Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Razvan Adrian Covache-Busuioc | -- | 2421 | 2023-09-20 13:44:38 | | | |
| 2 | Rita Xu | Meta information modification | 2421 | 2023-09-21 05:02:17 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Dumitru, A.V.; Stoica, E.; Covache-Busuioc, R.; Bratu, B.; Cirstoiu, M. Golgi Apparatus in Breast Cancer Progression. Encyclopedia. Available online: https://encyclopedia.pub/entry/49427 (accessed on 07 February 2026).
Dumitru AV, Stoica E, Covache-Busuioc R, Bratu B, Cirstoiu M. Golgi Apparatus in Breast Cancer Progression. Encyclopedia. Available at: https://encyclopedia.pub/entry/49427. Accessed February 07, 2026.
Dumitru, Adrian Vasile, Evelina-Elena Stoica, Razvan-Adrian Covache-Busuioc, Bogdan-Gabriel Bratu, Monica-Mihaela Cirstoiu. "Golgi Apparatus in Breast Cancer Progression" Encyclopedia, https://encyclopedia.pub/entry/49427 (accessed February 07, 2026).
Dumitru, A.V., Stoica, E., Covache-Busuioc, R., Bratu, B., & Cirstoiu, M. (2023, September 20). Golgi Apparatus in Breast Cancer Progression. In Encyclopedia. https://encyclopedia.pub/entry/49427
Dumitru, Adrian Vasile, et al. "Golgi Apparatus in Breast Cancer Progression." Encyclopedia. Web. 20 September, 2023.
Copy Citation
Breast cancer represents a paramount global health challenge, warranting intensified exploration of the molecular underpinnings influencing its progression to facilitate the development of precise diagnostic instruments and customized therapeutic regimens.
golgi apparatus
breast cancer
protein glycosylation
protein trafficking
1. Introduction
Camillo Golgi is credited with the identification of the Golgi apparatus, a fundamental organelle inherent to eukaryotic cells [1]. Characterized by its intricate and dynamic nature, the Golgi apparatus is pivotal in an array of cellular activities, predominantly protein modification, segregation, conveyance, and packaging before its designated delivery to specific intracellular locales. Structurally, the organelle comprises overlapping membranous sacs, termed cisternae, which possess a unique architectural design optimized for proficient modification and packaging before routing to defined cellular destinations.
Acting as a central hub, the Golgi apparatus is instrumental in the processing and classification of diverse soluble proteins and lipids, directing them to their intended cellular destinations [2]. Given its seminal position in the secretory continuum, any perturbation in its architecture or functionality can gravely impact cellular protein and lipid equilibrium. Notably, a growing body of research has demonstrated that aberrations in the Golgi apparatus are implicated in a spectrum of conditions ranging from neurodegenerative maladies [3][4][5][6][7] to ischemic strokes, cardiovascular ailments, pulmonary arterial hypertension, infectious diseases, and malignancies.
Intrinsically adaptable, the Golgi apparatus possesses the capacity to rapidly recalibrate in response to evolving cellular demands and extracellular cues. This involves undergoing morphological transitions, such as reorganization, fragmentation, and integration with ancillary organelles, to aptly address variances in protein production and cellular requisites. In this context, the Golgi apparatus engages in complex interplays with other cellular structures, notably the endoplasmic reticulum (ER) and endosomes, orchestrating a sophisticated intracellular transport and communication matrix [8].
The indispensability of the Golgi apparatus in cellular operations underscores the ramifications of its dysfunction on human physiology and health. Disruptions or anomalies in its form, functionality, protein shuttling, or associated metabolic pathways have been pinpointed as etiological agents in a diverse array of pathologies, including malignancies, neurodegenerative diseases, and metabolic anomalies.
