
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Kuo-Hu Chen | -- | 2769 | 2023-09-16 16:23:36 | | | |
| 2 | Peter Tang | Meta information modification | 2769 | 2023-09-18 05:08:17 | | |
Video Upload Options
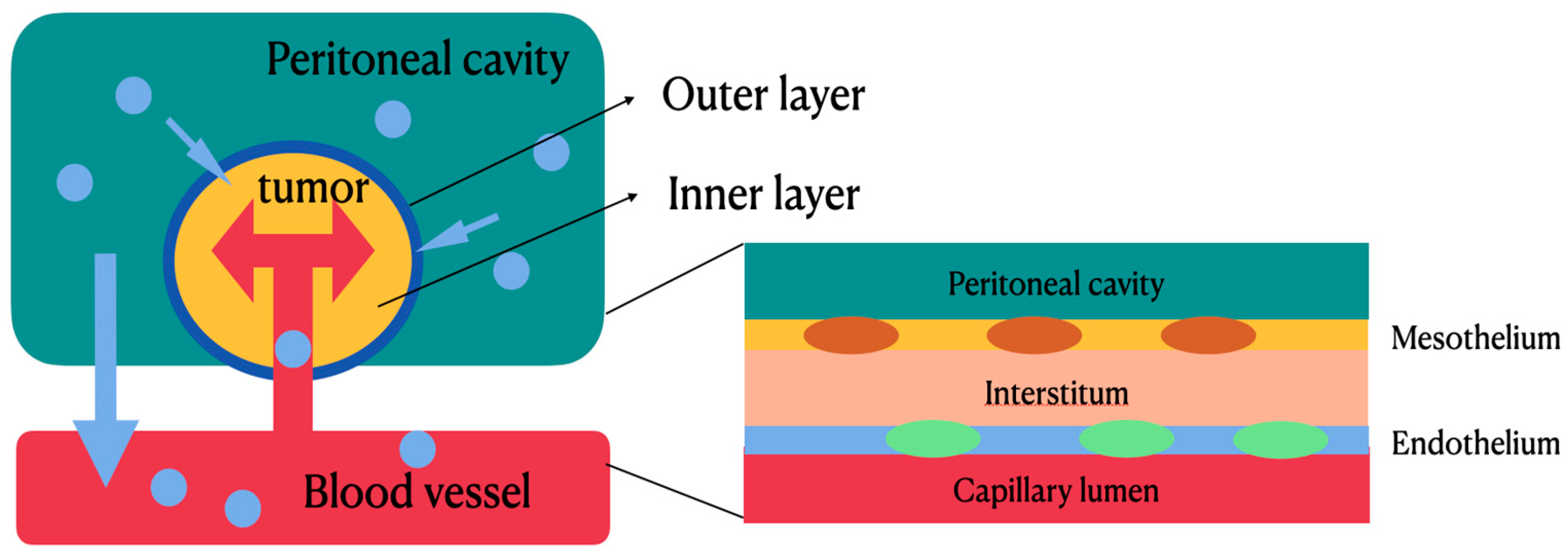
Most patients with epithelial ovarian cancers (EOCs) are at advanced stages (stage III–IV), for which the recurrence rate is high and the 5-year survival rate is low. The most effective treatment for advanced diseases involves a debulking surgery followed by adjuvant intravenous chemotherapy with carboplatin and paclitaxel. Nevertheless, systemic treatment with intravenous chemotherapeutic agents for peritoneal metastasis appears to be less effective due to the poor blood supply to the peritoneal surface with low drug penetration into tumor nodules. Based on this reason, hyperthermic intraperitoneal chemotherapy (HIPEC) emerges as a new therapeutic alternative. By convection and diffusion, the hyperthermic chemotherapeutic agents can directly contact intraperitoneal tumors and produce cytotoxicity. In a two-compartment model, the peritoneal–plasma barrier blocks the leakage of chemotherapeutic agents from peritoneal cavity and tumor tissues to local vessels, thus maintaining a higher concentration of chemotherapeutic agents within the tumor tissues to facilitate tumor apoptosis and a lower concentration of chemotherapeutic agents within the local vessels to decrease systemic toxicity.
