Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Jamilya Khizroeva | -- | 3854 | 2023-09-20 17:34:17 | | | |
| 2 | Sirius Huang | Meta information modification | 3854 | 2023-09-21 03:18:27 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Khizroeva, J.; Makatsariya, A.; Vorobev, A.; Bitsadze, V.; Elalamy, I.; Lazarchuk, A.; Salnikova, P.; Einullaeva, S.; Solopova, A.; Tretykova, M.; et al. Neonatal Hemostasis System Peculiarities and Neonatal Thrombosis. Encyclopedia. Available online: https://encyclopedia.pub/entry/49439 (accessed on 07 February 2026).
Khizroeva J, Makatsariya A, Vorobev A, Bitsadze V, Elalamy I, Lazarchuk A, et al. Neonatal Hemostasis System Peculiarities and Neonatal Thrombosis. Encyclopedia. Available at: https://encyclopedia.pub/entry/49439. Accessed February 07, 2026.
Khizroeva, Jamilya, Alexander Makatsariya, Alexander Vorobev, Victoria Bitsadze, Ismail Elalamy, Arina Lazarchuk, Polina Salnikova, Sabina Einullaeva, Antonina Solopova, Maria Tretykova, et al. "Neonatal Hemostasis System Peculiarities and Neonatal Thrombosis" Encyclopedia, https://encyclopedia.pub/entry/49439 (accessed February 07, 2026).
Khizroeva, J., Makatsariya, A., Vorobev, A., Bitsadze, V., Elalamy, I., Lazarchuk, A., Salnikova, P., Einullaeva, S., Solopova, A., Tretykova, M., Antonova, A., Mashkova, T., Grigoreva, K., Kvaratskheliia, M., Yakubova, F., Degtyareva, N., Tsibizova, V., Gashimova, N., & Blbulyan, D. (2023, September 20). Neonatal Hemostasis System Peculiarities and Neonatal Thrombosis. In Encyclopedia. https://encyclopedia.pub/entry/49439
Khizroeva, Jamilya, et al. "Neonatal Hemostasis System Peculiarities and Neonatal Thrombosis." Encyclopedia. Web. 20 September, 2023.
Copy Citation
Newborns are the most vulnerable patients for thrombosis development among all children, with critically ill and premature infants being in the highest risk group. The upward trend in the rate of neonatal thrombosis could be attributed to progress in the treatment of severe neonatal conditions and the increased survival in premature babies. There are physiological differences in the hemostatic system between neonates and adults.
neonatal hemostasis
neonatal thrombosis
risk factors for neonatal thrombosis
1. Introduction
Neonatal thrombosis is a rare (0.7–0.14 per 10,000) but dangerous condition, leading to significant morbidity and mortality among newborns [1]. From 1997 to 2018, the number of newborns with neonatal thrombosis increased by 13 times [1][2]. About 2% to 4% of neonates die due to venous thromboembolism (VTE) [3][4]. The risk of neonatal thrombosis increases after the admission of a child to the neonatal intensive care unit (NICU) and the overall incidence of thrombosis in NICU patients is about 0.2% [5]. According to a prospective 2-year registry of VTE in children in the Netherlands, 85% of patients develop thrombosis during the patient’s stay in the hospital [3]. Two children out of ninety-nine died as result of VTE.
The clinical presentation can be nonspecific or even asymptomatic, which is why the number of thrombosis cases in neonates is underestimated [4][6].
One of the essential pathogenetic factors is the condition of the coagulation system in newborns, which is different from that of the older children and adults [1]. Coagulation proteins do not cross the placenta, but are synthesized in the fetus from an early stage. At birth, activities of the vitamin K-dependent factors II, VII, IX, and X and the concentrations of the contact factors XI and XII are decreased to about 50% of normal adult values and approach adult level by 6 months of life [7]. Conversely, levels of natural anticoagulants (antithrombin, heparin cofactor II and protein C and S) are low at birth. The fibrinolysis system is characterized by reduced level of plasminogen and alpha-2-antiplasmin, an increased tissue plasminogen activator. These features all tend to be gestational dependent, and therefore are more present in the preterm infant. Despite these features of neonatal hemostasis, the healthy newborn rarely develops spontaneous VTE but appears more easily vulnerable to thrombosis. Different triggering factors are responsible for thrombosis in neonates.
2. Neonatal Hemostasis System Peculiarities and Neonatal Thrombosis
2.1. Epidemiology of Thrombosis in Neonates
According to studies conducted in Canada, Germany, and Denmark, the incidence of thrombosis among newborns is approximately 2.4/1000 children in ICU and 5.5/100,000 births in general. The meta-analysis summarizing the incidence of thrombosis in ICU children found that the incidence rate of ICU thrombosis is approximately 2%, which is consistent with previous studies [8].
The first Canadian registry of neonates with thrombosis was established in 1990 and included data from 15 tertiary-care pediatric centers [9]. One hundred and thirty-seven patients with thrombosis were identified. The overall incidence of DVT/PE was 5.3/10,000 hospital admissions or 0.07/10,000 children in this registry.
A case registry at McMaster University included data from physicians in 64 centers in North America, Europe and Australia [10]. Ninety-seven cases of venous thromboembolism (VTE) were registered. Twenty-one neonates developed renal vein thrombosis and thirty-nine children had other localizations of VTE. In 89% of the cases, thrombosis development was associated with catheter placement, but sepsis and extensive surgery were also the most important risk factors.