2. Golgi Sorting, Protein Trafficking, and Glycosylation Abnormalities
Preserving the structural coherence of the Golgi apparatus is paramount for its optimal operation, as structural perturbations could usher in an array of pathologies. Operational aberrations of the Golgi apparatus encompass modifications in its pH equilibrium, anomalous glycosylation trajectories, and compromised membrane transport. Notably, fragmentation of the Golgi has been postulated as a precursor event in cellular apoptosis [9][10]. In scenarios of pharmacological or oxidative duress, the Golgi apparatus undergoes transformations, such as cargo saturation, ion concentration disequilibrium, and irregular luminal acidity, which collectively can induce membrane transport defects. Researchers have coined the term “Golgi stress” to encapsulate this specific Golgi apparatus response, and two well-discussed molecular pathways are the structural preservation of Golgi apparatus by the TFE3 (transcription factor binding to IGHM enhancer 3) pathway and the proteoglycan pathway, which uptake the expression of enzymes for glycosylation [11].
Glycosylation stands as a pervasive posttranslational modification of proteins and plays a pivotal role in protein-mediated signaling. The glycans situated at glycosylation loci can span a spectrum in terms of complexity, from singular sugar chains to polymers boasting over 200 sugar units. Furthermore, glycans can be subjected to auxiliary modifications, encompassing the addition of entities like phosphate, sulfate, acetate, or phosphorylcholine for further diversification. It is noteworthy that a multitude of glycans manifest branch-like structures. An N-glycan entity can house up to six branches, each embedded with several recurrent disaccharide segments. The work by Stanley et al. (2011) offers insights into the traits and operations of Golgi glycosyltransferases (GTs), encompassing their activity spectra from their initiation at the cis-Golgi to their passage through the trans-Golgi network (TGN) [12].
The glycosylation of proteins is executed at two discrete intracellular locales, each defined by unique attributes. Proteins resident in the cytosol and nucleus undergo O-GlcNAcylation, wherein singular sugar entities termed N-acetylglucosamine (GlcNAc) directly bind to serine or threonine amino acids. This mechanism is instrumental in fine-tuning protein interactions, stability, functionality, and a gamut of cellular undertakings such as transcription, metabolism, apoptosis, and organelle genesis and transport [13][14]. In contrast, within the ER and Golgi apparatus lumen, secretory and transmembrane proteins are subjected to glycosylation by affixing specific glycosaccharides, or glycans, to particular amino acid chains. This modus operandi facilitates their functional diversification, allowing them to partake in multifarious cellular events [15].
The Golgi apparatus houses an array of glycosylation enzymes capable of either cleaving monosaccharides (glycosidases) or attaching them (GTs). Intriguingly, these enzymes can form both heteromeric and homomeric assemblies [16]. Structurally, GTs are membrane-bound proteins characterized by a brief N-terminal segment, a singular membrane domain, and a luminal domain. Due to this intricate configuration, they frequently establish enzyme complexes with other active enzymes within specific glycosylation pathways. N-glycosyltransferases within the Golgi can manifest in either homomeric or heteromeric groupings. The cyclical process of these GTs entails transitions influenced by the microenvironment, oscillating between heteromeric and homomeric states. While homomeric enzyme formations are pivotal in facilitating the folding and transportation of GTs to the Golgi apparatus, the more active heteromers are predominantly utilized for streamlined glycosylation [17][18]. Noteworthy GTs include GalNAc-T2 (N-acetylgalactosaminyltransferase-2) and GalT (β1,4-galactosyltransferase), which, due to their specificity for the Golgi apparatus, highlight that any depletion of juxtanuclear Golgi staining might be indicative of the organelle’s attributes and the associated membrane proteins [19].
A dysfunctional Golgi glycosylation process has been associated with invasive behavior in various cancer types, encompassing prostate and breast malignancies [20][21]. The glycosylation process within the Golgi plays a cardinal role in numerous oncogenic molecular and cellular sequences, such as signal transduction, cellular communication, dissociation and invasion of cancer cells, cell–matrix attachment, angiogenesis, immunomodulation, and metastasis [22]. Analogous to the function of epithelial cadherin in mediating epithelial cellular cohesion, the Golgi-mediated glycosylation of N-linked glycans on epithelial cadherin might influence the epithelial-to-mesenchymal transition, thereby catalyzing the emergence of metastatic outgrowths. Such a mechanism is postulated to facilitate the migratory capacity of neoplastic cells from their inception point, be it during reparative processes post-injury or other standard physiological events, and becomes instrumental in the metastatic spread and proliferation of cancer [8][23].
