CD133 and the Tumor Microenvironment
Recent research has explored the impact that microenvironmental factors have on CD133, suggesting the possibility that changes in the microenvironment may mediate its expression. The exploration of the impact of microenvironmental factors has focused mainly on hypoxia, as rapid tumor proliferation leads to diminished blood supply causing hypoxia and necrosis, as well as driving the stem cell state. As a result, investigating how cells survive in this environment while still allowing GBM in particular to maintain resistance to conventional therapies is crucial for potential treatment approaches. Musah-Eroje and Watson explored this correlation by placing three GBM cell lines in 1% oxygen microenvironments and discovered that there was a significant upregulation of CD133 under hypoxic conditions [15] (Figure 1A). Similar research has confirmed this increased expression due to hypoxia in the microenvironment [16][17][18], with Ahmed et al. confirming this in both 2D and 3D models [19].
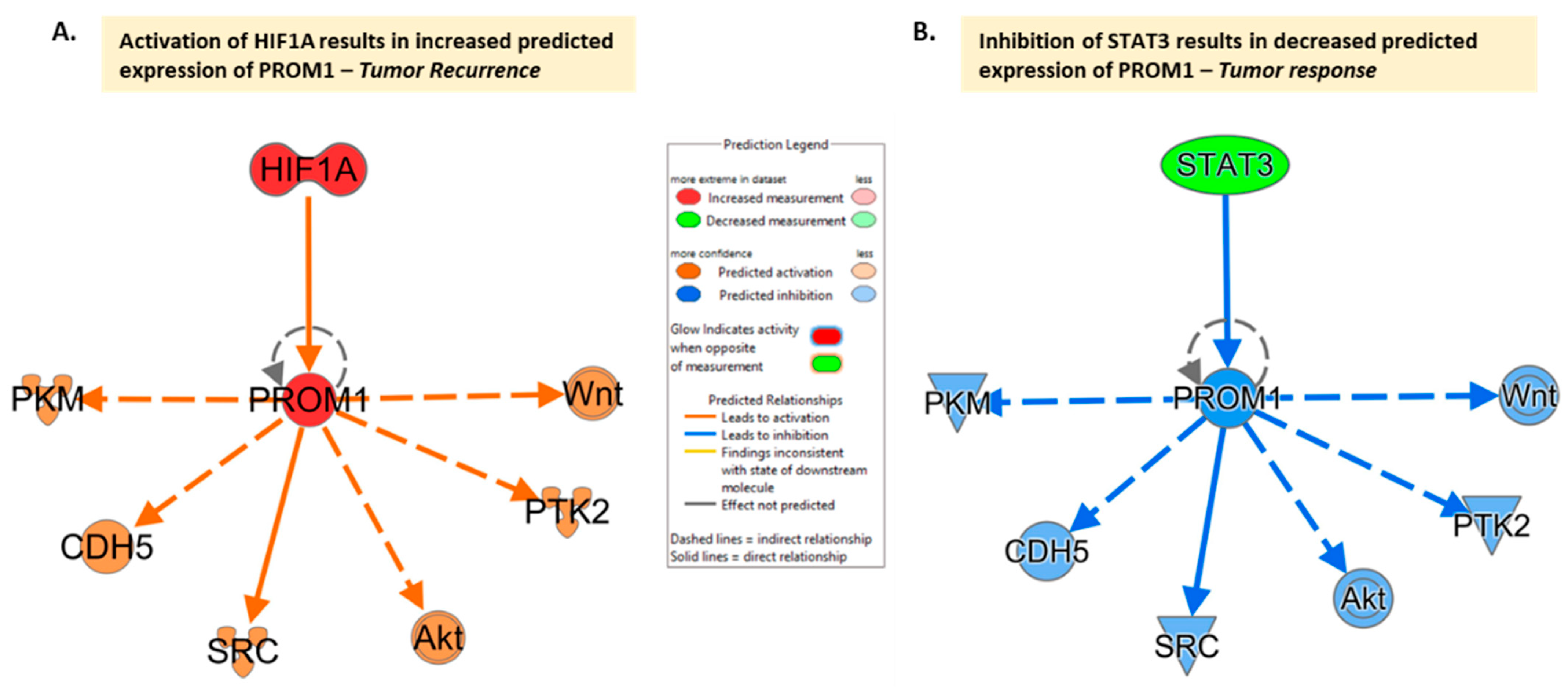
Figure 1. CD133 (PROM1) interaction network limiting interaction to molecules in cancer based on the IPA molecule activity prediction ((QIAGEN Inc., https://www.qiagenbioinformatics.com/products/ingenuitypathway-analysis) accessed on 24 July 2023)[20]. Network comparison analysis with (A) Activation of HIF1A (e.g., under hypoxia conditions) results in increased predicted expression of PROM1 and the hypothesis is that this drives tumor recurrence. (B) Inhibition of STAT3 results in decreased predicted expression of PROM1 and the hypothesis is that this can lead to tumor response. The same downstream mediators appear to be involved, given current evidence, with PROM1 activation or inhibition driving activation/inhibition of downstream molecules.
