
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Upendar Rao Golla | -- | 3056 | 2023-09-20 07:38:57 | | | |
| 2 | Peter Tang | Meta information modification | 3056 | 2023-09-20 07:48:17 | | |
Video Upload Options
Selenium is an essential, naturally occurring trace mineral element, implicated in a diverse set of biological processes that impact health and disease. Supplementing chemotherapy and radiotherapy with selenium has been shown to have benefits against various cancers. This approach has also been shown to alleviate the side effects associated with standard cancer therapies and improve the quality of life in patients.
1. Introduction
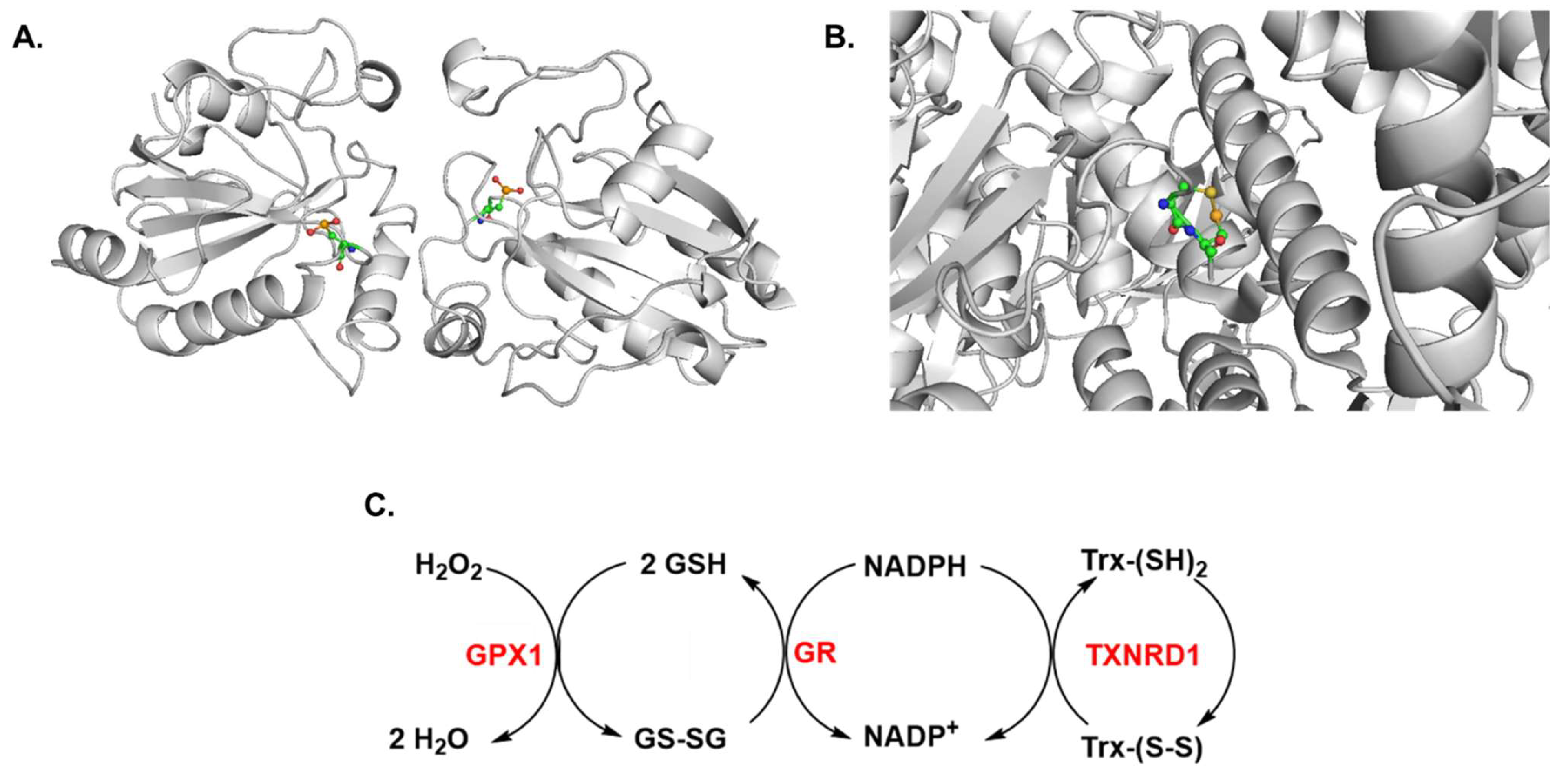
2. Regulation of Signaling Pathways by Selenium in Cancer
2.1. Selenoproteins Play a Role in Cancer
2.2. Role of Selenoproteins in Hematological Malignancies
2.3. Inorganic and Organic Selenium Compounds Exert Therapeutic Activities
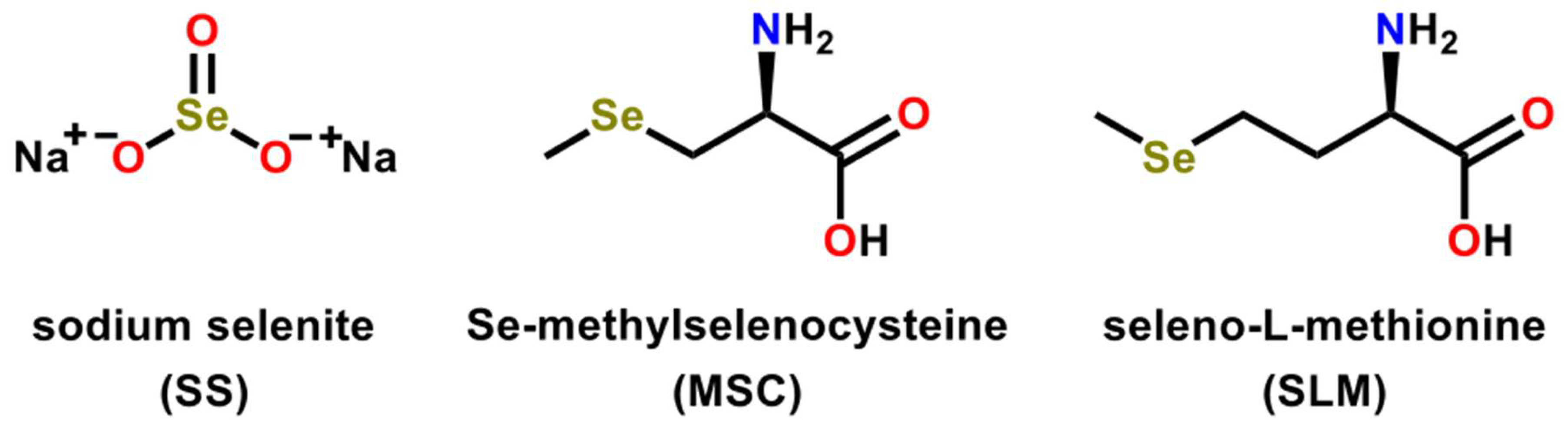
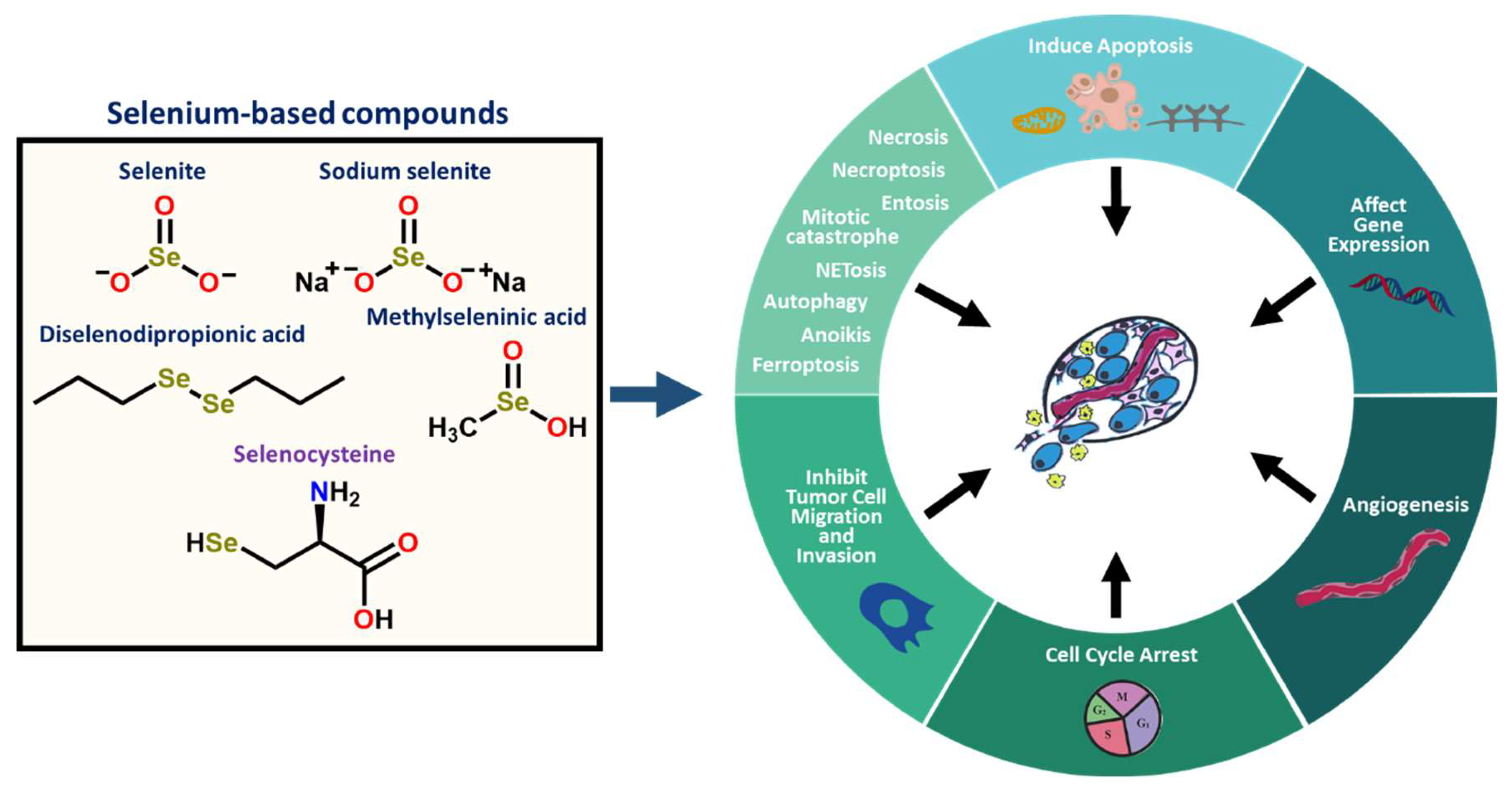
2.4. Selenium-Based Compounds or Proteins Act by Various Modes of Action
|
S. No. |
Compound |
Redox Property |
Cytotoxicity Mechanism |
References |
|---|---|---|---|---|
|
1 |
Selenate |
Proapoptotic, genotoxic |
Activates protein phosphatase 2A, which inhibits various signaling cascades such as phosphatidylinositol 3-kinase (PI3K)/Akt pathway. Induces apoptosis, but at a relatively high concentration. |
[78] |
|
2 |
Selenite/Sodium selenite (SS) |
Proapoptotic, prooxidative, genotoxic, inhibits cell proliferation |
Activation of extracellular signal-regulated protein kinase (ERK) pathway. Inhibition of autophagy through PI3K/Akt pathway |
|
|
3 |
Selenocysteine (SeCys) |
Antioxidant |
Induces apoptosis through the cell cycle arrest, and oxidative damage. Paraptotic-like effect mediated by ER stress. |
|
|
4 |
Selenomethionine (SeMet) |
Proapoptotic, proliferation inhibition |