The GOLPH3 complex, recognized as Golgi phosphoprotein 3, stands as a pivotal molecular entity in the realm of Golgi-facilitated oncogenesis. Its centrality in cancer can be attributed to a myriad of critical functionalities. GOLPH3 not only orchestrates Golgi glycosylation pivotal for the cancerous phenotype manifestation but also amplifies the DNA (deoxyribonucleic acid) damage response, bolstering survival amidst DNA-injurious scenarios. Additionally, it synergizes with retromer elements to enhance the mTOR (mammalian target of rapamycin) signaling upon growth factor induction and facilitates cell motility by orienting the Golgi apparatus toward the cellular forefront. Beyond GOLPH3, the Golgi spectrum hosts another consequential protein, GM130 (Golgi matrix protein 130). Integral to Golgi glycosylation and membranous protein trafficking, the downregulation of GM130 culminates in autophagy, diminished angiogenesis, and suppressed tumorigenesis [6][23][24][25].
Dysregulated Golgi glycosylation not only holds implications for carcinogenesis but might also propel cancer progression. Given the intertwined nature of Golgi-related operations and oncology, delving into and therapeutically targeting these processes should be foundational in cancer research endeavors.
Divergences in glycosylation can engender alterations in the conformation and function of numerous membranous proteins, with particular significance to collagen, fibronectin, integrins, and laminin at the extracellular interface. The paramount role of transmembrane integrins lies in fortifying the cytoskeleton via myriad cell–cell and cell–matrix interactions, thereby catalyzing cellular maturation and proliferation [26]. Glycosylation aberrancies might culminate in the flawed anchorage of these proteins, engendering a plethora of pathologies encompassing neurodegenerative conditions, malignancies, and cardiovascular afflictions [27][28][29].
The Golgi apparatus, with its cardinal role in modulating core cellular mechanisms, like adhesion and migration, stands as a keystone in the panorama of cancer evolution and metastatic dissemination. A prominent influencer in these oncogenic processes is identified as phosphatidylinositol 4-phosphate (PI4P). Hence, its role in human breast cancer can markedly sway cell–cell adhesion and migratory patterns [24].
Recent investigations underscore the paramount regulatory role of PI4P in the structural and functional intricacies of the Golgi apparatus, notably affecting glycosylation and the trafficking of proteins pivotal to cell–cell adhesion. By modulating the Golgi PI4P concentrations, the localization and activity of cardinal adhesion molecules, such as E-cadherin, are affected, thereby reshaping the intensity and dynamics of cell–cell interactions. Beyond its role in adhesion, PI4P governs activities linked with enzymes crucial for the synthesis or restructuring of glycosphingolipids, which are indispensable for cell surface interactions and signaling modalities. Furthermore, PI4P is integral in governing invasive cellular motility, a critical phenomenon in oncologic metastasis. Its regulatory role in Golgi-centric vesicular trafficking and membranous dynamism facilitates the modulation of invasive cell polarization and protrusive activities, augmenting their migratory and invasive propensities. In this orchestration, PI4P collaborates with a cohort of Golgi-associated proteins and lipid-mediated signaling pathways to modulate cytoskeletal transformations and matrix degradation, thereby facilitating the metastatic voyage of cancerous cells [21][30].
The traversal of cargoes through the Golgi apparatus is a multifaceted event and remains a focal point of discourse in the scientific literature. This research sheds light on five contemporary models postulated for assessing Golgi traffic, weighing their merits and demerits. The inaugural model posits anterograde vesicular transport amidst stable compartments of the Golgi. Conversely, the second hypothesis advocates for cisternal progression/maturation, wherein Golgi cisternae transition through sequential maturation phases. The third paradigm fuses progression/maturation with heterotypic tubular conveyance between cisternae. The penultimate model champions swift protein partitioning within a heterogenous Golgi and the terminal model envisions stable compartments as precursors for ensuing cisternal development.
