Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Jianshe Wei | -- | 1774 | 2023-09-20 11:24:00 | | | |
| 2 | Jason Zhu | Meta information modification | 1774 | 2023-09-21 04:04:11 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Chu, J.; Li, J.; Sun, L.; Wei, J. Cellular Defense System for Ferroptosis. Encyclopedia. Available online: https://encyclopedia.pub/entry/49417 (accessed on 07 February 2026).
Chu J, Li J, Sun L, Wei J. Cellular Defense System for Ferroptosis. Encyclopedia. Available at: https://encyclopedia.pub/entry/49417. Accessed February 07, 2026.
Chu, Jie, Jingwen Li, Lin Sun, Jianshe Wei. "Cellular Defense System for Ferroptosis" Encyclopedia, https://encyclopedia.pub/entry/49417 (accessed February 07, 2026).
Chu, J., Li, J., Sun, L., & Wei, J. (2023, September 20). Cellular Defense System for Ferroptosis. In Encyclopedia. https://encyclopedia.pub/entry/49417
Chu, Jie, et al. "Cellular Defense System for Ferroptosis." Encyclopedia. Web. 20 September, 2023.
Copy Citation
Parkinson’s disease (PD) and Alzheimer’s disease (AD) are the most common rapidly developing neurodegenerative diseases that lead to serious health and socio-economic consequences. Ferroptosis is a non-apoptotic form of cell death; there is growing evidence to support the notion that ferroptosis is involved in a variety of pathophysiological contexts, and there is increasing interest in the role of ferroptosis in PD and AD. Simultaneously, cells may have evolved four defense systems to counteract the toxic effects of ferroptosis occasioned by lipid peroxidation.
ferroptosis
cellular defense systems
Parkinson’s disease
2. Cellular Defense System for Ferroptosis
Iron is a crucial trace element involved in a variety of biological processes, including DNA synthesis, cellular respiration, and immune function, and is essential for the survival of living organisms [1]. In normal healthy cells, iron homeostasis is tightly regulated to balance systemic uptake, distribution, cellular uptake, storage, and export [2][3][4]. Imbalance in iron homeostasis is a key cause of ferroptosis, which is often accompanied by excessive cellular iron uptake and the release of intracytoplasmic iron, such as ferritin phagocytosis [5][6]. The core events of ferroptosis involve increased iron accumulation, impaired lipid repair systems, and lipid peroxidation, ultimately leading to membrane disruption and cell death [7][8]. Intracellular GSH depletion and reduced GPX4 activity, the inability of lipid peroxides to be metabolized by GPX4-catalyzed reduction reactions, and the generation of large amounts of reactive oxygen radicals (ROS) by Fe2+ via the Fenton reaction increase intracellular and lipid oxidative stress levels [9][10]. The lethal accumulation of lipid peroxides in cell membranes can eventually yield membrane damage and rupture, which leads to ferroptosis, damage to cell membrane structure’s, and severe effects on various organelle functions [11][12][13].
Accordingly, cells may have evolved four defense systems to counteract the toxic effects of ferroptosis induced by lipid peroxidation [14]. The most notable defense mechanism against ferroptosis is mediated by GPX4, which acts as a lipid peroxidase, reducing toxic lipid peroxides from cell membranes to nontoxic lipid alcohols, thereby alleviating ferroptosis [15][16][17]. In addition, ferroptosis suppressant protein 1 (FSP1) acts as a REDOX reductase to inhibit GPX4-independent ferroptosis by reducing coenzyme Q (CoQ) to dihydroubiquione (CoQH2) on the plasma membrane. Subsequently, CoQH2 acts as a lipophilic radical to trap antioxidants; thus, lipid peroxy radicals are detoxified [18][19]. GPX4 acts synergically with dihydrowhey dehydrogenase (DHODH) to convert CoQ to CoQH2; thus, lipid peroxidation and ferroptosis are inhibited [20][21]. Finally, GTP cyclohydrolase 1 (GCH1) mediates the synthesis of the powerful endogenous antioxidant tetrahydrobiopterin/dihydrobiopterin (BH4/BH2), which inhibits ferroptosis in a manner independent of GPX4 [22][23]. The specific regulatory mechanism is depicted in Figure 1.