Pro-apoptotic effects in several cancer cell lines. Activation of p53-dependent proteins. Non-toxic and non-genotoxic. |
|
|
5 |
Methylselenocysteine (MSC) |
Proapoptotic, anti-angiogenic, proliferation inhibition |
Anti-cancer effects in various cell lines, including promyelocytic leukemia. ER stress and mitochondrial dysfunction/signaling |
|
|
6 |
Methylselenic acid (MSA) |
Pro-apoptotic, anti-inflammatory, pro-oxidant, anti-angiogenic |
Induces cytotoxicity through DNA damage. Regulation of PI3k/Akt, ERK1/2, and p38 pathways |
|
|
7 |
Selenodiglutathione (SDG) |
Antioxidant, pro-apoptotic |
Induction of apoptosis through ROS and oxidative damage. |
[87] |
|
8 |
Methylselenol |
Pro-apoptotic; inhibits cell growth |
Inhibition of the ERK1/2 pathway activation and c-Myc expression. Induces cell cycle arrest. |
[88] |
|
9 |
Ebselen |
Anti-inflammatory, antioxidant, protects against oxidative stress as well as DNA damage |
As an antioxidant, Ebselen induces apoptosis through many pathways. Induces ROS generation and oxidative damage |
[89] |
|
10 |
Ethaselen |
Proliferation suppression, synergistically effective with cisplatin against resistant leukemic cells |
Induces ROS and apoptosis by TrxR inhibition |
|
|
11 |
Dimethyl diselenide |
Antioxidant |
Induces NADPH quinone oxidoreductase |
[92] |
|
12 |
Zidovudine derivatives |
Pro-apoptotic |
Induced apoptosis through the mitochondrial pathway |
[93] |
|
13 |
Phenylindolyl Ketone Derivative |
Pro-apoptotic |
Induced apoptosis through cell cycle arrest and inhibition of tubulin polymerization. |
[94] |
|
14 |
Combretastatin 4-A analog |
Inhibit tubulin polymerization |
Inhibition of cell growth. |
[95] |
|
15 |
Diphenyl diselenide |
Antioxidant, inhibitor of nociception |
Protective against genotoxic substances. Induces apoptosis through oxidative damage. |
|
|
16 |
Selol |
Cytotoxic effects, inhibits proliferation, apoptotic |