A meticulous analysis reveals that no singular model can holistically encapsulate all documented phenomena across varied organisms. It might be more tenable to perceive cisternal progression/maturation as a foundational and evolutionarily conserved mechanism governing Golgi traffic. Certain cellular systems might integrate heterotypic tubular transport within Golgi cisternae. A judicious exploration of these models will illuminate the intricate facets of Golgi traffic, bestowing deeper insights into its operational mechanisms and elucidating this quintessential cellular undertaking. Grasping its foundational tenets is indispensable for decoding its influence on cellular equilibrium as well as pathological states linked to protein trafficking or excretion [31].
3. Golgi Apparatus Involvement in Breast Cancer
Breast cancer, a pressing global health challenge, leads to female mortality rates, and its prevalence is anticipated to surge in the forthcoming years. Diagnostic techniques like mammography and clinical breast inspections are pivotal for its early identification. While therapeutic modalities encompass surgical interventions, chemotherapy, and radiation treatments, each come with a set of concerns. Chemotherapy, despite its efficacy in neutralizing cancerous cells, presents a suite of adverse reactions. Radiotherapy, typically paired with surgery, may inflict enduring harm to critical organs. More promising therapeutic avenues encompass the deployment of anti-ErbB2 antibodies, exemplified by trastuzumab, especially for HER2-positive breast cancer variants. Additionally, antiestrogens and aromatase inhibitors serve to suppress the manifestation of estrogen-associated genes, proffering treatment avenues with diminished side effects [32][33].
The Rab GTPases, pivotal orchestrators of vesicular transportation, hold profound implications for the malignancy and invasiveness of cancer cells. Delving into estrogen receptor-positive breast cancer cellular frameworks reveals the instrumental role of Rab27B. Its heightened expression correlates with an augmented cellular elongation and an escalated invasiveness when interacting with collagen matrices. Such effects can be counteracted through miRNA-mediated interventions. Moreover, the amplification of Rab27B expression bears a direct relation to the surge in HSP90 alpha expression, a molecular custodian pivotal for upholding the structural integrity of MMP2 [34][35].
Rab40B’s influence is palpably seen in maneuvering the trafficking pathways of metalloproteases MMP2 and MMP9 within the MDA-MB-231 breast cancer cellular context, facilitating the degradation of the external cellular matrix. Another metalloprotease, MT1-MMP, falls under the regulatory domain of Rab2A, which further fuels metastatic behaviors via its interaction with the VPS39 protein and is crucial for the amalgamation and clustering of late endosomes/lysosomes.
SiRNA screening has unmasked Rab2A’s regulatory influence over the Golgi transport mechanisms of surface E-cadherin in breast cancer cells. Given the pivotal role of E-cadherin loss as an oncogenic transformation indicator, these revelations accentuate the significance of Rab GTPases in dictating vesicular transportation mechanisms. Such processes wield influence over cellular structural dynamics, invasion capacities, and external matrix degradation in these particular breast cancer cells [36][37]. Refer to Figure 1 for a visual representation.