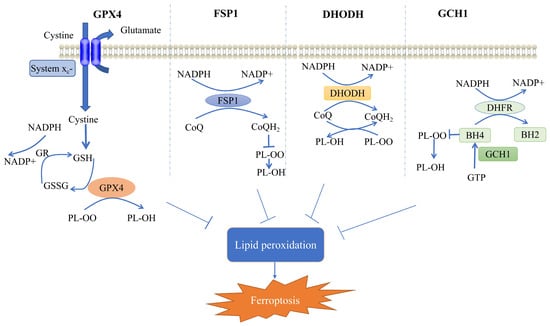
Figure 1. Mechanisms of ferroptosis cellular defense systems. Shows the four cellular defense systems of ferroptosis. GR, glutathione reductase; GSH, glutathione; GSSG, glutathione disulfide; GPX4, glutathione peroxidase 4; CoQH2, dihydroubiquione; CoQ, coenzyme Q; FSP1, ferroptosis suppressant protein 1; DHODH, dihydrowhey dehydrogenase; GCH1, GTP cyclohydrolase 1; DHFR, dihydrofolate reductase; BH4, tetrahydrobiopterin; BH2, dihydrobiopterin; PL-OO, phospholipid hydroperoxide; PL-OH, phospholipid alcohols; and NADPH, nicotinamide adenine dinucleotide phosphate. (Modified from [24][25]).
2. GPX4-Mediated Cellular Defense System
GPX4 (i.e., glutathione-dependent peroxidase) is a member of the GPXS family. Glutathione peroxidase (GPX), which is a highly conserved evolutionary enzyme, utilizes reduced glutathione (GSH) as a cofactor to detoxify lipid peroxidation [16][17][26][27]. GSH, the main cofactor of GPX4, is a tripeptide composed of glutamate, cysteine, and glycine [8]. In contrast, the synthesis of GSH entails an amino acid reverse transporter protein (System Xc-) that mediates the uptake of cystine in exchange for intracellular glutamate and is a heterodimer consisting of two subunits, namely, SLC7A11 and SLC3A2 [28]. System Xc-, which is widely distributed in phospholipids, exchanges glutamate and cystine in a 1:1 ratio, with cystine being taken up into cells, where it is reduced to cysteine [29]. GSH is then utilized as a cofactor for GPX4, which acts as a lipid peroxidase, reducing lipid peroxides to lipid alcohols; thus, propagation of lipid peroxidation in the membrane is limited [8]. Therefore, GPX4 is one of the most crucial defense mechanisms for the cellular detoxification of lipid peroxides. GPX4 activity is essential for maintaining lipid homeostasis in cells, preventing the accumulation of toxic lipid ROS and maintaining redox homeostasis [30].
GPX4 knockdown may lead to substantial cell death and cell degeneration (e.g., hepatocytes and neurons), which can be alleviated by ferroptosis inhibitors. GPX4-regulated ferroptosis is involved in cancer development and progression [31], drug-resistant persistent cells are highly dependent on GPX4 for survival, and loss of GPX4 function leads to cellular ferroptosis and prevents drug resistance; thus, a novel avenue of cancer therapy is proposed [32]. GPX4-ablation-induced ferroptosis is responsible for cognitive impairment and neurodegeneration in neurodegenerative diseases and can be ameliorated by ferroptosis inhibitors [33]. Regarding tissue or cellular injury, the inhibition of ferroptosis by adaptive GPX4 upregulation provides protection against the various adverse factors that contribute to the injury [34][35]. Overall, GPX4 crucially facilitates the regulation of ferroptosis, affecting the development and progression of a wide range of diseases.