Induced apoptosis in resistant cancer cell lines including leukemia through oxidative damage. |
[98] |
References
- Sinha, R.; El-Bayoumy, K. Apoptosis is a critical cellular event in cancer chemoprevention and chemotherapy by selenium compounds. Curr. Cancer Drug Targets 2004, 4, 13–28.
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2016. CA Cancer J. Clin. 2016, 66, 7–30.
- Zugazagoitia, J.; Guedes, C.; Ponce, S.; Ferrer, I.; Molina-Pinelo, S.; Paz-Ares, L. Current Challenges in Cancer Treatment. Clin. Ther. 2016, 38, 1551–1566.
- Percival, M.-E.M.; Tao, L.; Medeiros, B.C.; Clarke, C.A. Improvements in the early death rate among 9380 patients with acute myeloid leukemia after initial therapy: A SEER database analysis. Cancer 2015, 121, 2004–2012.
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674.
- Hanahan, D.; Weinberg, R.A. The hallmarks of cancer. Cell 2000, 100, 57–70.
- Miller, K.D.; Nogueira, L.; Mariotto, A.B.; Rowland, J.H.; Yabroff, K.R.; Alfano, C.M.; Jemal, A.; Kramer, J.L.; Siegel, R.L. Cancer treatment and survivorship statistics, 2019. CA Cancer J. Clin. 2019, 69, 363–385.
- Newcomb, P.A.; Carbone, P.P. Cancer Treatment and Age: Patient Perspectives. JNCI J. Natl. Cancer Inst. 1993, 85, 1580–1584.
- Pui, C.H.; Mullighan, C.G.; Evans, W.E.; Relling, M.V. Pediatric acute lymphoblastic leukemia: Where are we going and how do we get there? Blood 2012, 120, 1165–1174.
- Rayman, M.P. The importance of selenium to human health. Lancet 2000, 356, 233–241.
- Patra, A.R.; Hajra, S.; Baral, R.; Bhattacharya, S. Use of selenium as micronutrients and for future anticancer drug: A review. Nucleus 2020, 63, 107–118.
- Hatfield, D.L.; Tsuji, P.A.; Carlson, B.A.; Gladyshev, V.N. Selenium and selenocysteine: Roles in cancer, health, and development. Trends Biochem. Sci. 2014, 39, 112–120.
- Rayman, M.P. Selenium and human health. Lancet 2012, 379, 1256–1268.