4. CD133 and Resistance
5. CD133 and Recurrence
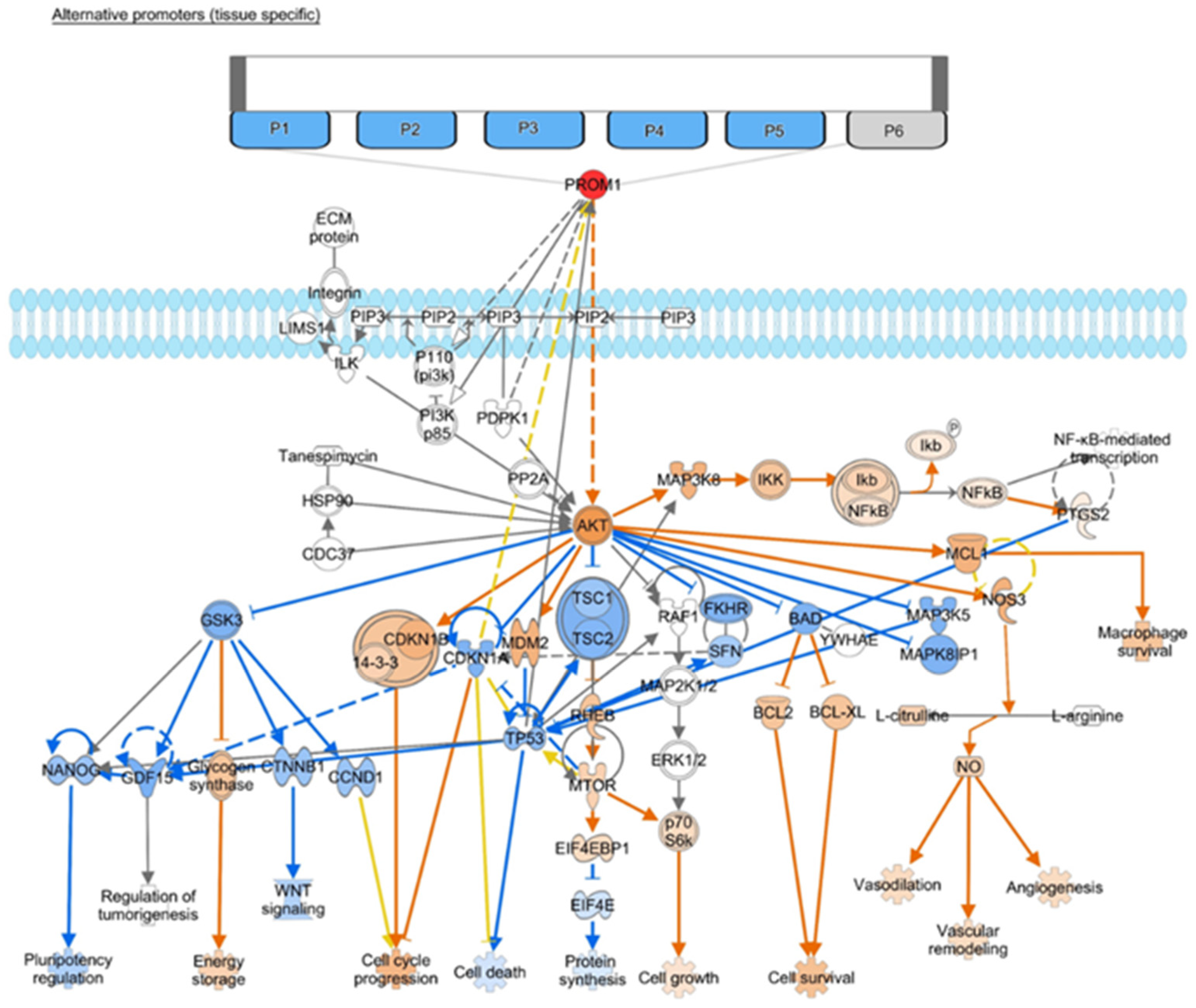
6. Conclusion
Although there is still a lack of consensus regarding the exact mechanistic relationships of CD133 to prognosis, the tumor microenvironment, resistance, and recurrence, these fields of research are rapidly progressing. The role of CD133 as a functional unit involved in each of these continues to gain momentum. A comprehensive understanding of the role of CD133 remains elusive but is crucial as omic data becomes increasingly accessible, since CD133 will be included in panels aimed at measuring and classifying tumor resistance. As a result, being aware of its functions and the meaning of its alteration will become incredibly significant due to its potential ability to track prognosis, as well as its possible role as a treatment target. Clarity into which of these mechanisms is most significant creates immense potential for the utilization of CD133 to develop treatment mechanisms, as its inhibition may reduce the tumorigenic and resistance capabilities of GBM. In conclusion, further advancement regarding the role and linkage of CD133 could allow for additional prognostic and predictive uses as well as specific pathways that can be exploited in treatment approaches and mechanisms in the future.
References