3. FSP1-Mediated Cellular Defense System
GPX4 is the main enzyme regulating ferroptosis; however, a number of cancer cell lines (e.g., non-small cell lung cancer PC9, melanoma A375, and Kuramochi ovarian cancer JCRB cells) are resistant to GPX4 inhibitors [19][32][36][37][38], and the sensitivity to ferroptosis induced by GPX4 inactivation varies considerably between cancer cell types, which indicates that a parallel ferroptosis defense system to GPX4 may exist [36]. In U-2 OS osteosarcoma cells treated with GPX4 inhibitor 1S, 3R-RSL3 (hereafter RSL3), a CRISPR/Cas9 screening was conducted using apoptosis and a cancer single-stranded RNA (sgRNA) subpool to identify FSP1 as a potent ferroptosis defense factor [18]. FSP1 expression was positively correlated with resistance to multiple GPX4 inhibitors (RSL3, ML210, and ML162), and the protective effect of FSP1 against cell death was specific to ferroptosis and not to cell death induced by cytotoxic compounds and/or pro-apoptotic conditions [19]. FSP1 functions as an NADH-dependent coenzyme Q10 (CoQ10) oxidoreductase; it converts CoQ in the cell membrane to CoQH2 [39][40], which acts as a lipophilic radical-trapping antioxidant that prevents ferroptosis from occurring and is unique in this role [41].
FSP1 was originally named flavoprotein apoptosis-inducing factor mitochondrial-associated 2 (AIFM2) because it is homologous to apoptosis-inducing factor (AIF or AIFM1), a mitochondrial pro-apoptotic protein [42]. However, FSP1 lacks the N-terminal mitochondrial targeting sequence in AIF, is not located in mitochondria, and does not promote apoptosis. Therefore, AIFM2 was renamed FSP1 [18][19]. Although the mechanism of regulation with FSP1 is not appropriately understood, the level of resistance to ferroptosis is positively correlated with the level of FSP1 expression in many cultured human cancer cell lines, and the overexpression of FSP1 protects cancer cells from ferroptosis both in vivo and in vitro [18]. FSP1 exists as an independent parallel system that can be utilized with GPX4 to inhibit phospholipid peroxidation and ferroptosis [43]. FSP1 is a molecular target that facilitates neural repair, and administration of phenylephrine treatment can inhibit ferroptosis in mice after cerebral hemorrhage by upregulating FSP1, thereby reducing white matter damage and promoting the recovery of motor and coordination abilities [44]. Experiments with human cartilage and mouse chondrocytes show that FSP1 treatment attenuates the development of osteoarthritis in GPX4 knockout mice [45]. Ginsenoside Rg1 alleviates sepsis-induced acute kidney injury, probably by inhibiting ferroptosis in tubular epithelial cells in the kidney via FSP1. Ginsenoside Rg1 reduced iron content, ferroptosis-related protein, and MDA levels, increased GPX4, FSP1, and GSH levels, and inhibited lipid peroxidation and ferroptosis responses. In addition, the inhibitory effect of ginsenoside Rg1 on ferroptosis response was counteracted by FSP1 knockdown. In cellular experiments, ginsenoside Rg1 increased the viability of renal tubular epithelial cells and reduced iron accumulation and lipid peroxidation during ferroptosis; its anti-ferroptosis activity was dependent on FSP1. Ginsenoside Rg1 alleviated sepsis-induced acute kidney injury, possibly by inhibiting ferroptosis in renal tubular epithelial cells in the kidney via FSP1 [46][47]. In conclusion, FSP1 plays different roles in various diseases and may be a good molecular target; the upregulation of FSP1 or the stabilization of FSP1 expression could provide a novel direction for the treatment of related diseases.