A study conducted in Germany reported the incidence of VTE as 5.1 per 100,000 births, with a total of 79 cases of neonatal thrombosis registered [11]. The diagnosis was confirmed by Doppler ultrasonography. Renal vein thrombosis occurred in 35 neonates, with another VTE localization in 25, and arterial thrombosis developed in other cases. Thrombosis associated with various risk factors developed in 59 cases: central venous catheter placement (n = 25), asphyxia (n = 13), septicemia (n = 11), dehydration (n = 6), maternal diabetes (n = 2), and heart disease (n = 1). Genetic thrombophilia was diagnosed in seven cases.
The Denmark study from 1994 to 2006 included patients from 0 to 18 years old diagnosed with VTE and found age- and sex-related disparities in the incidence of pediatric venous and arterial thrombosis [12]. The highest incidence was registered in children under 1 year, especially males. Risk factors were presented in 86.6% of cases, and 47.9% of newborns were diagnosed with inherited thrombophilia.
The report from the Italian Registry of Pediatric Thrombosis represented 75 neonates (0–28 days) with thromboembolism. The data were collected from neonatology centers from 2007 to 2013. VTE was observed in 41 (55%) neonates, arterial thrombosis in 22 (29%), and other participants had cerebral venous thrombosis. A total of 65% of children were male and in 29 (25%) cases thrombosis was diagnosed on the first day of life [6]. A total of 70% of cases were associated with maternal/placental risk factors in the early-onset group, and 33% of patients were diagnosed with inherited thrombophilia. Postnatal risk factors were associated with catheter use and infection in 73% of all cases. The study also suggested that corticosteroid use in preterm infants is an additional risk factor. Systemic glucocorticoids exert prothrombotic effects via the reducing the clearance of activated clotting factors and through direct vasoconstriction. However, the association between thrombosis development and glucocorticoid use is still inexplicit.
The incidence of stroke in neonates and premature neonates is 25 per 100,000 population per year. Half of these are ischemic [1]. In North America, the incidence of neonatal ischemic stroke was estimated to be 2.5 to 2.7 cases per 100,000 children per year, and in France it was 3 cases per 100,000 children per year. Ischemic stroke is among the top 10 causes of death among children in the United States, with the highest rate during the first year of life [7].
The incidence of cerebral venous sinus thrombosis is approximately 0.4 per 1000 newborns.
2.2. Hemostasis System in the Neonate
2.2.1. Neonatal Hemostatic Balance
The fetal/neonatal hemostatic system differs from the adult one. The classical clotting pathway undergoes a series of chemical reactions through two pathways. The first pathway is extrinsic and starts with the activation of tissue factor. The second cascade is an intrinsic pathway that begins with the contact activation of factor XII. Fetus coagulation factors do not cross the placenta, and the first synthesis of them is appears in the 11th week of gestational age. At weeks 19–27 there are still low levels of many factors, except the von Willebrand factor (VWF). At 28–31 weeks of pregnancy, VWF and fibrinogen levels are normal but other factors are still low. Most components of the coagulation system in neonates achieve an adult level by 6 months (Table 1) [13][14].
Table 1. Coagulation factors and natural anticoagulant levels changes in full-term neonates at term and at 6 months.
| Hemostasis System Parameter |
At Birth | 6 Months |
|---|---|---|
| Antithrombin | 40–60% | adult level at day 90 |
| Protein S | 40–60% | adult level at day 90 |
| Protein C | low | adult level not reached |
| VWF | mean 153% | drops to ≈ 100% |
| Fibrinogen | adult level | adult level |
| Vitamin K-dependent factors (II, VII, IX, X) | mean ≈ 40–50% | 80–90% of adult level |
| Contact system factors (factors XI, XII, prekallikrein, HMWK) | mean ≈ 40–50% | 80–90% of adult level |
| Factor V and XIII (both a- and b-unit) | mean 70–80% | adult levels at day 5 |
| FVIII | mean 100% | drops slowly to ≈ 75% |
| Factor V and XIII | mean 70–80% | adult levels at day 5 |
| a2-macroglobulin | high | increasing further |
At first glance, neonates have a tendency to bleed, due to specific physiological mechanisms. However, their bleeding time and the time it takes to form a clot is paradoxically less than in adults [15][16][17]. Despite the prolongation of APTT, PT and delayed thrombin generation, a healthy newborn is not more prone to bleeding or thrombosis. There is an evolving “hemostatic balance” of pro-and anticoagulant factors due to which a healthy newborn hardly ever develops VTE (Figure 1) [14]. Despite these age-related differences between coagulation factors, the production of thrombin in newborns is equivalent to about 90% of that in adults, which is enough for the formation of a hemostatic clot [18].
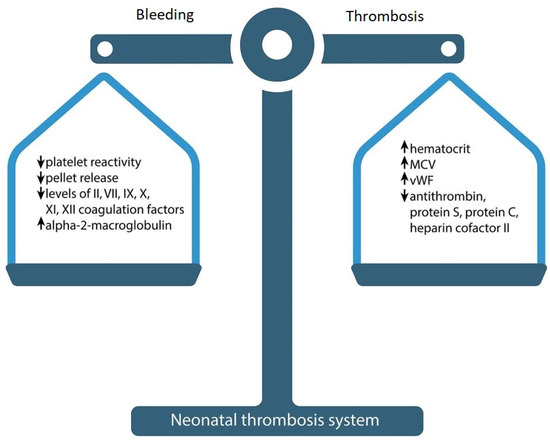
Figure 1. Neonatal hemostasis.