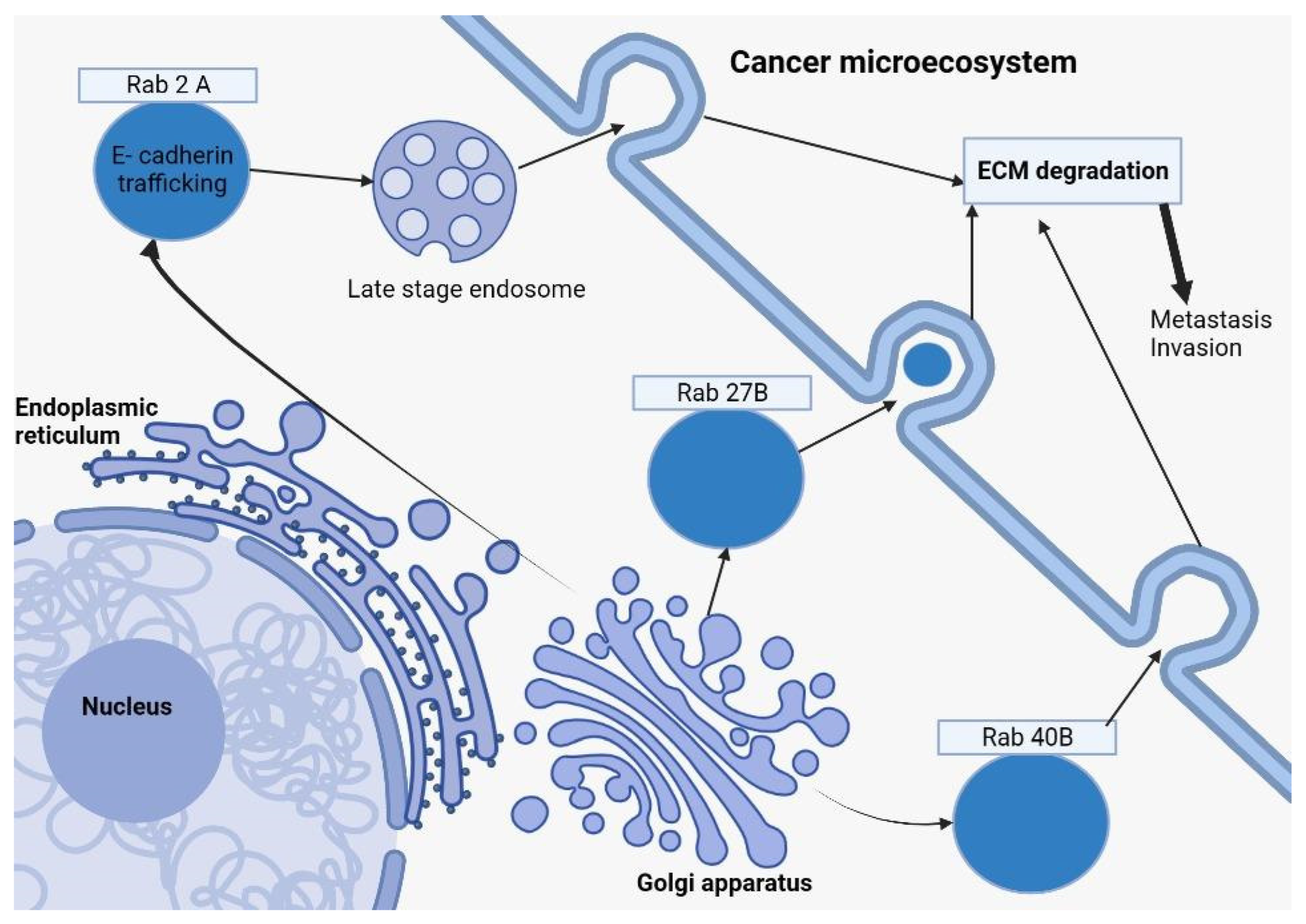
Figure 1. This diagram depicts how Rab27B, Rab2A (in a late-stage endosome form), and Rab40B from the Rab family of proteins, formed by the Golgi apparatus, are involved in breast cancer. After exocytosis, those Rab proteins play a key role in ECM (Extracellular Matrix Degradation), a representative element in the cancer microecosystem, which will further determine cell proliferation, tumoral invasion, and the formation of metastatic masses.
As highlighted in the cited study [38], GOLPH3’s pronounced overexpression in breast cancer cells and tissues contrasts starkly with its presence in normal breast tissue. Escalated GOLPH3 levels correlate with advanced tumor development, metastatic spread, and a grim prognosis for breast cancer sufferers.
In the breast cancer scenario, GOLPH3 emerges as a pivotal entity, underpinning cancer cell proliferation and longevity by modulating its DNA damage response apparatus. A noteworthy interaction of GOLPH3 is with ATM (ataxia-telangiectasia mutated), a quintessential DNA damage response protein located at the Golgi. This interaction amplifies survival rates, rendering cancer cells more resilient against DNA damage-driven cellular demise. Such a mechanism equips cancer cells with heightened resistance against the genotoxic assaults unleashed by treatments like chemotherapy and radiation [39].