- Song Nie; Mikel Gurrea; Jianhui Zhu; Smathorn Thakolwiboon; Jason A. Heth; Karin M. Muraszko; Xing Fan; David M. Lubman; Tenascin-C: A Novel Candidate Marker for Cancer Stem Cells in Glioblastoma Identified by Tissue Microarrays. J. Proteome Res. 2014, 14, 814-822.
- Roger Stupp; Warren P. Mason; Martin J. van Den Bent; Michael Weller; Barbara Fisher; Martin J.B. Taphoorn; Karl Belanger; Alba A. Brandes; Christine Marosi; Ulrich Bogdahn; et al.Jürgen CurschmannRobert C. JanzerSamuel K. LudwinThierry GorliaAnouk AllgeierDenis LacombeJ. Gregory CairncrossElizabeth EisenhauerRené O. Mirimanoff Radiotherapy plus Concomitant and Adjuvant Temozolomide for Glioblastoma. New Engl. J. Med. 2005, 352, 987-996.
- Ting Sun; Guilin Chen; Yanyan Li; Xueshun Xie; Youxin Zhou; Ziwei DU; Aggressive invasion is observed in CD133−/A2B5+ glioma-initiating cells. Oncol. Lett. 2015, 10, 3399-3406.
- Khalilullah Mia-Jan; So Young Jung; Ik-Yong Kim; Sung Soo Oh; EunHee Choi; Sei Jin Chang; Tae Young Kang; Mee-Yon Cho; CD133 expression is not an independent prognostic factor in stage II and III colorectal cancer but may predict the better outcome in patients with adjuvant therapy. BMC Cancer 2013, 13, 166-166.
- Gina Lee; Brenda Auffinger; Donna Guo; Tanwir Hasan; Marc Deheeger; Alex L. Tobias; Jeong Yeon Kim; Fatemeh Atashi; Lingjiao Zhang; Maciej S. Lesniak; et al.C. David JamesAtique U. Ahmed Dedifferentiation of Glioma Cells to Glioma Stem-like Cells By Therapeutic Stress-induced HIF Signaling in the Recurrent GBM Model. Mol. Cancer Ther. 2016, 15, 3064-3076.