Physiologically low levels of inhibitors could compensate for low concentrations of clotting factors and ensure sufficient thrombin production, but the results obtained with standard clotting tests do not confirm this assumption: it has been shown that the ability to produce thrombin in the plasma of a healthy newborn is markedly reduced and delayed compared to an adult. Only 30–50% of peak thrombin activity can be produced in neonatal plasma compared to adults.
Cvirn et al. demonstrated that the simultaneous effect of low anticoagulant capacity of the three inhibitors (activated protein C (APC), tissue factor pathway inhibitor (TFPI) and antithrombin) leads to a reduction in clotting time and faster formation of factor (F) Xa and thrombin in the umbilical cord compared to adult plasma when small amounts of TF (<10 microns) are used to initiate clotting [19]. This can explain the clinically observed excellent hemostasis of neonates despite low levels of procoagulant factors [20].
In most healthy newborns, infants, and young children, normal hemostasis mechanisms can compensate for differences in pro- and anticoagulant factors, preventing serious bleeding and thrombotic complications. However, neonatal hemostasis has less buffer capacity and can be easily tipped over into thrombosis by acquired risk factors such as concomitant diseases, decreased fibrinolytic capacity, heparin resistance due to low antithrombin (AT), higher clearance of unfractionated heparin (UFH), and increased sensitivity to anticoagulants. The upward trend in the rate of neonatal thrombosis could be attributed to progress in the treatment of severe neonatal conditions and increased survival in the premature babies. The compensatory mechanisms of premature neonates are less developed. Premature babies have even bigger differences of pro- and anticoagulant factors due to lower levels of clotting factors, but they accelerate fast towards “normal”.
In 1987, Andrew et al. published the first reference values for PT and activation time of blood platelets among healthy term babies [13]. In 1988, a study was conducted on healthy babies born within 30 to 36 weeks [21]. Studies mentioned above included children aged 1, 5, 30, or 90 days old. The postnatal maturation of clotting factors to the level of an adult occurs at an accelerated rate in premature infants compared to full-term infants. By 6 months, most of the components of the blood clotting system in premature infants reach values close to those of adults [21]. In the premature birth protein C, the mean value is 38%; protein S is even lower (26–28%), and is not compensated by a2-macroglobulin (110%)—which, however, starts rising quickly. The activity of vitamin K-dependent coagulation factors is particularly reduced in preterm infants as compared to term-born infants. The majority of premature neonates have increased platelet-VWF interaction and are more prone to sepsis. The bleeding time of preterm neonates born after 37 weeks’ gestation is approximately two times longer than those born earlier (p < 0.001) [15].
In the largest Irish cross-sectional study of very premature infants (<30 weeks gestational age) there was no difference in the potential of endogenous thrombin between the plasma of premature and full-term infants. Despite the prolongation of clotting time, thrombin generation was similar in very preterm and full-term neonates [22]. Neary et al. showed that in preterm children, levels of coagulation proteins II, VII, and IX and protein S, as well as antithrombin, are lower than in term children.
The role of heparin cofactor II (so called “minor” antithrombin) in the modulation of neonatal hemostasis system is still questioned. HCII selectively inhibits thrombin and has a moderate binding affinity to heparin and dermatan sulfate. The rate of thrombin inhibition by HCII is significantly slower that by antithrombin, and the plasma level of HCII is 25–50% that of AT. Term infants and healthy preterm newborns have a lower level of HCII, which more likely reflects immature liver function, and they attain adult levels at 5 to 7 months of life. Neither adult or children with a thrombosis history had significantly lower levels of HCII activity. Considering that the absence of HCII does not strongly correlate with thrombosis, its physiological functions are still under investigation.
The level of plasma soluble thrombomodulin, which reflects endothelial damage, is increased at birth in asphyxiated full-term infants.
Thrombomodulin (TM) is a multidomain glycoprotein receptor for thrombin and is best known for its role as a cofactor in a clinically important natural anticoagulant pathway of protein C. It binds thrombin and converts it from a procoagulant to an anticoagulant enzyme that activates protein C. TM is expressed at the surface of endothelial cells and exists in a soluble form (sTM) in plasma and urine. Loss of TM from the surface of endothelial cells may favor thrombosis. TM not only has anticoagulant activity but also exerts anti-inflammatory properties using APC-dependent and APC-independent mechanisms. In addition to its anticoagulant and anti-inflammatory function, the TM–thrombin complex activates thrombin-activatable fibrinolysis inhibitor (TAFI), which inhibits tissue plasminogen activator (tPA)-induced fibrinolysis (antifibrinolytic properties of TM). Inflammation has been shown to reduce TM expression on the endothelial surface and this decrease may contribute to the hypercoagulable condition which is characteristic of inflammatory states. Plasma thrombomodulin concentration and plasma thrombomodulin-to-serum creatinine ratio at birth were even higher in very-low-birthweight infants than those in full-term infants [23].
2.2.2. Platelet Count and Function in the Neonate
Platelet function and physiology also depend on age. The results of the largest study on the hemostasis system formation in neonates has demonstrated that the platelet count in the fetus during pregnancy increases by ~2 × 109/L for each week of gestation. Even in premature infants, the average platelet count was ≥200 × 109/L (within the normal range for an adult) [24]. The study showed that the postpartum period had a significant effect on the number of platelets; during the first 9 weeks, the indicators corresponded to a sinusoidal pattern with two peaks: one at 2–3 weeks, and the second at 6–7 weeks. The upper limit of the expected values (95th percentile) during these peaks reached 750,000 per microliter of blood.