1. Introduction
2. Molecular and Cellular Mechanisms of Actions: HIPEC and Chemotherapeutic Agents
2.1. HIPEC
|
Physical parameter, treatment, variable, carrier properties |
|
|
Hydrostatic pressure |
Osmolarity |
|
Temperature |
pH |
|
Viscosity |
Exposure time |
|
Drug properties |
|
|
Concentration Molecular weight Hydrodynamic diameter |
Configuration Water solubility Protein binding Charge ionization |
|
TME properties |
|
|
Interstitial fluid pressure |
Retardation coefficient |
|
Solid pressure |
Cellular composition |
|
Hydraulic conductivity |
Stromal and vascular density |
|
Viscoelasticity, stiffness |
Geometrical arrangement |
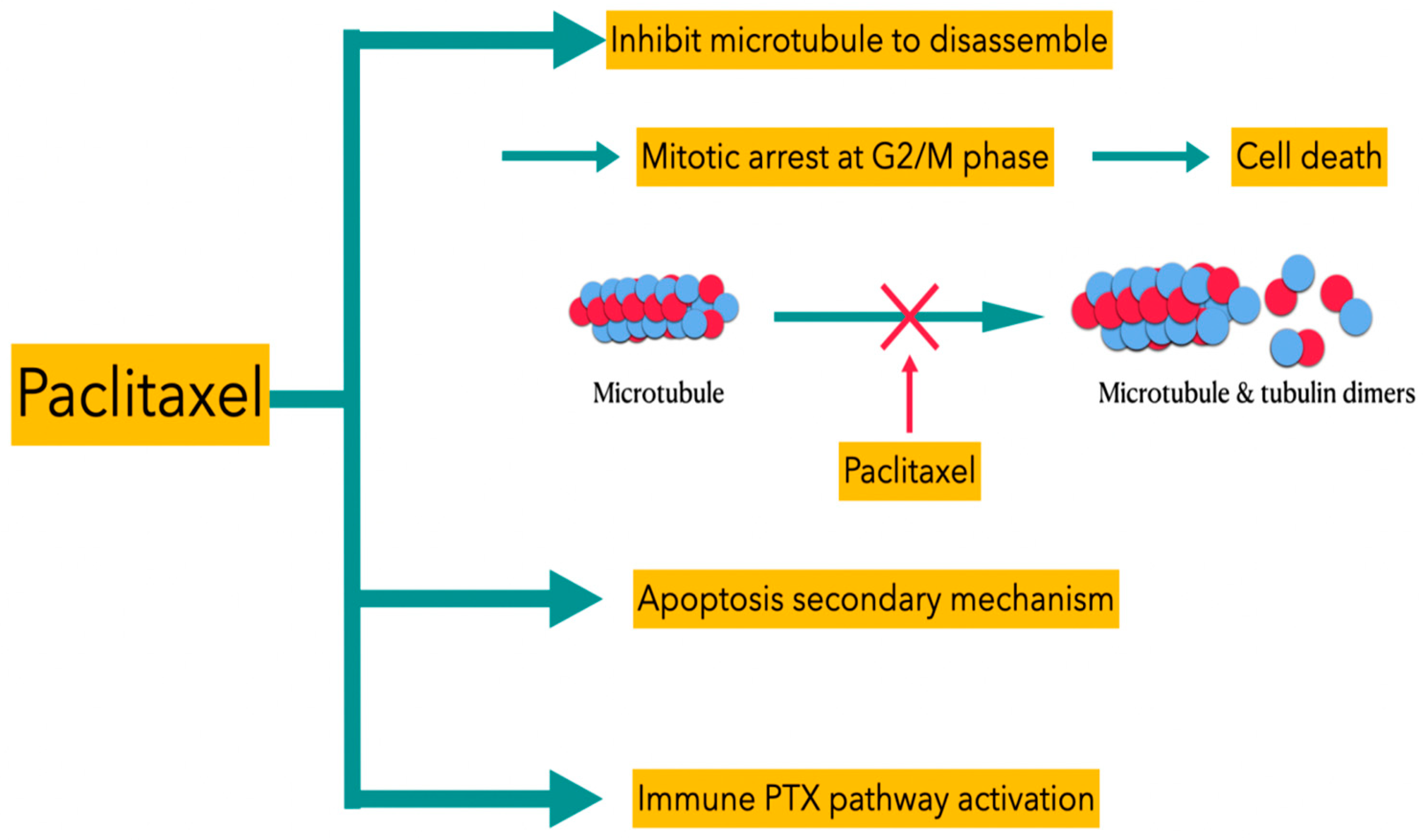
2.2. Paclitaxel
2.3. Cisplatin
3. Therapeutic Effects of HIPEC in Epithelial Ovarian Cancers
|
Authors |
Study Design |
Patients |
Treatment |
Results |
|---|---|---|---|---|
|
Lim MC (2017) |
Prospective, randomized multicenter trial |
1. Patients with stage III/IV primary advanced epithelial ovarian cancer who have optimal cytoreductive surgery; 2. Total: 184 patients. |
Groups: 1. Intraoperative HIPEC with cisplatin (75 mg/m2, 90 min); 2. Control arm (no HIPEC) |
1. HIPEC: 2-year PFS: 43.2%; 5-year PFS: 20.9%; 5-year OS: 51.0%. 2. Control group: 2-year PFS: 43.5% 5-year PFS: 16.0% (p = 0.569) 5-year OS: 49.4% (p = 0.574) |
|
W.J. van Driel (2018) |
Multicenter, prospective, randomized phase III trial |
1. Newly diagnosed stage III epithelial ovarian, fallopian tube, or peritoneal cancers; 2. Not eligible for primary cytoreductive surgery; 3. 245 patients. |
Three cycles of carboplatin (AUC of 5 to 6 mg/mL/min) and paclitaxel (175 mg per square meter of body-surface area) after interval cytoreductive surgery for all patient. Groups: 1. HIPEC with cisplatin (100 mg per square meter), with intra-abdominal temperature of 40 °C (104 °F), open technique; 2. Without HIPEC. |
1. Median follow up: 4.7 years 2. Disease recurrence or death:
3. Median recurrence-free survival (RFS):
4. Median OS:
|
|
Zhang G (2019) |
Meta-analysis including randomized controlled trials and case–control trials |
1. Patients with primary stage III/IV ovarian cancers; 2. 13 comparative studies. |
Groups: 1. Interval CRS plus HIPEC; 2. Primary CRS plus HIPEC; 3. Without HIPEC. |