- Alexei V. Salnikov; Jury Gladkich; Gerhard Moldenhauer; Manfred Volm; Ingrid Herr; Jürgen Mattern; CD133 is indicative for a resistance phenotype but does not represent a prognostic marker for survival of non-small cell lung cancer patients. Int. J. Cancer 2009, 126, 950-958.
- Yu, J.W.; Zhang, P.; Wu, J.G.; Wu, S.H.; Li, X.Q.; Wang, S.T.; Lu, R.Q.; Ni, X.C.; Jiang, B.J. Expressions and clinical significances of CD133 protein and CD133 mRNA in primary lesion of gastric adenocacinoma. J. Exp. Clin. Cancer Res. 2010, 29, 141.
- Ewa Missol-Kolka; Jana Karbanová; Peggy Janich; Michael Haase; Christine A. Fargeas; Wieland B. Huttner; Denis Corbeil; Prominin-1 (CD133) is not restricted to stem cells located in the basal compartment of murine and human prostate. Prostate 2010, 71, 254-267.
- Bin Wu; Caixing Sun; Fang Feng; Minghua Ge; Liang Xia; Do relevant markers of cancer stem cells CD133 and Nestin indicate a poor prognosis in glioma patients? A systematic review and meta-analysis. J. Exp. Clin. Cancer Res. 2015, 34, 1-12.
- Han, M.; Guo, L.; Zhang, Y.; Huang, B.; Chen, A.; Chen, W.; Liu, X.; Sun, S.; Wang, K.; Liu, A; et al. Clinicopathological and Prognostic Significance of CD133 in Glioma Patients: A Meta-Analysis. Mol. Neurobiol. 2016, 53, 720-727.
- Wei Zhang; Huanran Chen; Shengqing Lv; Hui Yang; High CD133 Expression Is Associated with Worse Prognosis in Patients with Glioblastoma. Mol. Neurobiol. 2015, 53, 2354-2360.
- Mahdi Abdoli Shadbad; Negar Hosseinkhani; Zahra Asadzadeh; Oronzo Brunetti; Nicola Silvestris; Behzad Baradaran; The Prognostic Value of CD133 in Predicting the Relapse and Recurrence Pattern of High-Grade Gliomas on MRI: A Meta-Analysis. Front. Oncol. 2021, 11, 722833.
- Li, B.; McCrudden, C.M.; Yuen, H.F.; Xi, X.; Lyu, P.; Chan, K.W.; Zhang, S.D.; Kwok, H.F. CD133 in brain tumor: The prognostic factor. Oncotarget 2017, 8, 11144-11159.
- Elda-Georgina Chavez-Cortez; Gustavo Vargas Felix; Edgar Rangel López; Julio Sotelo; Carlos Martínez-Canseco; Verónica Pérez-De la Cruz; Benjamin Pineda; Production and Evaluation of an Avian IgY Immunotoxin against CD133+ for Treatment of Carcinogenic Stem Cells in Malignant Glioma: IgY Immunotoxin for the Treatment of Glioblastoma. J. Oncol. 2019, 2019, 1-15.
- Ahmed Musah-Eroje; Sue Watson; Adaptive Changes of Glioblastoma Cells Following Exposure to Hypoxic (1% Oxygen) Tumour Microenvironment. Int. J. Mol. Sci. 2019, 20, 2091.
- Daniel V. Brown; Gulay Filiz; Paul M. Daniel; Frédéric Hollande; Sebastian Dworkin; Stephanie Amiridis; Nicole Kountouri; Wayne Ng; Andrew P. Morokoff; Theo Mantamadiotis; et al. Expression of CD133 and CD44 in glioblastoma stem cells correlates with cell proliferation, phenotype stability and intra-tumor heterogeneity. PLOS ONE 2017, 12, e0172791.
- Lee, S.Y.; Kim, J.K.; Jeon, H.Y.; Ham, S.W.; Kim, H. CD133 Regulates IL-1β Signaling and Neutrophil Recruitment in Glioblastoma. Mol. Cells 2017, 40, 515-522.