The average platelet count in children is similar to that in adults. The normal range of platelet counts in newborns and infants is from 150 × 103 to 450 × 103/mcL, although some data indicate a slightly lower limit for normal, especially in premature infants [25]. Preterm neonates may have a higher incidence of thrombocytopenia and bleeding, most commonly in the brain. Nevertheless, the function of the blood cells shows the great differences.
Platelet activation in vitro demonstrates decreased activation and response to various inducers, e.g., collagen, ADP, thromboxane A2 (TxA2), thrombin, and epinephrine. Neonatal platelet reactivity increases with gestational age, demonstrating that platelet reactivity is age-dependent. In preterm neonates, platelet hyporeactivity occurs due to decreased membrane glycoprotein expression [26]. Platelets collected from infants of less than 30 weeks of gestation expressed lower levels of membrane glycoproteins (GP), exposed less P-selectin on their surface [27], and were less reactive than platelets from term newborns [28]. In the same study, a significantly lower level of glycoprotein (GPIIb/IIIa) expression on platelets from peripheral blood was seen in term newborns as well as preterm infants, compared to adults.
It is now clear that platelets perform not only hemostatic but also important non-hemostatic functions, especially in angiogenesis, immune responses and inflammation [29][30].
The umbilical cord blood platelets of full-term and premature newborns showed a reduced response to most physiological agonists. This hyporeactivity is partly due to both insufficient synthesis and reaction to an important mediator of platelet function—thromboxane A2 (TxA2). The poor response of newborn platelets to TxA2 is not due to differences in the characteristics of binding to the TxA2 receptor compared to platelets of the control group of adults. It was reported the post-receptor signal transduction pathway through the TxA2 receptor was affected in newborns, and this defect in signal transduction through phospholipase C-β (PLCβ) contributes to the observed poor response of newborns’ platelets to TxA2 and consequently to TxA2-dependent agonists such as collagen. This post-receptor defect in signal transduction in cord blood platelets may explain different abnormalities in platelet function of newborn infants, including poor aggregation and secretion responses, decreased PLC activity, impaired calcium mobilization, decreased thromboxane production, and decreased response to an important mediator of platelet function TxA2 [31].
Neonatal platelets are also hyporesponsive to the tyrosine kinase-linked receptor agonist collagen, which is related to a reduced expression of glycoprotein VI (GPVI) and C-type lectin-like receptor 2 (CLEC-2) [32].
Decreased platelet function in newborns is associated with different factors:
-
Platelet hyporeactivity to epinephrine is caused by a reduced number of alpha-2 (α2) adrenergic receptors on the cell surface.
-
Decreased thrombin platelet response occurs due to the lack of protease-activated receptor 1 (PAR-1) and PAR-4 receptors on the neonatal platelets [33].
-
Reduced signal transduction results in thromboxane hyporeactivity in the newborn.
-
Reduced platelet activation after collagen inducing is associated with a lack of GPVI receptors, combined with defects in intracellular signaling pathways. Evidence may be seen in an insufficient phosphorylation of Syk and CLEC-2 in neonatal platelets [32].
A recent study showed a neonate’s platelets’ special features compared to an adult. Platelets of a newborn display hypersensitivity while inhibited by the prostaglandin E1 (PGE1). The blood cells also show increased PGE1-induced cAMP levels. However, the biological significance of this mechanism is still not clear, and the tests show the increased functioning of the PGE2-ADP-Proteinkinase II axis [34].
Platelet alpha granules (α-granules) contain clotting system growth factors and proteins, e.g., von Willebrand factor, P-selectin, coagulation factors, and others. Caparros et al. demonstrated that SNARE family proteins like Stx11 and its regulator Munc18b and β1-tubulin have reduced secretion. This phenomenon is associated with decreased α-degranulation in platelets. However, a decreased β1-tubulin level has no effect on platelet morphology [35].
2.3. Clinical Features of Neonatal Thrombosis
The new Italian Registry of Infantile Thrombosis (RITI) is the largest available European registry of neonatal and pediatric thrombosis, and includes a total of 2668 questions. The RITI collected the data of 1001 neonates and children affected by cerebral or systemic thrombosis from 48 Italian pediatric and intensive care units. Available data showed that 57.8% of affected neonatal and pediatric patients were male; the age at first thrombotic event was median 0.9 years; 24.8% had neonatal cerebral thrombosis, 8.7% had neonatal systemic thrombosis, 41.5% had pediatric cerebral thrombosis and 25.1% had pediatric systemic thrombosis [36]. Attention is drawn to the high frequency of cerebral thrombosis.
Perinatal arterial ischemic stroke (PAIS) is a cerebrovascular disorder which includes a group of arterial ischemic injuries that can affect full-term and premature infants in the prenatal, perinatal and postpartum periods. Different types of perinatal arterial ischemic stroke have different clinical manifestations, risk factors, and long-term outcomes. The clinical manifestations of PAIS are characterized by acute encephalopathy, seizures, and central nervous system depression, and it carries significant long-term disabilities [37]. The true frequency of perinatal stroke is unknown, but various cases have been reported, in which the frequency ranged from 0.025% in live births [38] to 17% during autopsy of full-term newborns [39]. An imaging study of the brain is necessary to confirm a parenchymal hemorrhage with occlusion of the corresponding artery [40]. The route of possible cardiogenic emboli is shorter when passing through the aorta and the left carotid artery. That is why the left middle cerebral artery is the most frequent artery involved in stroke.