- Kieliszek, M.; Błażejak, S. Current Knowledge on the Importance of Selenium in Food for Living Organisms: A Review. Molecules 2016, 21, 609.
- Reich, H.J.; Hondal, R.J. Why Nature Chose Selenium. ACS Chem. Biol. 2016, 11, 821–841.
- Holmgren, A.; Lu, J. Thioredoxin and thioredoxin reductase: Current research with special reference to human disease. Biochem. Biophys. Res. Commun. 2010, 396, 120–124.
- Abdalla, M.A.; Sulieman, S.; Muhling, K.H. Regulation of Selenium/Sulfur Interactions to Enhance Chemopreventive Effects: Lessons to Learn from Brassicaceae. Molecules 2020, 25, 5846.
- Meyer, J.; Moulis, J.M.; Gaillard, J.; Lutz, M. Replacement of Sulfur by Selenium in Iron-Sulfur Proteins. Adv. Inorg. Chem. 1992, 38, 73–115.
- Ip, C.; Ganther, H.E. Comparison of selenium and sulfur analogs in cancer prevention. Carcinogenesis 1992, 13, 1167–1170.
- Schweizer, U.; Fradejas-Villar, N. Why 21? The significance of selenoproteins for human health revealed by inborn errors of metabolism. FASEB J. 2016, 30, 3669–3681.
- Wu, W.; Li, D.; Feng, X.; Zhao, F.; Li, C.; Zheng, S.; Lyu, J. A pan-cancer study of selenoprotein genes as promising targets for cancer therapy. BMC Med. Genomics 2021, 14, 78.
- Harris, I.S.; Treloar, A.E.; Inoue, S.; Sasaki, M.; Gorrini, C.; Lee, K.C.; Yung, K.Y.; Brenner, D.; Knobbe-Thomsen, C.B.; Cox, M.A.; et al. Glutathione and thioredoxin antioxidant pathways synergize to drive cancer initiation and progression. Cancer Cell 2015, 27, 211–222.
- Jablonska, E.; Vinceti, M. Selenium and Human Health: Witnessing a Copernican Revolution? J. Environ. Sci. Health C Environ. Carcinog. Ecotoxicol. Rev. 2015, 33, 328–368.
- Vinceti, M.; Filippini, T.; Del Giovane, C.; Dennert, G.; Zwahlen, M.; Brinkman, M.; Zeegers, M.P.; Horneber, M.; D’Amico, R.; Crespi, C.M. Selenium for preventing cancer. Cochrane Database Syst. Rev. 2018, 1, CD005195.
- Vinceti, M.; Filippini, T.; Cilloni, S.; Crespi, C.M. The Epidemiology of Selenium and Human Cancer. Adv. Cancer Res. 2017, 136, 1–48.
- Vinceti, M.; Filippini, T.; Wise, L.A.; Rothman, K.J. A systematic review and dose-response meta-analysis of exposure to environmental selenium and the risk of type 2 diabetes in nonexperimental studies. Environ. Res. 2021, 197, 111210.
- Kuršvietienė, L.; Mongirdienė, A.; Bernatonienė, J.; Šulinskienė, J.; Stanevičienė, I. Selenium Anticancer Properties and Impact on Cellular Redox Status. Antioxidants 2020, 9, 80.
- Liu, L.L.; Zhang, J.L.; Zhang, Z.W.; Yao, H.D.; Sun, G.; Xu, S.W. Protective roles of selenium on nitric oxide-mediated apoptosis of immune organs induced by cadmium in chickens. Biol. Trace Elem. Res. 2014, 159, 199–209.
- Prabhu, K.S.; Zamamiri-Davis, F.; Stewart, J.B.; Thompson, J.T.; Sordillo, L.M.; Reddy, C.C. Selenium deficiency increases the expression of inducible nitric oxide synthase in RAW 264.7 macrophages: Role of nuclear factor-kappaB in up-regulation. Biochem. J. 2002, 366, 203–209.
- The PyMOL Molecular Graphics System, Version 2.2 Schrödinger, LLC. Available online: https://pymol.org/ (accessed on 28 June 2021).
- Papp, L.V.; Lu, J.; Holmgren, A.; Khanna, K.K. From selenium to selenoproteins: Synthesis, identity, and their role in human health. Antioxid. Redox Signal 2007, 9, 775–806.
- Allan, C.B.; Lacourciere, G.M.; Stadtman, T.C. Responsiveness of selenoproteins to dietary selenium. Annu. Rev. Nutr. 1999, 19, 1–16.
- Rayman, M.P. Selenium in cancer prevention: A review of the evidence and mechanism of action. Proc. Nutr. Soc. 2005, 64, 527–542.
- Traverso, N.; Ricciarelli, R.; Nitti, M.; Marengo, B.; Furfaro, A.L.; Pronzato, M.A.; Marinari, U.M.; Domenicotti, C. Role of glutathione in cancer progression and chemoresistance. Oxid. Med. Cell. Longev. 2013, 2013, 972913.
- Handa, E.; Puspitasari, I.M.; Abdulah, R.; Yamazaki, C.; Kameo, S.; Nakano, T.; Koyama, H. Recent advances in clinical studies of selenium supplementation in radiotherapy. J. Trace Elem. Med. Biol. 2020, 62, 126653.
- Muecke, R.; Schomburg, L.; Buentzel, J.; Kisters, K.; Micke, O. Selenium or no selenium--that is the question in tumor patients: A new controversy. Integr. Cancer Ther. 2010, 9, 136–141.