4. DHODH-Mediated Cellular Defense System
DHODH is an inner mitochondrial membrane enzyme, an enzyme essential for the de novo biosynthesis of pyrimidine-based nucleotides, which catalyzes the de novo synthesis of pyrimidine ribonucleotide and crucially affects the metabolism of cancer cells [48][49]. DHODH catalyzes the oxidation of dihydroorotic acid (DHO) to orotate, which is essential for the production of uridine-5′-phosphate (UMP), and DHODH inhibition leads to pyrimidine depletion; thus, the cell lacks the essential nucleotides it requires [50]. DHODH-CoQ10 and mitochondrial (mGPX4) act as local mitochondrial defense systems, inhibiting mitochondrial lipid peroxidation and preventing ferroptosis. DHODH couples with the reduction of CoQ10 to CoQ10H2 after the oxidation of DHO to whey acid [49]. CoQ10H2 acts as a radical-trapping antioxidant, reducing lipid ROS in the inner mitochondrial membrane. DHODH and mGPX4 form the two main defense arms to inhibit lipid peroxidation in the mitochondrial membrane, which must be disabled to promote mitochondrial lipid peroxidation and induce ferroptosis [51]. DHODH has been extensively analyzed as a potential target for cancer therapy due to the increased demand for nucleotides by rapidly proliferating cells [49]. DHODH exhibits a unique function in attenuating mitochondrial lipid peroxidation and ferroptosis, which is independent of its traditional role in the production of pyrimidine nucleotides. The inactivation of DHODH sensitizes GPX4-high cancer cells to ferroptosis inducers and enhances ferroptosis in GPX4-low cancer cells [52]. Mechanistically, DHODH acts in parallel with GPX4, independent of other pathways, to inhibit ferroptosis through the reduction of CoQ to CoQH2.
5. GCH1-Mediated Cellular Defense System
To identify alternative approaches to ferroptosis regulation, a set of novel ferroptosis suppressor genes was identified through a genome-wide activation library screen, which culminated in the identification of a novel GCH1-centred ferroptosis suppressor axis [22]. Tetrahydrobiopterin (BH4) is an active cofactor involved in redox processes and exhibits antioxidant properties both in vitro and in vivo [53]. Overexpression of GCH1, the synthetic rate-limiting enzyme of BH4, not only abolished lipid peroxidation but also exhibited strong protection against the two ferroptosis inducers (i.e., RSL3 and IKE) and against ferroptosis induced by the absence of GPX4, independently of other ferroptosis-related antioxidant systems [54][55][56]. Furthermore, GCH1 did not protect against apoptosis induced by apoptosis inducers, which indicated that GCH1 could act as a selective target for ferroptosis [57]. Elevated levels of CoQ10, a potent antioxidant that neutralizes lethal lipid peroxidation by trapping free radicals, were found in GCH1-overexpressing cells [22]. A subsequent metabolic analysis of GCH1 overexpression cells indicated that BH4 was responsible for the effective anti-ferroptosis effect of GCH1 overexpression and that the supplementation of GCH1 knockout cells with BH2/BH4 was sufficient to rescue the cells from ferroptosis; however, it was observed that this BH4 function did not considerably influence its protective effect against ferroptosis [54]. In conclusion, GCH1, which acts as a ferroptosis defense system, prevents the onset of ferroptosis and acts independently of known ferroptosis-pathway-related proteins.
References
- Mayneris-Perxachs, J.; Moreno-Navarrete, J.M.; Fernandez-Real, J.M. The role of iron in host-microbiota crosstalk and its effects on systemic glucose metabolism. Nat. Rev. Endocrinol. 2022, 18, 683–698.
- Bogdan, A.R.; Miyazawa, M.; Hashimoto, K.; Tsuji, Y. Regulators of Iron Homeostasis: New Players in Metabolism, Cell Death, and Disease. Trends Biochem. Sci. 2016, 41, 274–286.
- Zhang, C.; Zhang, F. Iron homeostasis and tumorigenesis: Molecular mechanisms and therapeutic opportunities. Protein Cell 2015, 6, 88–100.
- Torti, S.V.; Torti, F.M. Iron: The cancer connection. Mol. Asp. Med. 2020, 75, 100860.
- Ajoolabady, A.; Aslkhodapasandhokmabad, H.; Libby, P.; Tuomilehto, J.; Lip, G.Y.H.; Penninger, J.M.; Richardson, D.R.; Tang, D.; Zhou, H.; Wang, S.; et al. Ferritinophagy and ferroptosis in the management of metabolic diseases. Trends Endocrinol. Metab. 2021, 32, 444–462.