Furthermore, GOLPH3’s tentacles extend into cancer progression by exerting regulatory control over a slew of signaling pathways, notably the PI3K-AKT-mTOR axis. GOLPH3 bolsters AKT activation, a kinase pivotal for cell proliferation and survival. This ultimately accelerates tumor growth and endows them with fortified resistance against treatments. From a therapeutic lens, targeting GOLPH3 emerges as a promising stratagem in the battle against breast cancer. A nuanced inhibition of its expression or functional prowess could prime cancer cells for increased susceptibility to DNA-damaging agents, effectively crippling tumor proliferation and metastatic spread [40].
In breast cancer patients, a surge in gene expression linked to ER-Golgi transport processes is evident, exemplified by genes like ARF4, COPB1, and USO1. These genes play an instrumental role in ferrying proteins between the ER and Golgi apparatus. To elucidate further, COPII vesicles shepherd proteins from the ER to the Golgi, whereas ARFs pilot the retrograde journey from the Golgi to the ER, which is facilitated by COPI vesicle formation [41].
Delving into the transportation dynamics of these genes reveals intriguing insights. The overexpression of ARF4, COPB1, and USO1 accelerates protein shuttling from the ER to Golgi. Introducing biotin amplifies this trafficking tempo even more, hinting at the pivotal role these ER-Golgi trafficking genes play in optimizing transportation kinetics [41].
References
- Rios, R.M.; Bornens, M. The Golgi apparatus at the cell centre. Curr. Opin. Cell Biol. 2002, 15, 60–66.
- Saceleanu, V.M.; Covache-Busuioc, R.-A.; Costin, H.-P.; Glavan, L.-A.; Ciurea, A.V. An Important Step in Neuroscience: Camillo Golgi and His Discoveries. Cells 2022, 11, 4112.
- Rendón, W.O.; Martínez-Alonso, E.; Tomás, M.; Martínez-Martínez, N.; Martínez-Menárguez, J.A. Golgi fragmentation is Rab and SNARE dependent in cellular models of Parkinson’s disease. Histochem. Cell Biol. 2012, 139, 671–684.
- Brandstaetter, H.; Kruppa, A.J.; Buss, F. Huntingtin is required for ER-to-Golgi transport and for secretory vesicle fusion at the plasma membrane. Dis. Model. Mech. 2014, 7, 1335–1340.
- Yuan, D.; Liu, C.; Hu, B. Dysfunction of Membrane Trafficking Leads to Ischemia-Reperfusion Injury After Transient Cerebral Ischemia. Transl. Stroke Res. 2017, 9, 215–222.
- Li, T.; You, H.; Mo, X.; He, W.; Tang, X.; Jiang, Z.; Chen, S.; Chen, Y.; Zhang, J.; Hu, Z. GOLPH3 Mediated Golgi Stress Response in Modulating N2A Cell Death upon Oxygen-Glucose Deprivation and Reoxygenation Injury. Mol. Neurobiol. 2015, 53, 1377–1385.
- Ciurea, A.V.; Mohan, A.G.; Covache-Busuioc, R.A.; Costin, H.P.; Glavan, L.A.; Corlatescu, A.D.; Saceleanu, V.M. Unraveling Molecular and Genetic Insights into Neurodegenerative Diseases: Advances in Understanding Alzheimer’s, Parkinson’s, ALS, and Huntington’s Disease. Medicine and Pharmacology. 2023. Available online: https://www.preprints.org/manuscript/202305.1229/v1 (accessed on 5 June 2023).
- Liu, J.; Huang, Y.; Li, T.; Jiang, Z.; Zeng, L.; Hu, Z. The role of the Golgi apparatus in disease (Review). Int. J. Mol. Med. 2021, 47, 38.
- Sundaramoorthy, V.; Sultana, J.M.; Atkin, J.D. Golgi fragmentation in amyotrophic lateral sclerosis, an overview of possible triggers and consequences. Front. Neurosci. 2015, 9, 400.
- Gonatas, N.K.; Stieber, A.; Gonatas, J.O. Fragmentation of the Golgi apparatus in neurodegenerative diseases and cell death. J. Neurol. Sci. 2006, 246, 21–30.