- Shaaban, Y.; Aref, S.; Taalab, M.; Ayed, M.; Mabed, M. Implications of Glutathione Peroxidase 3 Expression in a Cohort of Egyptian Patients with Acute Myeloid Leukemia. Asian Pac. J. Cancer Prev. 2020, 21, 3567–3572.
- Xie, W.; Ma, W.; Liu, P.; Zhou, F. Overview of thioredoxin system and targeted therapies for acute leukemia. Mitochondrion 2019, 47, 38–46.
- Wang, X.; Gao, M.; Zhang, J.; Ma, Y.; Qu, W.; Liang, J.; Wu, H.; Wen, H. Peperomin E and its orally bioavailable analog induce oxidative stress-mediated apoptosis of acute myeloid leukemia progenitor cells by targeting thioredoxin reductase. Redox Biol. 2019, 24, 101153.
- Zhou, F.; Pan, Y.; Wei, Y.; Zhang, R.; Bai, G.; Shen, Q.; Meng, S.; Le, X.-F.; Andreeff, M.; Claret, F.X. Jab1/Csn5–Thioredoxin Signaling in Relapsed Acute Monocytic Leukemia under Oxidative Stress. Clin. Cancer Res. 2017, 23, 4450–4461.
- Fedirko, V.; Jenab, M.; Meplan, C.; Jones, J.S.; Zhu, W.; Schomburg, L.; Siddiq, A.; Hybsier, S.; Overvad, K.; Tjonneland, A.; et al. Association of Selenoprotein and Selenium Pathway Genotypes with Risk of Colorectal Cancer and Interaction with Selenium Status. Nutrients 2019, 11, 935.
- Brown, K.M.; Arthur, J.R. Selenium, selenoproteins and human health: A review. Public Health Nutr. 2001, 4, 593–599.
- Hatfield, D.L.; Yoo, M.H.; Carlson, B.A.; Gladyshev, V.N. Selenoproteins that function in cancer prevention and promotion. Biochim. Biophys. Acta 2009, 1790, 1541–1545.
- Kaweme, N.M.; Zhou, S.; Changwe, G.J.; Zhou, F. The significant role of redox system in myeloid leukemia: From pathogenesis to therapeutic applications. Biomark. Res. 2020, 8, 63.
- Conrad, M.; Jakupoglu, C.; Moreno, S.G.; Lippl, S.; Banjac, A.; Schneider, M.; Beck, H.; Hatzopoulos, A.K.; Just, U.; Sinowatz, F.; et al. Essential role for mitochondrial thioredoxin reductase in hematopoiesis, heart development, and heart function. Mol. Cell. Biol. 2004, 24, 9414–9423.
- Yang, Y.; Li, D.; Wu, W.; Huang, D.; Zheng, H.; Aihaiti, Y. A Pan-Cancer Analysis of the Role of Selenoprotein P mRNA in Tumorigenesis. Int. J. Gen. Med. 2021, 14, 7471–7485.
- Wei, J.; Xie, Q.; Liu, X.; Wan, C.; Wu, W.; Fang, K.; Yao, Y.; Cheng, P.; Deng, D.; Liu, Z. Identification the prognostic value of glutathione peroxidases expression levels in acute myeloid leukemia. Ann. Transl. Med. 2020, 8, 678.
- Kinowaki, Y.; Kurata, M.; Ishibashi, S.; Ikeda, M.; Tatsuzawa, A.; Yamamoto, M.; Miura, O.; Kitagawa, M.; Yamamoto, K. Glutathione peroxidase 4 overexpression inhibits ROS-induced cell death in diffuse large B-cell lymphoma. Lab. Investig. 2018, 98, 609–619.
- Taguchi, T.; Kurata, M.; Onishi, I.; Kinowaki, Y.; Sato, Y.; Shiono, S.; Ishibashi, S.; Ikeda, M.; Yamamoto, M.; Kitagawa, M.; et al. SECISBP2 is a novel prognostic predictor that regulates selenoproteins in diffuse large B-cell lymphoma. Lab. Investig. 2021, 101, 218–227.
- Eagle, K.; Jiang, Y.; Shi, X.; Li, M.; Obholzer, N.P.; Hu, T.; Perez, M.W.; Koren, J.V.; Kitano, A.; Yi, J.S.; et al. An oncogenic enhancer encodes selective selenium dependency in AML. Cell Stem Cell 2022, 29, 386–399.e387.
- Ali, W.; Benedetti, R.; Handzlik, J.; Zwergel, C.; Battistelli, C. The innovative potential of selenium-containing agents for fighting cancer and viral infections. Drug Discov. Today 2021, 26, 256–263.
- Chuai, H.; Zhang, S.-Q.; Bai, H.; Li, J.; Wang, Y.; Sun, J.; Wen, E.; Zhang, J.; Xin, M. Small molecule Selenium-Containing Compounds: Recent Development and Therapeutic Applications. Eur. J. Med. Chem. 2021, 223, 113621.
- Radomska, D.; Czarnomysy, R.; Radomski, D.; Bielawski, K. Selenium Compounds as Novel Potential Anticancer Agents. Int. J. Mol. Sci. 2021, 22, 1009.
- Soriano-Garcia, M. Organoselenium compounds as potential therapeutic and chemopreventive agents: A review. Curr. Med. Chem. 2004, 11, 1657–1669.
- Zeng, H.; Wu, M. The Inhibitory Efficacy of Methylseleninic Acid Against Colon Cancer Xenografts in C57BL/6 Mice. Nutr. Cancer 2015, 67, 831–838.