1. Better outcomes of surgery and HIPEC in patients with primary advanced ovarian cancer (pooled HR for OS: 0.54; 95% CI: 0.45–0.66; pooled HR for PFS: 0.45; 95% CI: 0.32–0.62); 2. Favorable clinical outcome for stage III/IV ovarian cancer with initial diagnosis (HR: 0.64,95% CI: 0.50–0.82, HR: 0.36,95% CI: 0.20–0.65). |
|
Lei Z (2020) |
Multicenter retrospective cohort study |
1. Patients with stage III primary epithelial ovarian cancers; 2. 584 patients. |
1. Closed technique; 2. Circulating heated saline with cisplatin at a dose of 50 mg/m2 3. 43 °C, 60 min. |
Median survival time: 1. HIPEC: 49.8 months; 2. Non-HIPEC 34.0 months
3-year overall survival rate: 1. Surgery + HIPEC: 60.3% (95% CI: 55.3–65.0%). 2. Surgery alone: 49.5% (95% CI: 41.0–57.4%) (weighted HR: 0.64; 95% CI: 0.50–0.82; p < 0.001). |
|
Authors |
Study Design |
Patients |
Treatment |
Results |
|---|---|---|---|---|
|
Cascales-Campos (2011) |
Descriptive study of outcomes in both primary and recurrent epithelial ovarian cancer |
1. Patients previously diagnosed with primary stage IIIc (35 patients) or recurrent ovarian cancer (11 patients) treated using peritonectomy procedures and HIPEC; 2. Total: 46 patients. |
A total of 37 patients (80.4%) received systemic chemotherapy (3–18 cycles per patient) before HIPEC and surgery. Regimen dose of HIPEC: 1. Paclitaxel (60 mg/m2); 2. Cisplatin (75 mg/m2) in taxol-allergic patients 3. 60 min, 42 °C. |
1. Median operation time: 380 min (200–540 min); 2. CC-0 (no macroscopic tumor residue at the end of cytoreduction) achieved in 38 patients (82.6%). |
|
Spiliotis (2015) |
Prospective randomized phase III study |
1. Patients with advanced ovarian cancer (FIGO) IIIc and IV) who experienced disease recurrence after initial treatment with conservative or debulking surgery and systemic chemotherapy; 2. 120 patients. |
Groups: HIPEC (group A): 1. CRS was followed by the administration of HIPEC and subsequent systemic chemotherapy; 2. Platinum-sensitive disease (n = 34): cisplatin 100 mg/m2 + paclitaxel 175 mg/m2, 60 min at 42.5 °C; 3. Platinum-resistant disease (n = 26): doxorubicin 35 mg/m2 + (paclitaxel 175 mg/m2 or mitomycin 15 mg/m2), 60 min at 42.5 °C. Non-HIPEC (group B): CRS followed by systemic chemotherapy. |
Overall mean survival: HIPEC: 26.7 months; Non-HIPEC: 13.4 months (p < 0.006). 3-year survival: HIPEC: 75%; Non-HIPEC: 18% (p < 0.01). |
|
Zhang G (2019) |
Meta-analysis including randomized controlled trials and case–control trials |