Studies suggest that multiple risk factors are involved in perinatal stroke and placental pathology may be a triggering factor [41]. Certain maternal and fetal conditions, including thrombotic vasculopathy and antiphospholipid syndrome, can lead to emboli entering the fetal circulation through the placental bloodstream.
Besides acquired contributing factors, the data suggests that genetic prothrombotic risk factors (the factor V (FV) G1691A mutation and the prothrombin (PT) G20210A variant) play a role in symptomatic neonatal stroke. Lipoprotein(a) elevated levels (>30 mg/dL) and protein C deficiency were also observed in neonates with PAIS [42].
Cerebral venous sinus (sinovenous) thrombosis (CSVT) is a rare but often underrecognized disorder with neurologic sequelae in up to 40% of survivors and a mortality approaching 10% [43]. Clinical manifestations of CSVT are similar to those of PAIS [44]. The neonate may experience sleep apnea or seizures (58%), diffuse neurologic signs (76%), focal neurologic signs (42%) [43], coma (30%), headache (18%), and motor weakness (21%) [45]. Rarely, anemia or thrombocytopenia can occur. Clinical symptoms may be nonspecific, which may obscure the diagnosis and delay treatment. Important manifestations of venous sinus thrombosis are papilledema and signs of increasing intracranial pressure. Vessels of the superior sagittal and transverse sinuses are most often involved in the pathological process. The most accurate method of diagnosis is contrast-enhanced MRI, but ultrasonogram is also used [46]. Mortality in venous sinus thrombosis has been estimated as from 2% to 24%, and the main complications are cerebral palsy, epilepsy and cognitive impairment [47]. Severe cases can lead to loss of limb function [48].
The development of neonatal intracardiac thrombosis is mainly associated with the installation of a central venous catheter (CVC), which is essential for the treatment of critically ill neonates. This type of thrombosis is associated with endocarditis development, pulmonary artery obstruction, ventricular dysfunction, and high mortality rate [49].
Intracardiac thrombosis often occurs in children after major surgical interventions associated with severe heart defects [50]. The study of newborns who underwent palliative treatment demonstrated that in 23–33% of cases, intracardiac thrombosis developed after the surgery [51]. Echocardiography is the most common diagnostic method with a minimally invasive strategy. The main clinical features in the newborn are the appearance of abnormal murmurs, persistent thrombocytopenia, and heart failure.
Renal vein thrombosis is the most common thrombosis that is not associated with the insertion of a catheter. The prevalence rate is 0.5 per 1000 admissions to the ICU. Unilateral renal vein thrombosis occurs in 70% of cases, and in 64% it localizes on the left side. The patients are mostly male [52]. The main clinical manifestations are macroscopic hematuria, palpable abdominal mass and thrombocytopenia. Other symptoms can be oliguria, proteinuria, acute renal damage, and increased blood pressure. The most common risk factors are prematurity and birth asphyxia. The main diagnostic method is a Doppler ultrasound. Echogenic clot, venous dilation, or lack of blood flow can be seen on the image. The most common complications of renal vein thrombosis are adrenal hemorrhage, inferior vena cava thrombosis, and hypertension.
The most common risk factors of portal vein thrombosis are omphalitis, sepsis and phototherapy in premature infants. Making a diagnosis can be challenging due to lack of clinical manifestations. In many children, portal vein thrombosis resolves on its own, but portal hypertension may manifest a decade after the neonatal period. A rather severe complication of thrombosis is cavernous transformation of the portal vein with splenomegaly and reverse portal blood flow [53].
In acute femoral artery thrombosis, there is usually a limb color change from pale to cyanotic. Femoral arterial pulse absence is an indication for ultrasound examination, which usually identifies the clot blocking the blood flow of the lower limb [54].
Neonatal purpura fulminans is a life-threatening condition that often develops a few hours after birth. This disorder manifests with an acute, rapidly progressive thrombosis of small-diameter vessels located primarily on the skin of the extremities. Purpura is characterized by the sudden development of intravascular thrombosis and hemorrhagic skin infarction, rapidly leading to disseminated intravascular coagulation (DIC) with consumption coagulopathy and shock symptoms. The first description of the disease dates back to 1962, but it was only several decades later that a link was established between purpura fulminans and a protein S deficiency [55]. There is a high mortality rate without immediate diagnosis and therapy intervention. The severity of clinical manifestation is dependent on the genetic variation of congenital proteins C and S deficiency. Skin lesions start out dark red, but eventually turn purple–black and necrotic. Spots often appear at trauma sites, such as where the intravenous catheter is inserted. A severe protein C deficiency is associated with retinal detachment, vitreous hemorrhage, and cerebral vascular thrombosis [56]. The following laboratory parameters are indicative of acute disease: thrombocytopenia, hypofibrinogenemia, increased fibrin degradation product levels, prothrombin time, and the activation time of blood platelet prolongation. Microangiopathic hemolytic anemia may also occur [57]. Neonatal fulminant purpura caused by congenital or acquired protein C or S deficiency remains a life-threatening condition. Early recognition of symptoms, fast-track diagnostics, and immediate substitution therapy can reduce the possible fatal outcome [58].