- Powers, M.; Liu, L.; Deemer, D.; Chen, S.; Scholl, A.; Yoshinaga, M.; Liu, Z. Selenite Inhibits Notch Signaling in Cells and Mice. Int. J. Mol. Sci. 2021, 22, 2518.
- Liu, X.; He, S.; Peng, J.; Guo, X.; Tan, W. Expression Profile Analysis of Selenium-Related Genes in Peripheral Blood Mononuclear Cells of Patients with Keshan Disease. Bio. Med. Res. Int. 2019, 2019, 4352905.
- Spengler, G.; Gajdács, M.; Marć, M.A.; Domínguez-Álvarez, E.; Sanmartín, C. Organoselenium Compounds as Novel Adjuvants of Chemotherapy Drugs—A Promising Approach to Fight Cancer Drug Resistance. Molecules 2019, 24, 336.
- Jahangard-Rafsanjani, Z.; Gholami, K.; Hadjibabaie, M.; Shamshiri, A.R.; Alimoghadam, K.; Sarayani, A.; Mojtahedzadeh, M.; Ostadali-Dehaghi, M.; Ghavamzadeh, A. The efficacy of selenium in prevention of oral mucositis in patients undergoing hematopoietic SCT: A randomized clinical trial. Bone Marrow Transplant. 2013, 48, 832–836.
- Muecke, R.; Schomburg, L.; Glatzel, M.; Berndt-Skorka, R.; Baaske, D.; Reichl, B.; Buentzel, J.; Kundt, G.; Prott, F.J.; Devries, A.; et al. Multicenter, phase 3 trial comparing selenium supplementation with observation in gynecologic radiation oncology. Int. J. Radiat. Oncol. Biol. Phys. 2010, 78, 828–835.
- Varela-Lopez, A.; Battino, M.; Navarro-Hortal, M.D.; Giampieri, F.; Forbes-Hernandez, T.Y.; Romero-Marquez, J.M.; Collado, R.; Quiles, J.L. An update on the mechanisms related to cell death and toxicity of doxorubicin and the protective role of nutrients. Food Chem. Toxicol. 2019, 134, 110834.
- Cao, S.; Durrani, F.A.; Tóth, K.; Rustum, Y.M. Se-methylselenocysteine offers selective protection against toxicity and potentiates the antitumour activity of anticancer drugs in preclinical animal models. Br. J. Cancer 2014, 110, 1733–1743.
- Evans, S.O.; Khairuddin, P.F.; Jameson, M.B. Optimising Selenium for Modulation of Cancer Treatments. Anticancer Res. 2017, 37, 6497–6509.
- Evans, S.O.; Jacobson, G.M.; Goodman, H.J.B.; Bird, S.; Jameson, M.B. Comparative Safety and Pharmacokinetic Evaluation of Three Oral Selenium Compounds in Cancer Patients. Biol. Trace Elem. Res. 2019, 189, 395–404.
- Ip, C.; Hayes, C.; Budnick, R.M.; Ganther, H.E. Chemical form of selenium, critical metabolites, and cancer prevention. Cancer Res. 1991, 51, 595–600.
- Pinto, J.T.; Krasnikov, B.F.; Alcutt, S.; Jones, M.E.; Dorai, T.; Villar, M.T.; Artigues, A.; Li, J.; Cooper, A.J. Kynurenine aminotransferase III and glutamine transaminase L are identical enzymes that have cysteine S-conjugate beta-lyase activity and can transaminate L-selenomethionine. J. Biol. Chem. 2014, 289, 30950–30961.
- Kieliszek, M.; Lipinski, B.; Błażejak, S. Application of Sodium Selenite in the Prevention and Treatment of Cancers. Cells 2017, 6, 39.
- Brodin, O.; Eksborg, S.; Wallenberg, M.; Asker-Hagelberg, C.; Larsen, E.H.; Mohlkert, D.; Lenneby-Helleday, C.; Jacobsson, H.; Linder, S.; Misra, S.; et al. Pharmacokinetics and Toxicity of Sodium Selenite in the Treatment of Patients with Carcinoma in a Phase I Clinical Trial: The SECAR Study. Nutrients 2015, 7, 4978–4994.
- Valdiglesias, V.; Pásaro, E.; Méndez, J.; Laffon, B. In Vitro evaluation of selenium genotoxic, cytotoxic, and protective effects: A review. Arch. Toxicol. 2010, 84, 337–351.
- Soukupová, K.; Rudolf, E. Suppression of proliferation and activation of cell death by sodium selenite involves mitochondria and lysosomes in chemoresistant bladder cancer cells. J. Trace Elem. Med. Biol. 2019, 52, 58–67.
- Goel, A.; Fuerst, F.; Hotchkiss, E.; Boland, C.R. Selenomethionine induces p53 mediated cell cycle arrest and apoptosis in human colon cancer cells. Cancer Biol. Ther. 2006, 5, 529–535.
- Jiang, C.; Wang, Z.; Ganther, H.; Lu, J. Distinct effects of methylseleninic acid versus selenite on apoptosis, cell cycle, and protein kinase pathways in DU145 human prostate cancer cells. Mol. Cancer Ther. 2002, 1, 1059–1066.
- Sanmartín, C.; Plano, D.; Sharma, A.K.; Palop, J.A. Selenium Compounds, Apoptosis and Other Types of Cell Death: An Overview for Cancer Therapy. Int. J. Mol. Sci. 2012, 13, 9649–9672.