- Jiang, Z.; Hu, Z.; Zeng, L.; Lu, W.; Zhang, H.; Li, T.; Xiao, H. The role of the Golgi apparatus in oxidative stress: Is this organelle less significant than mitochondria? Free Radic. Biol. Med. 2011, 50, 907–917.
- Stanley, P. Golgi glycosylation. Cold Spring Harb. Perspect. Biol. 2011, 3, a005199.
- Bond, M.R.; Hanover, J.A. A little sugar goes a long way: The cell biology of O-GlcNAc. J. Cell Biol. 2015, 208, 869–880.
- Torres, C.R.; Hart, G.W. Topography and polypeptide distribution of terminal N-acetylglucosamine residues on the surfaces of intact lymphocytes. Evidence for O-linked GlcNAc. J. Biol. Chem. 1984, 259, 3308–3317.
- Zhang, X.; Wang, Y. Glycosylation Quality Control by the Golgi Structure. J. Mol. Biol. 2016, 428, 3183–3193.
- Yamaguchi, N.; Fukuda, M.N. Golgi retention mechanism of beta-1,4-galactosyltransferase. Membrane-spanning domain-dependent homodimerization and association with alpha- and beta-tubulins. J. Biol. Chem. 1995, 270, 12170–12176.
- Schmitz, K.R.; Liu, J.; Li, S.; Setty, T.G.; Wood, C.S.; Burd, C.G.; Ferguson, K.M. Golgi Localization of Glycosyltransferases Requires a Vps74p Oligomer. Dev. Cell 2008, 14, 523–534.
- Hassinen, A.; Kellokumpu, S. Organizational Interplay of Golgi N-Glycosyltransferases Involves Organelle Microenvironment-Dependent Transitions between Enzyme Homo- and Heteromers. J. Biol. Chem. 2014, 289, 26937–26948.
- Storrie, B.; White, J.; Röttger, S.; Stelzer, E.H.; Suganuma, T.; Nilsson, T. Recycling of Golgi-resident Glycosyltransferases through the ER Reveals a Novel Pathway and Provides an Explanation for Nocodazole-induced Golgi Scattering. J. Cell Biol. 1998, 143, 1505–1521.
- Petrosyan, A.; Holzapfel, M.S.; Muirhead, D.E.; Cheng, P.W. Restoration of compact Golgi morphology in advanced prostate cancer enhances susceptibility to galectin-1-induced apoptosis by modifying mucin O-glycan synthesis. Mol. Cancer Res. 2014, 12, 1704–1716.
- Tokuda, E.; Itoh, T.; Hasegawa, J.; Ijuin, T.; Takeuchi, Y.; Irino, Y.; Fukumoto, M.; Takenawa, T. Phosphatidylinositol 4-Phosphate in the Golgi Apparatus Regulates Cell–Cell Adhesion and Invasive Cell Migration in Human Breast Cancer. Cancer Res 2014, 74, 3054–3066.
- Pinho, S.S.; Reis, C.A. Glycosylation in cancer: Mechanisms and clinical implications. Nat. Rev. Cancer 2015, 15, 540–555.
- Baschieri, F.; Confalonieri, S.; Bertalot, G.; Di Fiore, P.P.; Dietmaier, W.; Leist, M.; Crespo, P.; Macara, I.G.; Farhan, H. Spatial control of Cdc42 signalling by a GM130–RasGRF complex regulates polarity and tumorigenesis. Nat. Commun. 2014, 5, 4839.
- Rizzo, R.; Parashuraman, S.; D’Angelo, G.; Luini, A. GOLPH3 and oncogenesis: What is the molecular link? Tissue Cell 2017, 49 Pt A, 170–174.
- Xing, M.; Peterman, M.C.; Davis, R.L.; Oegema, K.; Shiau, A.K.; Field, S.J.; Chapnick, D.A.; Liu, X.; Luo, M.E.K.; Koch, S.; et al. GOLPH3 drives cell migration by promoting Golgi reorientation and directional trafficking to the leading edge. Mol. Biol. Cell 2016, 27, 3828–3840.
- Thomas, D.; Rathinavel, A.K.; Radhakrishnan, P. Altered glycosylation in cancer: A promising target for biomarkers and therapeutics. Biochim. Biophys. Acta Rev. Cancer 2021, 1875, 188464.