- Holbein, B.E.; Lehmann, C. Dysregulated Iron Homeostasis as Common Disease Etiology and Promising Therapeutic Target. Antioxidants 2023, 12, 671.
- La Rosa, P.; Petrillo, S.; Fiorenza, M.T.; Bertini, E.S.; Piemonte, F. Ferroptosis in Friedreich’s Ataxia: A Metal-Induced Neurodegenerative Disease. Biomolecules 2020, 10, 1551.
- Ursini, F.; Maiorino, M. Lipid peroxidation and ferroptosis: The role of GSH and GPx4. Free Radic. Biol. Med. 2020, 152, 175–185.
- Li, J.; Cao, F.; Yin, H.L.; Huang, Z.J.; Lin, Z.T.; Mao, N.; Sun, B.; Wang, G. Ferroptosis: Past, present and future. Cell Death Dis. 2020, 11, 88.
- Gordan, R.; Wongjaikam, S.; Gwathmey, J.K.; Chattipakorn, N.; Chattipakorn, S.C.; Xie, L.H. Involvement of cytosolic and mitochondrial iron in iron overload cardiomyopathy: An update. Heart Fail. Rev. 2018, 23, 801–816.
- Yan, B.; Ai, Y.; Sun, Q.; Ma, Y.; Cao, Y.; Wang, J.; Zhang, Z.; Wang, X. Membrane Damage during Ferroptosis Is Caused by Oxidation of Phospholipids Catalyzed by the Oxidoreductases POR and CYB5R1. Mol. Cell 2021, 81, 355–369.e310.
- Niu, B.; Liao, K.; Zhou, Y.; Wen, T.; Quan, G.; Pan, X.; Wu, C. Application of glutathione depletion in cancer therapy: Enhanced ROS-based therapy, ferroptosis, and chemotherapy. Biomaterials 2021, 277, 121110.
- Matsushita, M.; Freigang, S.; Schneider, C.; Conrad, M.; Bornkamm, G.W.; Kopf, M. T cell lipid peroxidation induces ferroptosis and prevents immunity to infection. J. Exp. Med. 2015, 212, 555–568.
- Jiang, X.; Stockwell, B.R.; Conrad, M. Ferroptosis: Mechanisms, biology and role in disease. Nat. Rev. Mol. Cell Biol. 2021, 22, 266–282.
- Tang, D.; Chen, X.; Kang, R.; Kroemer, G. Ferroptosis: Molecular mechanisms and health implications. Cell Res. 2021, 31, 107–125.
- Moujalled, D.; Strasser, A.; Liddell, J.R. Molecular mechanisms of cell death in neurological diseases. Cell Death Differ. 2021, 28, 2029–2044.
- Yang, W.S.; SriRamaratnam, R.; Welsch, M.E.; Shimada, K.; Skouta, R.; Viswanathan, V.S.; Cheah, J.H.; Clemons, P.A.; Shamji, A.F.; Clish, C.B.; et al. Regulation of ferroptotic cancer cell death by GPX4. Cell 2014, 156, 317–331.
- Bersuker, K.; Hendricks, J.M.; Li, Z.; Magtanong, L.; Ford, B.; Tang, P.H.; Roberts, M.A.; Tong, B.; Maimone, T.J.; Zoncu, R.; et al. The CoQ oxidoreductase FSP1 acts parallel to GPX4 to inhibit ferroptosis. Nature 2019, 575, 688–692.
- Doll, S.; Freitas, F.P.; Shah, R.; Aldrovandi, M.; da Silva, M.C.; Ingold, I.; Grocin, A.G.; da Silva, T.N.X.; Panzilius, E.; Scheel, C.H.; et al. FSP1 is a glutathione-independent ferroptosis suppressor. Nature 2019, 575, 693–698.
- Amos, A.; Amos, A.; Wu, L.; Xia, H. The Warburg effect modulates DHODH role in ferroptosis: A review. Cell Commun. Signal 2023, 21, 100.