- Sun, H.; Zhang, M.; Cheng, K.; Li, P.; Han, S.; Li, R.; Su, M.; Zeng, W.; Liu, J.; Guo, J.; et al. Resistance of glioma cells to nutrient-deprived microenvironment can be enhanced by CD133-mediated autophagy. Oncotarget 2016, 7, 76238-76249.
- Ahmed, E.M.; Bandopadhyay, G.; Coyle, B.; Grabowska, A. A HIF-independent, CD133-mediated mechanism of cisplatin resistance in glioblastoma cells. Cell. Oncol. 2018, 41, 319-328.
- Andreas Krämer; Jeff Green; Jack Pollard; Stuart Tugendreich; Causal analysis approaches in Ingenuity Pathway Analysis. Bioinform. 2013, 30, 523-530.
- Perazzoli, G.; Prados, J.; Ortiz, R.; Caba, O.; Cabeza, L.; Berdasco, M.; Gonzalez, B.; Melguizo, C. Temozolomide Resistance in Glioblastoma Cell Lines: Implication of MGMT, MMR, P-Glycoprotein and CD133 Expression. PLoS ONE 2015, 10, e0140131.
- Viktorija Juric; Heiko Düssmann; Martine L. M. Lamfers; Jochen H. M. Prehn; Markus Rehm; Brona M. Murphy; Transcriptional CDK Inhibitors CYC065 and THZ1 Induce Apoptosis in Glioma Stem Cells Derived from Recurrent GBM. Cells 2021, 10, 1182.
- Wang Miao; Xiaodong Liu; Hongqin Wang; Yimin Fan; Shizhong Lian; Xin Yang; Xinxing Wang; Geng Guo; Qichao Li; Sifei Wang; et al. p53 upregulated modulator of apoptosis sensitizes drug-resistant U251 glioblastoma stem cells to temozolomide through enhanced apoptosis. Mol. Med. Rep. 2015, 11, 4165-4173.
- Wen-Shin Song; Yi-Ping Yang; Chi-Shuan Huang; Kai-Hsi Lu; Wei-Hsiu Liu; Wai-Wah Wu; Yi-Yen Lee; Wen-Liang Lo; Shou-Dong Lee; Yi-Wei Chen; et al.Pin-I HuangMing-Teh Chen Sox2, a stemness gene, regulates tumor-initiating and drug-resistant properties in CD133-positive glioblastoma stem cells. J. Chin. Med Assoc. 2016, 79, 538-545.
- Bülent Polat; Gisela Wohlleben; Rebekka Kosmala; Dominik Lisowski; Frederick Mantel; Victor Lewitzki; Mario Löhr; Robert Blum; Petra Herud; Michael Flentje; et al.Camelia-Maria Monoranu Differences in stem cell marker and osteopontin expression in primary and recurrent glioblastoma. Cancer Cell Int. 2022, 22, 1-11.
- Chia-Hsin Chang; Wei-Ting Liu; Hui-Chi Hung; Chia-Yu Gean; Hong-Ming Tsai; Chun-Lin Su; Po-Wu Gean; Synergistic inhibition of tumor growth by combination treatment with drugs against different subpopulations of glioblastoma cells. BMC Cancer 2017, 17, 1-10.
- Sun, B.; Wan, Z.; Shen, J.; Ni, L.; Chen, J.; Cui, M.; Ni, H.; Shi, W.; Shi, J. DNA hypomethylation of CD133 promoter is associated with recurrent glioma. Oncol. Rep. 2016, 36, 1062-1068.
- W. P. Zhao; Q. X. Chen; Effects of HMGB1 on proliferation and apoptosis of human brain glioma CD133 cells. Bratisl. Med J. 2015, 116, 480-485.
- Sandra Bien-Möller; Ellen Balz; Susann Herzog; Laura Plantera; Silke Vogelgesang; Kerstin Weitmann; Carolin Seifert; Matthias A. Fink; Sascha Marx; Angela Bialke; et al.Chitra VenugopalSheila K. SinghWolfgang HoffmannBernhard H. RauchHenry W. S. Schroeder Association of Glioblastoma Multiforme Stem Cell Characteristics, Differentiation, and Microglia Marker Genes with Patient Survival. Stem Cells Int. 2018, 2018, 1-19.