References
- Haley, K.M. Neonatal venous thromboembolism. Front. Pediatr. 2017, 5, 136.
- Bhat, R.; Kumar, R.; Kwon, S.; Murthy, K.; Liem, R.I. Risk Factors for Neonatal Venous and Arterial Thromboembolism in the Neonatal Intensive Care Unit—A Case Control Study. J. Pediatr. 2018, 195, 28–32.
- van Ommen, C.H.; Heijboer, H.; Büller, H.R.; Hirasing, R.A.; Heijmans, H.S.; Peters, M. Venous thromboembolism in childhood: A prospective two-year registry in The Netherlands. J. Pediatr. 2001, 139, 676–681.
- Chalmers, E.A. Neonatal thrombosis. J. Clin. Pathol. 2000, 53, 419–423.
- Robinson, V.; Achey, M.A.; Nag, U.P.; Reed, C.R.; Pahl, K.S.; Greenberg, R.G.; Clark, R.H.; Tracy, E.T. Thrombosis in infants in the neonatal intensive care unit: Analysis of a large national database. J. Thromb. Haemost. 2021, 19, 400–407.
- Saracco, P.; Bagna, R.; Gentilomo, C.; Magarotto, M.; Viano, A.; Magnetti, F.; Giordano, P.; Luciani, M.; Molinari, A.C.; Suppiej, A.; et al. Clinical Data of Neonatal Systemic Thrombosis. J. Pediatr. 2016, 171, 60–66.e1.
- Makatsariya, A.; Bitsadze, V.; Khizroeva, J.; Vorobev, A.; Makatsariya, N.; Egorova, E.; Mischenko, A.; Mashkova, T.; Antonova, A. Neonatal thrombosis. J. Matern. Fetal Neonatal Med. 2022, 35, 1169–1177.
- Song, S.; Li, Z.; Zhao, G.; Li, X.; Wang, R.; Li, B.; Liu, Q. Epidemiology and risk factors for thrombosis in children and newborns: Systematic evaluation and meta-analysis. BMC Pediatr. 2023, 23, 292.
- Andrew, M.; David, M.; Adams, M.; Ali, K.; Anderson, R.; Barnard, D.; Bernstein, M.; Brisson, L.; Cairney, B.; DeSai, D.; et al. Venous thromboembolic complications (VTE) in children: First analyses of the Canadian Registry of VTE. Blood 1994, 83, 1251–1257.
- Schmidt, B.; Andrew, M. Neonatal thrombosis: Report of a prospective Canadian and international registry. Pediatrics 1995, 96 Pt 1, 939–943.
- Nowak-Göttl, U.; von Kries, R.; Göbel, U. Neonatal symptomatic thromboembolism in Germany: Two-year survey. Arch. Dis. Child. Fetal Neonatal Ed. 1997, 76, F163–F167.
- Tuckuviene, R.; Christensen, A.L.; Helgestad, J.; Johnsen, S.P.; Kristensen, S.R. Pediatric venous and arterial noncerebral thromboembolism in Denmark: A nationwide population-based study. J. Pediatr. 2011, 159, 663–669.
- Andrew, M.; Paes, B.; Milner, R.; Johnston, M.; Mitchell, L.; Tollefsen, D.M.; Powers, P. Development of the human coagulation system in the full-term infant. Blood 1987, 70, 165–172.
- Achey, M.A.; Nag, U.P.; Robinson, V.L.; Reed, C.R.; Arepally, G.M.; Levy, J.H.; Tracy, E.T. The Developing Balance of Thrombosis and Hemorrhage in Pediatric Surgery: Clinical Implications of Age-Related Changes in Hemostasis. Clin. Appl. Thromb. Hemost. 2020, 26, 1076029620929092.
- Del Vecchio, A.; Latini, G.; Henry, E.; Christensen, R.D. Template bleeding times of 240 neonates born at 24 to 41 weeks gestation. J. Perinatol. 2008, 28, 427–431.
- Andrew, M.; Paes, B.; Bowker, J.; Vegh, P. Evaluation of an automated bleeding time device in the newborn. Am. J. Hematol. 1990, 35, 275–277.
- Boudewijns, M.; Raes, M.; Peeters, V.; Mewis, A.; Cartuyvels, R.; Magerman, K.; Rummens, J.L. Evaluation of platelet function on cord blood in 80 healthy term neonates using the Platelet Function Analyser (PFA-100); shorter in vitro bleeding times in neonates than adults. Eur. J. Pediatr. 2003, 162, 212–213.
- Andrew, M.; Vegh, P.; Johnston, M.; Bowker, J.; Ofosu, F.; Mitchell, L. Maturation of the hemostatic system during childhood. Blood 1992, 80, 1998–2005.
- Cvirn, G.; Gallistl, S.; Leschnik, B.; Muntean, W. Low tissue factor pathway inhibitor (TFPI) together with low antithrombin allows sufficient thrombin generation in neonates. J. Thromb. Haemost. 2003, 1, 263–268.
- Cvirn, G.; Gallistl, S.; Rehak, T.; Jürgens, G.; Muntean, W. Elevated thrombin-forming capacity of tissue factor-activated cord compared with adult plasma. J. Thromb. Haemost. 2003, 1, 1785–1790.
- Andrew, M.; Paes, B.; Milner, R.; Johnston, M.; Mitchell, L.; Tollefsen, D.M.; Castle, V.; Powers, P. Development of the Human Coagulation System in the Healthy Premature Infant. Blood 1988, 72, 1651–1657.