- Wang, F.; Min, J. DHODH tangoing with GPX4 on the ferroptotic stage. Signal Transduct. Target. Ther. 2021, 6, 244.
- Kraft, V.A.N.; Bezjian, C.T.; Pfeiffer, S.; Ringelstetter, L.; Müller, C.; Zandkarimi, F.; Merl-Pham, J.; Bao, X.; Anastasov, N.; Kössl, J.; et al. GTP Cyclohydrolase 1/Tetrahydrobiopterin Counteract Ferroptosis through Lipid Remodeling. ACS Cent. Sci. 2020, 6, 41–53.
- Jiang, Y.; Zhao, J.; Li, R.; Liu, Y.; Zhou, L.; Wang, C.; Lv, C.; Gao, L.; Cui, D. CircLRFN5 inhibits the progression of glioblastoma via PRRX2/GCH1 mediated ferroptosis. J. Exp. Clin. Cancer Res. 2022, 41, 307.
- Wang, Y.; Wu, S.; Li, Q.; Sun, H.; Wang, H. Pharmacological Inhibition of Ferroptosis as a Therapeutic Target for Neurodegenerative Diseases and Strokes. Adv. Sci. 2023, 10, e2300325.
- Lei, G.; Zhuang, L.; Gan, B. Targeting ferroptosis as a vulnerability in cancer. Nat. Rev. Cancer 2022, 22, 381–396.
- Xie, L.H.; Fefelova, N.; Pamarthi, S.H.; Gwathmey, J.K. Molecular Mechanisms of Ferroptosis and Relevance to Cardiovascular Disease. Cells 2022, 11, 2726.
- Zhang, Y.; Swanda, R.V.; Nie, L.; Liu, X.; Wang, C.; Lee, H.; Lei, G.; Mao, C.; Koppula, P.; Cheng, W.; et al. mTORC1 couples cyst(e)ine availability with GPX4 protein synthesis and ferroptosis regulation. Nat. Commun. 2021, 12, 1589.
- Chen, X.; Yu, C.; Kang, R.; Kroemer, G.; Tang, D. Cellular degradation systems in ferroptosis. Cell Death Differ. 2021, 28, 1135–1148.
- Kim, S.; Kang, S.W.; Joo, J.; Han, S.H.; Shin, H.; Nam, B.Y.; Park, J.; Yoo, T.H.; Kim, G.; Lee, P.; et al. Characterization of ferroptosis in kidney tubular cell death under diabetic conditions. Cell Death Dis. 2021, 12, 160.
- Jia, M.; Qin, D.; Zhao, C.; Chai, L.; Yu, Z.; Wang, W.; Tong, L.; Lv, L.; Wang, Y.; Rehwinkel, J.; et al. Redox homeostasis maintained by GPX4 facilitates STING activation. Nat. Immunol. 2020, 21, 727–735.
- Wu, J.; Wang, Y.; Jiang, R.T.; Xue, R.; Yin, X.H.; Wu, M.C.; Meng, Q.H. Ferroptosis in liver disease: New insights into disease mechanisms. Cell Death Discov. 2021, 7, 276.
- Hangauer, M.J.; Viswanathan, V.S.; Ryan, M.J.; Bole, D.; Eaton, J.K.; Matov, A.; Galeas, J.; Dhruv, H.D.; Berens, M.E.; Schreiber, S.L.; et al. Drug-tolerant persister cancer cells are vulnerable to GPX4 inhibition. Nature 2017, 551, 247–250.
- Hambright, W.S.; Fonseca, R.S.; Chen, L.; Na, R.; Ran, Q. Ablation of ferroptosis regulator glutathione peroxidase 4 in forebrain neurons promotes cognitive impairment and neurodegeneration. Redox Biol. 2017, 12, 8–17.