- Menni, C.; Gudelj, I.; Macdonald-Dunlop, E.; Mangino, M.; Zierer, J.; Bešić, E.; Joshi, P.K.; Trbojević-Akmačić, I.; Chowienczyk, P.J.; Spector, T.D.; et al. Glycosylation Profile of Immunoglobulin G Is Cross-Sectionally Associated with Cardiovascular Disease Risk Score and Subclinical Atherosclerosis in Two Independent Cohorts. Circ. Res. 2018, 122, 1555–1564.
- Schedin-Weiss, S.; Winblad, B.; Tjernberg, L.O. The role of protein glycosylation in Alzheimer disease. FEBS J. 2013, 281, 46–62.
- Stowell, S.R.; Ju, T.; Cummings, R.D. Protein glycosylation in cancer. Annu. Rev. Pathol. 2015, 10, 473–510.
- Durrant, L.G.; Noble, P.; Spendlove, I. Immunology in the clinic review series; focus on cancer: Glycolipids as targets for tumour immunotherapy. Clin. Exp. Immunol. 2012, 167, 206–215.
- Glick, B.S.; Luini, A. Models for Golgi Traffic: A Critical Assessment. Cold Spring Harb. Perspect. Biol. 2011, 3, a005215.
- Mughees, M.; Chugh, H.; Wajid, S. Vesicular trafficking–related proteins as the potential therapeutic target for breast cancer. Protoplasma 2020, 257, 345–352.
- Chevalier, C.; Roche, S.; Bénistant, C. Vesicular trafficking regulators are new players in breast cancer progression: Role of TOM1L1 in ERBB2-dependent invasion. Mol. Cell. Oncol. 2016, 3, e1182241.
- Shahabi, A.; Naghili, B.; Ansarin, K.; Zarghami, N. The relationship between microRNAs and Rab family GTPases in human cancers. J. Cell. Physiol. 2019, 234, 12341–12352.
- Li, Z.; Fang, R.; Fang, J.; He, S.; Liu, T. Functional implications of Rab27 GTPases in Cancer. Cell Commun. Signal. 2018, 16, 44.
- Jacob, A.; Jing, J.; Lee, J.; Schedin, P.; Gilbert, S.M.; Peden, A.A.; Junutula, J.R.; Prekeris, R. Rab40b regulates MMP2 and MMP9 trafficking during invadopodia formation and breast cancer cell invasion. J. Cell Sci. 2013, 126, 4647–4658.
- Kajiho, H.; Kajiho, Y.; Frittoli, E.; Confalonieri, S.; Bertalot, G.; Viale, G.; Di Fiore, P.P.; Oldani, A.; Garre, M.; Beznoussenko, G.V.; et al. RAB2A controls MT1-MMP endocytic and E-cadherin polarized Golgi trafficking to promote invasive breast cancer programs. EMBO Rep. 2016, 17, 1061–1080.
- Buschman, M.D.; Rahajeng, J.; Field, S.J. GOLPH3 Links the Golgi, DNA Damage, and Cancer. Cancer Res. 2015, 75, 624–627.
- Farber-Katz, S.E.; Dippold, H.C.; Buschman, M.D.; Peterman, M.C.; Xing, M.; Noakes, C.J.; Tat, J.; Ng, M.M.; Rahajeng, J.; Cowan, D.M.; et al. DNA Damage Triggers Golgi Dispersal via DNA-PK and GOLPH3. Cell 2014, 156, 413–427.
- Ciccia, A.; Elledge, S.J. The DNA Damage Response: Making It Safe to Play with Knives. Mol. Cell 2010, 40, 179–204.
- Howley, B.V.; Link, L.A.; Grelet, S.; El-Sabban, M.; Howe, P.H. A CREB3-regulated ER–Golgi trafficking signature promotes metastatic progression in breast cancer. Oncogene 2018, 37, 1308–1325.
More
Information
Subjects:
Oncology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
694
Revisions:
2 times
(View History)
Update Date:
21 Sep 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