- Neary, E.; McCallion, N.; Kevane, B.; Cotter, M.; Egan, K.; Regan, I.; Kirkham, C.; Mooney, C.; Coulter-Smith, S.; Ní Áinle, F. Coagulation indices in very preterm infants from cord blood and postnatal samples. J. Thromb. Haemost. 2015, 13, 2021–2030.
- Nako, Y.; Ohki, Y.; Harigaya, A.; Tomomasa, T.; Morikawa, A. Plasma thrombomodulin level in very low birthweight infants at birth. Acta Paediatr. 1997, 86, 1105–1109.
- Wiedmeier, S.E.; Henry, E.; Sola-Visner, M.C.; Christensen, R.D. Platelet reference ranges for neonates, defined using data from over 47,000 patients in a multihospital healthcare system. J. Perinatol. 2009, 29, 130–136.
- Sillers, L.; Van Slambrouck, C.; Lapping-Carr, G. Neonatal Thrombocytopenia: Etiology and Diagnosis. Pediatr. Ann. 2015, 44, e175–e180.
- Bednarek, F.J.; Bean, S.; Barnard, M.R.; Frelinger, A.L.; Michelson, A.D. The platelet hyporeactivity of extremely low birth weight neonates is age-dependent. Thromb. Res. 2009, 124, 42–45.
- Waller, A.K.; Lantos, L.; Sammut, A.; Salgin, B.; McKinney, H.; Foster, H.R.; Kriek, N.; Gibbins, J.M.; Stanworth, S.J.; Garner, S.F.; et al. Flow cytometry for near-patient testing in premature neonates reveals variation in platelet function: A novel approach to guide platelet transfusion. Pediatr. Res. 2019, 85, 874–884.
- Sitaru, A.G.; Holzhauer, S.; Speer, C.P.; Singer, D.; Obergfell, A.; Walter, U.; Grossmann, R. Neonatal platelets from cord blood and peripheral blood. Platelets 2005, 16, 203–210.
- Andres, O.; Schulze, H.; Speer, C.P. Platelets in neonates: Central mediators in haemostasis, antimicrobial defence and inflammation. Thromb. Haemost. 2015, 113, 3–12.
- Davenport, P.; Sola-Visner, M. Platelets in the neonate: Not just a small adult. Res. Pract. Thromb. Haemost. 2022, 6, e12719.
- Israels, S.J.; Cheang, T.; Roberston, C.; McMillan-Ward, E.M.; McNicol, A. Impaired Signal Transduction in Neonatal Platelets. Pediatr. Res. 1999, 45 (Suppl. S5), 687–691.
- Hardy, A.T.; Palma-Barqueros, V.; Watson, S.K.; Malcor, J.D.; Eble, J.A.; Gardiner, E.E.; Blanco, J.E.; Guijarro-Campillo, R.; Delgado, J.L.; Lozano, M.L.; et al. Significant Hypo-Responsiveness to GPVI and CLEC-2 Agonists in Pre-Term and Full-Term Neonatal Platelets and following Immune Thrombocytopenia. Thromb. Haemost. 2018, 118, 1009–1020.
- Schlagenhauf, A.; Schweintzger, S.; Birner-Grünberger, R.; Leschnik, B.; Muntean, W. Comparative evaluation of PAR1, GPIb-IX-V, and integrin αIIbβ3 levels in cord and adult platelets. Hamostaseologie 2010, 30 (Suppl. S1), S164–S167.
- Palma-Barqueros, V.; Torregrosa, J.M.; Caparrós-Pérez, E.; Mota-Pérez, N.; Bohdan, N.; Llanos, M.D.C.; Begonja, A.J.; Sola-Visner, M.; Vicente, V.; Teruel-Montoya, R.; et al. Developmental Differences in Platelet Inhibition Response to Prostaglandin E1. Neonatology 2020, 117, 15–23.
- Caparrós-Pérez, E.; Teruel-Montoya, R.; Palma-Barquero, V.; Torregrosa, J.M.; Blanco, J.E.; Delgado, J.L.; Lozano, M.L.; Vicente, V.; Sola-Visner, M.; Rivera, J.; et al. Down Regulation of the Munc18b-syntaxin-11 Complex and β1-tubulin Impairs Secretion and Spreading in Neonatal Platelets. Thromb. Haemost. 2017, 117, 2079–2091.
- Pelizza, M.F.; Martinato, M.; Rosati, A.; Nosadini, M.; Saracco, P.; Giordano, P.; Luciani, M.; Ilardi, L.; Lasagni, D.; Molinari, A.C.; et al. The new Italian registry of infantile thrombosis (RITI): A reflection on its journey, challenges and pitfalls. Front. Pediatr. 2023, 11, 1094246.
- Martinez-Biarge, M.; Ferriero, D.M.; Cowan, F.M. Perinatal arterial ischemic stroke. Handb. Clin. Neurol. 2019, 162, 239–266.
- Lynch, J.K.; Hirtz, D.G.; DeVeber, G.; Nelson, K.B. Report of the National Institute of Neurological Disorders and Stroke workshop on perinatal and childhood stroke. Pediatrics 2002, 109, 116–123.
- Hunt, R.W.; Inder, T.E. Perinatal and neonatal ischaemic stroke: A review. Thromb. Res. 2006, 118, 39–48.