- Zhang, J.; Bi, J.B.; Ren, Y.F.; Du, Z.Q.; Li, T.; Wang, T.; Zhang, L.; Wang, M.Z.; Wei, S.S.; Lv, Y.; et al. Involvement of GPX4 in irisin’s protection against ischemia reperfusion-induced acute kidney injury. J. Cell Physiol. 2021, 236, 931–945.
- Wu, J.; Minikes, A.M.; Gao, M.; Bian, H.; Li, Y.; Stockwell, B.R.; Chen, Z.N.; Jiang, X. Intercellular interaction dictates cancer cell ferroptosis via NF2-YAP signalling. Nature 2019, 572, 402–406.
- Zou, Y.; Palte, M.J.; Deik, A.A.; Li, H.; Eaton, J.K.; Wang, W.; Tseng, Y.Y.; Deasy, R.; Kost-Alimova, M.; Dancik, V.; et al. A GPX4-dependent cancer cell state underlies the clear-cell morphology and confers sensitivity to ferroptosis. Nat. Commun. 2019, 10, 1617.
- Viswanathan, V.S.; Ryan, M.J.; Dhruv, H.D.; Gill, S.; Eichhoff, O.M.; Seashore-Ludlow, B.; Kaffenberger, S.D.; Eaton, J.K.; Shimada, K.; Aguirre, A.J.; et al. Dependency of a therapy-resistant state of cancer cells on a lipid peroxidase pathway. Nature 2017, 547, 453–457.
- Tsoi, J.; Robert, L.; Paraiso, K.; Galvan, C.; Sheu, K.M.; Lay, J.; Wong, D.J.L.; Atefi, M.; Shirazi, R.; Wang, X.; et al. Multi-stage Differentiation Defines Melanoma Subtypes with Differential Vulnerability to Drug-Induced Iron-Dependent Oxidative Stress. Cancer Cell 2018, 33, 890–904.e895.
- Jin, D.Y.; Chen, X.; Liu, Y.; Williams, C.M.; Pedersen, L.C.; Stafford, D.W.; Tie, J.K. A genome-wide CRISPR-Cas9 knockout screen identifies FSP1 as the warfarin-resistant vitamin K reductase. Nat. Commun. 2023, 14, 828.
- Xavier da Silva, T.N.; Schulte, C.; Alves, A.N.; Maric, H.M.; Friedmann Angeli, J.P. Molecular characterization of AIFM2/FSP1 inhibition by iFSP1-like molecules. Cell Death Dis. 2023, 14, 281.
- Hendricks, J.M.; Doubravsky, C.E.; Wehri, E.; Li, Z.; Roberts, M.A.; Deol, K.K.; Lange, M.; Lasheras-Otero, I.; Momper, J.D.; Dixon, S.J.; et al. Identification of structurally diverse FSP1 inhibitors that sensitize cancer cells to ferroptosis. Cell Chem. Biol. 2023, in press.
- Koppula, P.; Lei, G.; Zhang, Y.; Yan, Y.; Mao, C.; Kondiparthi, L.; Shi, J.; Liu, X.; Horbath, A.; Das, M.; et al. A targetable CoQ-FSP1 axis drives ferroptosis- and radiation-resistance in KEAP1 inactive lung cancers. Nat. Commun. 2022, 13, 2206.
- Nakamura, T.; Hipp, C.; Santos Dias Mourao, A.; Borggrafe, J.; Aldrovandi, M.; Henkelmann, B.; Wanninger, J.; Mishima, E.; Lytton, E.; Emler, D.; et al. Phase separation of FSP1 promotes ferroptosis. Nature 2023, 619, 371–377.
- Li, W.; Liang, L.; Liu, S.; Yi, H.; Zhou, Y. FSP1: A key regulator of ferroptosis. Trends Mol. Med. 2023, 29, 753–764.
- Wang, S.; Li, W.; Zhang, P.; Wang, Z.; Ma, X.; Liu, C.; Vasilev, K.; Zhang, L.; Zhou, X.; Liu, L.; et al. Mechanical overloading induces GPX4-regulated chondrocyte ferroptosis in osteoarthritis via Piezo1 channel facilitated calcium influx. J. Adv. Res. 2022, 41, 63–75.