Patients with recurrent ovarian cancers. |
Groups: 1. HIPEC; 2. Without HIPEC. |
1. OS: improved for HIPEC group; (HR: 0.45, 95% CI: 0.24–0.83) 2. PFS: no correlation between HIPEC and non-HIPEC group (HR: 0.55, 95% CI: 0.27–1.11). |
4. The Safety, Adverse Effects and Quality of Life in Patients Who Undergo HIPEC
References
- Torre, L.A.; Bray, F.; Siegel, R.L.; Ferlay, J.; Lortet-Tieulent, J.; Jemal, A. Global Cancer Statistics, 2012. CA. Cancer J. Clin. 2015, 65, 87–108.
- Koole, S.N.; van Driel, W.J.; Sonke, G.S. Hyperthermic Intraperitoneal Chemotherapy for Ovarian Cancer: The Heat Is On. Cancer 2019, 125 (Suppl. 24), 4587–4593.
- van Driel, W.J.; Koole, S.N.; Sikorska, K.; Schagen van Leeuwen, J.H.; Schreuder, H.W.R.; Hermans, R.H.M.; de Hingh, I.H.J.T.; van der Velden, J.; Arts, H.J.; Massuger, L.F.A.G.; et al. Hyperthermic Intraperitoneal Chemotherapy in Ovarian Cancer. N. Engl. J. Med. 2018, 378, 230–240.
- Harter, P.; Mahner, S.; Hilpert, F.; Runnebaum, I.; Ortmann, O.; Mustea, A.; Sehouli, J.; du Bois, A.; Wagner, U. Statement by the Kommission OVAR of the AGO Study Group on the Use of HIPEC (Hyperthermic Intraperitoneal Chemotherapy) to Treat Primary and Recurrent Ovarian Cancer. Geburtshilfe Frauenheilkd. 2013, 73, 221–223.
- Ceelen, W.; Demuytere, J.; de Hingh, I. Hyperthermic Intraperitoneal Chemotherapy: A Critical Review. Cancers 2021, 13, 3114.
- Goodman, M.D.; McPartland, S.; Detelich, D.; Saif, M.W. Chemotherapy for Intraperitoneal Use: A Review of Hyperthermic Intraperitoneal Chemotherapy and Early Post-Operative Intraperitoneal Chemotherapy. J. Gastrointest. Oncol. 2016, 7, 45–57.
- Riggs, M.J.; Pandalai, P.K.; Kim, J.; Dietrich, C.S. Hyperthermic Intraperitoneal Chemotherapy in Ovarian Cancer. Diagnostics 2020, 10, 43.
- Howell, S.B. Pharmacologic Principles of Intraperitoneal Chemotherapy for the Treatment of Ovarian Cancer. Int. J. Gynecol. Cancer 2008, 18 (Suppl. 1), 20–25.
- Lemoine, L.; Sugarbaker, P.; Van der Speeten, K. Drugs, Doses, and Durations of Intraperitoneal Chemotherapy: Standardising HIPEC and EPIC for Colorectal, Appendiceal, Gastric, Ovarian Peritoneal Surface Malignancies and Peritoneal Mesothelioma. Int. J. Hyperth. 2017, 33, 582–592.
- Hasovits, C.; Clarke, S. Pharmacokinetics and Pharmacodynamics of Intraperitoneal Cancer Chemotherapeutics. Clin. Pharmacokinet. 2012, 51, 203–224.