- Subburayan, K.; Thayyullathil, F.; Pallichankandy, S.; Cheratta, A.R.; Galadari, S. Superoxide-mediated ferroptosis in human cancer cells induced by sodium selenite. Transl. Oncol. 2020, 13, 100843.
- Ingold, I.; Conrad, M. Selenium and iron, two elemental rivals in the ferroptotic death process. Oncotarget 2018, 9, 22241–22242.
- Chen, Y.-C.; Prabhu, K.S.; Mastro, A.M. Is Selenium a Potential Treatment for Cancer Metastasis? Nutrients 2013, 5, 1149–1168.
- Pazirandeh, A.; Assadi Nejad, M.; Vossogh, P. Determination of selenium in blood serum of children with acute leukemia and effect of chemotherapy on serum selenium level. J. Trace Elem. Med. Biol. 1999, 13, 242–246.
- Tsukamoto, T.; Hama, S.; Kogure, K.; Tsuchiya, H. Selenate induces epithelial-mesenchymal transition in a colorectal carcinoma cell line by AKT activation. Exp. Cell Res. 2013, 319, 1913–1921.
- Han, B.; Wei, W.; Hua, F.; Cao, T.; Dong, H.; Yang, T.; Yang, Y.; Pan, H.; Xu, C. Requirement for ERK activity in sodium selenite-induced apoptosis of acute promyelocytic leukemia-derived NB4 cells. J. Biochem. Mol. Biol. 2007, 40, 196–204.
- Ren, Y.; Huang, F.; Liu, Y.; Yang, Y.; Jiang, Q.; Xu, C. Autophagy inhibition through PI3K/Akt increases apoptosis by sodium selenite in NB4 cells. BMB Rep. 2009, 42, 599–604.
- Fan, C.D.; Fu, X.Y.; Zhang, Z.Y.; Cao, M.Z.; Sun, J.Y.; Yang, M.F.; Fu, X.T.; Zhao, S.J.; Shao, L.R.; Zhang, H.F.; et al. Selenocysteine induces apoptosis in human glioma cells: Evidence for TrxR1-targeted inhibition and signaling crosstalk. Sci. Rep. 2017, 7, 6465.
- Wallenberg, M.; Misra, S.; Wasik, A.M.; Marzano, C.; Bjornstedt, M.; Gandin, V.; Fernandes, A.P. Selenium induces a multi-targeted cell death process in addition to ROS formation. J. Cell. Mol. Med. 2014, 18, 671–684.
- Smith, M.L.; Lancia, J.K.; Mercer, T.I.; Ip, C. Selenium compounds regulate p53 by common and distinctive mechanisms. Anticancer Res. 2004, 24, 1401–1408.
- Gandin, V.; Khalkar, P.; Braude, J.; Fernandes, A.P. Organic selenium compounds as potential chemotherapeutic agents for improved cancer treatment. Free Radic. Biol. Med. 2018, 127, 80–97.
- Suzuki, M.; Endo, M.; Shinohara, F.; Echigo, S.; Rikiishi, H. Differential apoptotic response of human cancer cells to organoselenium compounds. Cancer Chemother. Pharmacol. 2010, 66, 475–484.
- Li, G.X.; Lee, H.J.; Wang, Z.; Hu, H.; Liao, J.D.; Watts, J.C.; Combs, G.F., Jr.; Lu, J. Superior In Vivo inhibitory efficacy of methylseleninic acid against human prostate cancer over selenomethionine or selenite. Carcinogenesis 2008, 29, 1005–1012.
- Tobe, T.; Ueda, K.; Ando, M.; Okamoto, Y.; Kojima, N. Thiol-mediated multiple mechanisms centered on selenodiglutathione determine selenium cytotoxicity against MCF-7 cancer cells. J. Biol. Inorg. Chem. 2015, 20, 687–694.
- Zeng, H.; Wu, M.; Botnen, J.H. Methylselenol, a selenium metabolite, induces cell cycle arrest in G1 phase and apoptosis via the extracellular-regulated kinase 1/2 pathway and other cancer signaling genes. J. Nutr. 2009, 139, 1613–1618.
- Zhang, L.; Zhou, L.; Du, J.; Li, M.; Qian, C.; Cheng, Y.; Peng, Y.; Xie, J.; Wang, D. Induction of apoptosis in human multiple myeloma cell lines by ebselen via enhancing the endogenous reactive oxygen species production. Biomed. Res. Int. 2014, 2014, 696107.
- Tan, Q.; Li, J.; Yin, H.W.; Wang, L.H.; Tang, W.C.; Zhao, F.; Liu, X.M.; Zeng, H.H. Augmented antitumor effects of combination therapy of cisplatin with ethaselen as a novel thioredoxin reductase inhibitor on human A549 cell In Vivo. Investig. New Drugs 2010, 28, 205–215.
- Wang, L.; Yang, Z.; Fu, J.; Yin, H.; Xiong, K.; Tan, Q.; Jin, H.; Li, J.; Wang, T.; Tang, W.; et al. Ethaselen: A potent mammalian thioredoxin reductase 1 inhibitor and novel organoselenium anticancer agent. Free Radic. Biol. Med. 2012, 52, 898–908.
- Alvarez-Perez, M.; Ali, W.; Marc, M.A.; Handzlik, J.; Dominguez-Alvarez, E. Selenides and Diselenides: A Review of Their Anticancer and Chemopreventive Activity. Molecules 2018, 23, 628.
- De Souza, D.; Mariano, D.O.; Nedel, F.; Schultze, E.; Campos, V.F.; Seixas, F.; da Silva, R.S.; Munchen, T.S.; Ilha, V.; Dornelles, L.; et al. New organochalcogen multitarget drug: Synthesis and antioxidant and antitumoral activities of chalcogenozidovudine derivatives. J. Med. Chem. 2015, 58, 3329–3339.