- Guo, J.; Wang, R.; Min, F. Ginsenoside Rg1 ameliorates sepsis-induced acute kidney injury by inhibiting ferroptosis in renal tubular epithelial cells. J. Leukoc. Biol. 2022, 112, 1065–1077.
- Tonnus, W.; Meyer, C.; Steinebach, C.; Belavgeni, A.; von Massenhausen, A.; Gonzalez, N.Z.; Maremonti, F.; Gembardt, F.; Himmerkus, N.; Latk, M.; et al. Dysfunction of the key ferroptosis-surveilling systems hypersensitizes mice to tubular necrosis during acute kidney injury. Nat. Commun. 2021, 12, 4402.
- Li, L.; Ng, S.R.; Colón, C.I.; Drapkin, B.J.; Hsu, P.P.; Li, Z.; Nabel, C.S.; Lewis, C.A.; Romero, R.; Mercer, K.L.; et al. Identification of DHODH as a therapeutic target in small cell lung cancer. Sci. Transl. Med. 2019, 11, eaaw7852.
- Madak, J.T.; Bankhead, A., 3rd; Cuthbertson, C.R.; Showalter, H.D.; Neamati, N. Revisiting the role of dihydroorotate dehydrogenase as a therapeutic target for cancer. Pharmacol. Ther. 2019, 195, 111–131.
- Abt, E.R.; Rosser, E.W.; Durst, M.A.; Lok, V.; Poddar, S.; Le, T.M.; Cho, A.; Kim, W.; Wei, L.; Song, J.; et al. Metabolic Modifier Screen Reveals Secondary Targets of Protein Kinase Inhibitors within Nucleotide Metabolism. Cell Chem. Biol. 2020, 27, 197–205.e6.
- Vasan, K.; Werner, M.; Chandel, N.S. Mitochondrial Metabolism as a Target for Cancer Therapy. Cell Metab. 2020, 32, 341–352.
- Mao, C.; Liu, X.G.; Zhang, Y.L.; Lei, G.; Yan, Y.L.; Lee, H.M.; Koppula, P.; Wu, S.Q.; Zhuang, L.; Fang, B.L.; et al. DHODH-mediated ferroptosis defence is a targetable vulnerability in cancer. Nature 2021, 596, 586–590.
- Eichwald, T.; da Silva, L.B.; Staats Pires, A.C.; Niero, L.; Schnorrenberger, E.; Filho, C.C.; Espíndola, G.; Huang, W.L.; Guillemin, G.J.; Abdenur, J.E.; et al. Tetrahydrobiopterin: Beyond Its Traditional Role as a Cofactor. Antioxidants 2023, 12, 1037.
- Soula, M.; Weber, R.A.; Zilka, O.; Alwaseem, H.; La, K.; Yen, F.; Molina, H.; Garcia-Bermudez, J.; Pratt, D.A.; Birsoy, K. Metabolic determinants of cancer cell sensitivity to canonical ferroptosis inducers. Nat. Chem. Biol. 2020, 16, 1351–1360.
- Lv, Y.; Wu, M.; Wang, Z.; Wang, J. Ferroptosis: From regulation of lipid peroxidation to the treatment of diseases. Cell Biol. Toxicol. 2022, 39, 827–851.
- Xue, J.; Yu, C.; Sheng, W.; Zhu, W.; Luo, J.; Zhang, Q.; Yang, H.; Cao, H.; Wang, W.; Zhou, J.; et al. The Nrf2/GCH1/BH4 Axis Ameliorates Radiation-Induced Skin Injury by Modulating the ROS Cascade. J. Investig. Derm. 2017, 137, 2059–2068.
- Xu, L.; Liu, Y.; Chen, X.; Zhong, H.; Wang, Y. Ferroptosis in life: To be or not to be. Biomed. Pharm. 2023, 159, 114241.
More
Information
Subjects:
Neurosciences
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
436
Revisions:
2 times
(View History)
Update Date:
21 Sep 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