- Gallego-Jara, J.; Lozano-Terol, G.; Sola-Martínez, R.A.; Cánovas-Díaz, M.; de Diego Puente, T. A Compressive Review about Taxol®: History and Future Challenges. Molecules 2020, 25, 5986.
- Wang, T.H.; Wang, H.S.; Soong, Y.K. Paclitaxel-Induced Cell Death: Where the Cell Cycle and Apoptosis Come Together. Cancer 2000, 88, 2619–2628.
- Dasari, S.; Bernard Tchounwou, P. Cisplatin in Cancer Therapy: Molecular Mechanisms of Action. Eur. J. Pharmacol. 2014, 740, 364–378.
- Makovec, T. Cisplatin and beyond: Molecular Mechanisms of Action and Drug Resistance Development in Cancer Chemotherapy. Radiol. Oncol. 2019, 53, 148–158.
- Chiva, L.M.; Gonzalez-Martin, A. A Critical Appraisal of Hyperthermic Intraperitoneal Chemotherapy (HIPEC) in the Treatment of Advanced and Recurrent Ovarian Cancer. Gynecol. Oncol. 2015, 136, 130–135.
- Lim, M.C.; Chang, S.J.; Yoo, H.J.; Nam, B.H.; Bristow, R.; Park, S.Y. Randomized Trial of Hyperthermic Intraperitoneal Chemotherapy (HIPEC) in Women with Primary Advanced Peritoneal, Ovarian, and Tubal Cancer. J. Clin. Oncol. 2017, 35 (Suppl. 15), 5520.
- Laplace, N.; Kepenekian, V.; Friggeri, A.; Vassal, O.; Ranchon, F.; Rioufol, C.; Gertych, W.; Villeneuve, L.; Glehen, O.; Bakrin, N. Sodium Thiosulfate Protects from Renal Impairement Following Hyperthermic Intraperitoneal Chemotherapy (HIPEC) with Cisplatin. Int. J. Hyperth. 2020, 37, 897–902.
- Arjona-Sánchez, A.; Cadenas-Febres, A.; Cabrera-Bermon, J.; Muñoz-Casares, F.C.; Casado-Adam, A.; Sánchez-Hidalgo, J.M.; López-Andreu, M.; Briceño-Delgado, J.; Rufián-Peña, S. Assessment of RIFLE and AKIN Criteria to Define Acute Renal Dysfunction for HIPEC Procedures for Ovarian and Non Ovarian Peritoneal Malignances. Eur. J. Surg. Oncol. 2016, 42, 869–876.
- Raspé, C.; Flöther, L.; Schneider, R.; Bucher, M.; Piso, P. Best Practice for Perioperative Management of Patients with Cytoreductive Surgery and HIPEC. Eur. J. Surg. Oncol. 2017, 43, 1013–1027.
- Koole, S.N.; Kieffer, J.; Sikorska, K.; van Leeuwen, J.S.; Schreuder, H.; Hermans, R.; de Hingh, I.; van der Velden, J.; Arts, H.; van Ham, M.A.P.C.; et al. Health-Related Quality of Life after Interval Cytoreductive Surgery with or without Hyperthermic Intraperitoneal Chemotherapy (HIPEC) in Patients with Stage III Ovarian Cancer. Eur. J. Surg. Oncol. 2021, 47, 101–107.
- Tsuyoshi, H.; Inoue, D.; Kurokawa, T.; Yoshida, Y. Hyperthermic Intraperitoneal Chemotherapy (HIPEC) for Gynecological Cancer. J. Obstet. Gynaecol. Res. 2020, 46, 1661–1671.
- Burguete, D.; Mokdad, A.A.; Augustine, M.M.; Minter, R.; Mansour, J.C.; Choti, M.A.; Polanco, P.M. Non-Home Discharge and Prolonged Length of Stay After Cytoreductive Surgery and HIPEC. J. Surg. Res. 2019, 233, 360–367.
- Keyes, A.M.; Kelly, M.E.; McInerney, N.; Khan, M.F.; Bolger, J.C.; McCormack, E.; Grundy, J.; McCormack, O.; MacHale, J.; Conneely, J.; et al. Short-Term Outcomes in Older Patients with Peritoneal Malignancy Treated with Cytoreductive Surgery and HIPEC: Experience with 245 Patients from a National Centre. Eur. J. Surg. Oncol. 2021, 47, 2358–2362.