- Zhang, S.; An, B.; Li, J.; Hu, J.; Huang, L.; Li, X.; Chan, A.S.C. Synthesis and evaluation of selenium-containing indole chalcone and diarylketone derivatives as tubulin polymerization inhibition agents. Org. Biomol. Chem. 2017, 15, 7404–7410.
- Dos Santos Edos, A.; Hamel, E.; Bai, R.; Burnett, J.C.; Tozatti, C.S.; Bogo, D.; Perdomo, R.T.; Antunes, A.M.; Marques, M.M.; Matos Mde, F.; et al. Synthesis and evaluation of diaryl sulfides and diaryl selenide compounds for antitubulin and cytotoxic activity. Bioorg. Med. Chem. Lett. 2013, 23, 4669–4673.
- Savegnago, L.; Jesse, C.R.; Nogueira, C.W. Structural modifications into diphenyl diselenide molecule do not cause toxicity in mice. Environ. Toxicol. Pharmacol. 2009, 27, 271–276.
- Ferreira, L.M.; Azambuja, J.H.; da Silveira, E.F.; Marcondes Sari, M.H.; da Cruz Weber Fulco, B.; Costa Prado, V.; Gelsleichter, N.E.; Beckenkamp, L.R.; da Cruz Fernandes, M.; Spanevello, R.M.; et al. Antitumor action of diphenyl diselenide nanocapsules: In Vitro assessments and preclinical evidence in an animal model of glioblastoma multiforme. J. Trace Elem. Med. Biol. 2019, 55, 180–189.
- Suchocki, P.; Misiewicz-Krzeminska, I.; Skupinska, K.; Niedzwiecka, K.; Lubelska, K.; Fijalek, Z.; Kasprzycka-Guttman, T. Selenitetriglicerydes affect CYP1A1 and QR activity by involvement of reactive oxygen species and Nrf2 transcription factor. Pharmacol. Rep. 2010, 62, 352–361.
- Plano, D.; Baquedano, Y.; Ibáñez, E.; Jiménez, I.; Palop, J.A.; Spallholz, J.E.; Sanmartín, C. Antioxidant-Prooxidant Properties of a New Organoselenium Compound Library. Molecules 2010, 15, 7292–7312.
- Chung, H.J.; Yoon, S.I.; Shin, S.H.; Koh, Y.A.; Lee, S.-J.; Lee, Y.-S.; Bae, S. p53-mediated enhancement of radiosensitivity by selenophosphate synthetase 1 overexpression. J. Cell. Phys. 2006, 209, 131–141.
- Fischer, J.L.; Mihelc, E.M.; Pollok, K.E.; Smith, M.L. Chemotherapeutic selectivity conferred by selenium: A role for p53-dependent DNA repair. Mol. Cancer Ther. 2007, 6, 355–361.
- Seo, Y.R.; Sweeney, C.; Smith, M.L. Selenomethionine induction of DNA repair response in human fibroblasts. Oncogene 2002, 21, 3663–3669.
- Seo, Y.R.; Kelley, M.R.; Smith, M.L. Selenomethionine regulation of p53 by a ref1-dependent redox mechanism. Proc. Natl. Acad. Sci. USA 2002, 99, 14548–14553.
- Jayaraman, L.; Murthy, K.G.; Zhu, C.; Curran, T.; Xanthoudakis, S.; Prives, C. Identification of redox/repair protein Ref-1 as a potent activator of p53. Genes Dev. 1997, 11, 558–570.
- Seemann, S.; Hainaut, P. Roles of thioredoxin reductase 1 and APE/Ref-1 in the control of basal p53 stability and activity. Oncogene 2005, 24, 3853–3863.
- Sun, F.; Wang, J.; Wu, X.; Yang, C.S.; Zhang, J. Selenium nanoparticles act as an intestinal p53 inhibitor mitigating chemotherapy-induced diarrhea in mice. Pharmacol. Res. 2019, 149, 104475.
- Cao, S.; Durrani, F.A.; Rustum, Y.M. Selective modulation of the therapeutic efficacy of anticancer drugs by selenium containing compounds against human tumor xenografts. Clin. Cancer Res. 2004, 10, 2561–2569.
- Fakih, M.; Cao, S.; Durrani, F.A.; Rustum, Y.M. Selenium protects against toxicity induced by anticancer drugs and augments antitumor activity: A highly selective, new, and novel approach for the treatment of solid tumors. Clin. Colorectal Cancer 2005, 5, 132–135.
- Reid, M.E.; Stratton, M.S.; Lillico, A.J.; Fakih, M.; Natarajan, R.; Clark, L.C.; Marshall, J.R. A report of high-dose selenium supplementation: Response and toxicities. J. Trace Elem. Med. Biol. 2004, 18, 69–74.
- Wu, J.C.; Wang, F.Z.; Tsai, M.L.; Lo, C.Y.; Badmaev, V.; Ho, C.T.; Wang, Y.J.; Pan, M.H. Se-Allylselenocysteine induces autophagy by modulating the AMPK/mTOR signaling pathway and epigenetic regulation of PCDH17 in human colorectal adenocarcinoma cells. Mol. Nutr. Food Res. 2015, 59, 2511–2522.
- Siebel, C.; Lendahl, U. Notch Signaling in Development, Tissue Homeostasis, and Disease. Physiolog. Rev. 2017, 97, 1235–1294.