- Gacio, S.; Muñoz Giacomelli, F.; Klein, F. Presumed perinatal ischemic stroke: A review. Arch. Argent Pediatr. 2015, 113, 449–455, English, Spanish.
- Elbers, J.; Viero, S.; MacGregor, D.; deVeber, G.; Moore, A.M. Placental Pathology in Neonatal Stroke. Pediatrics 2011, 127, e722–e729.
- Günther, G.; Junker, R.; Sträter, R.; Schobess, R.; Kurnik, K.; Heller, C.; Kosch, A.; Nowak-Göttl, U.; Childhood Stroke Study Group. Symptomatic ischemic stroke in full-term neonates: Role of acquired and genetic prothrombotic risk factors. Stroke 2000, 31, 2437–2441, Erratum in Stroke 2001, 32, 279.
- Dlamini, N.; Billinghurst, L.; Kirkham, F.J. Cerebral venous sinus (sinovenous) thrombosis in children. Neurosurg. Clin. N. Am. 2010, 21, 511–527.
- deVeber, G.; Andrew, M.; Adams, C.; Bjornson, B.; Booth, F.; Buckley, D.J.; Camfield, C.S.; David, M.; Humphreys, P.; Langevin, P.; et al. Cerebral sinovenous thrombosis in children. N. Engl. J. Med. 2001, 345, 417–423.
- Wasay, M.; Dai, A.I.; Ansari, M.; Shaikh, Z.; Roach, E.S. Cerebral venous sinus thrombosis in children: A multicenter cohort from the United States. J. Child Neurol. 2008, 23, 26–31.
- Manco-Johnson, M.J. How I treat venous thrombosis in children. Blood 2006, 107, 21–29.
- Moharir, M.D.; Shroff, M.; Pontigon, A.M.; Askalan, R.; Yau, I.; Macgregor, D.; Deveber, G.A. A prospective outcome study of neonatal cerebral sinovenous thrombosis. J. Child Neurol. 2011, 26, 1137–1144.
- Zhu, W.; Zhang, H.; Xing, Y. Clinical Characteristics of Venous Thrombosis Associated with Peripherally Inserted Central Venous Catheter in Premature Infants. Children 2022, 9, 1126.
- Ulloa-Ricardez, A.; Romero-Espinoza, L.; Estrada-Loza Mde, J.; González-Cabello, H.J.; Núñez-Enríquez, J.C. Risk Factors for Intracardiac Thrombosis in the Right Atrium and Superior Vena Cava in Critically Ill Neonates who Required the Installation of a Central Venous Catheter. Pediatr. Neonatol. 2016, 57, 288–294.
- Cholette, J.M.; Rubenstein, J.S.; Alfieris, G.M.; McDermott, M.P.; Harmon, W.G.; Vermilion, R.; Eaton, M.P.; Gangemi, J.J.; Lerner, N.B. Elevated risk of thrombosis in neonates undergoing initial palliative cardiac surgery. Ann. Thorac. Surg. 2007, 84, 1320–1325.
- Fenton, K.N.; Siewers, R.D.; Rebovich, B.; Pigula, F.A. Interim mortality in infants with systemic-to-pulmonary artery shunts. Ann. Thorac. Surg. 2003, 76, 152–156, discussion 156–157.
- Messinger, Y.; Sheaffer, J.W.; Mrozek, J.; Smith, C.M.; Sinaiko, A.R. Renal outcome of neonatal renal venous thrombosis: Review of 28 patients and effectiveness of fibrinolytics and heparin in 10 patients. Pediatrics 2006, 118, e1478–e1484.
- Moon, C.J.; Kwon, T.H.; Lee, H.S. Portal vein thrombosis and food protein-induced allergic proctocolitis in a premature newborn with hypereosinophilia: A case report. BMC Pediatr. 2021, 21, 49.
- Tsonis, O.; Gouvias, T.; Gkrozou, F.; Antonopoulou, I.; Giantsouli, A.; Paschopoulos, M.; Baltogianni, M. Neonatal femoral artery thrombosis at the time of birth: A case report. J. Pediatr. Neonatal Individ. Med. (JPNIM) 2020, 9, e090214.
- Mahasandana, C.; Suvatte, V.; Marlar, R.A.; Manco-Johnson, M.J.; Jacobson, L.J.; Hathaway, W.E. Neonatal purpura fulminans associated with homozygous protein S deficiency. Lancet 1990, 335, 61–62.
- Hattenbach, L.O.; Beeg, T.; Kreuz, W.; Zubcov, A. Ophthalmic manifestation of congenital protein C deficiency. J. AAPOS 1999, 3, 188–190.
- Chalmers, E.; Cooper, P.; Forman, K.; Grimley, C.; Khair, K.; Minford, A.; Morgan, M.; Mumford, A.D. Purpura fulminans: Recognition, diagnosis and management. Arch. Dis. Child. 2011, 96, 1066–1071.
- Marlar, R.A.; Montgomery, R.R.; Broekmans, A.W. Diagnosis and treatment of homozygous protein C deficiency. Report of the Working Party on Homozygous Protein C Deficiency of the Subcommittee on Protein C and Protein S, International Committee on Thrombosis and Haemostasis. J. Pediatr. 1989, 114 Pt 1, 528–534.
More
Information
Subjects:
Obstetrics & Gynaecology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
628
Revisions:
2 times
(View History)
Update Date:
21 Sep 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




