Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Stergios Boussios | -- | 1981 | 2023-09-15 03:58:58 | | | |
| 2 | Catherine Yang | -4 word(s) | 1977 | 2023-09-15 04:04:21 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Papadopoulou, S.; Pavlidou, E.; Argyris, G.; Flouda, T.; Koukoutsidi, P.; Krikonis, K.; Shah, S.; Chirosca-Vasileiou, D.; Boussios, S. Language Induced Epilepsy. Encyclopedia. Available online: https://encyclopedia.pub/entry/49218 (accessed on 07 February 2026).
Papadopoulou S, Pavlidou E, Argyris G, Flouda T, Koukoutsidi P, Krikonis K, et al. Language Induced Epilepsy. Encyclopedia. Available at: https://encyclopedia.pub/entry/49218. Accessed February 07, 2026.
Papadopoulou, Soultana, Efterpi Pavlidou, Georgios Argyris, Thaleia Flouda, Panagiota Koukoutsidi, Konstantinos Krikonis, Sidrah Shah, Dana Chirosca-Vasileiou, Stergios Boussios. "Language Induced Epilepsy" Encyclopedia, https://encyclopedia.pub/entry/49218 (accessed February 07, 2026).
Papadopoulou, S., Pavlidou, E., Argyris, G., Flouda, T., Koukoutsidi, P., Krikonis, K., Shah, S., Chirosca-Vasileiou, D., & Boussios, S. (2023, September 15). Language Induced Epilepsy. In Encyclopedia. https://encyclopedia.pub/entry/49218
Papadopoulou, Soultana, et al. "Language Induced Epilepsy." Encyclopedia. Web. 15 September, 2023.
Copy Citation
Language-induced epilepsy is a subcategory of reflex epilepsy during which specific language stimuli appear to be the triggering mechanism. Specifically, higher mental activities, such as reading, speaking, writing, calculating, concentrating, playing chess, reading music, and playing a musical instrument, among others, have been reported as triggering focal or generalized seizures, under certain circumstances. To avoid misconceptions, it is deemed important here to exclude seizures triggered by non-verbal higher brain activities related to spatial processing and ideation or movements from the category of language-induced epilepsy, as such are considered praxis-induced seizures.
epilepsy
seizures
stuttering
psychogenic nonepileptic seizures
1. Introduction
It has been widely proven that epilepsy can affect language. Admittedly, the type, severity, and main cause of epilepsy, along with the type of treatment, define the extent and nature of the derived language disturbance (Figure 1) [1]. It has been specifically reported through case studies that certain antiepileptic drugs, such as phenytoin, carbamezepine, lamotrigine, topiramate, valproate and levetiracetam gabapentin, and divalporoex sodium have either induced the appearance or prevented the onset of language dysfunctioning, depending on the patients’ clinical background and drug idiosyncrasy, thus, strengthening the initial hypothesis of this work [2][3]. Similarly, one of the studies reviewed reports that ethosuximide and phenobarbital therapy prescribed to a 47 year old male patient with epileptic seizures reduced clinical seizures, including stuttering, while no receipt of anticonvulsant medication during certain periods revealed that clinical seizures were repeatedly percipitated by certain stimuli [4]. As presented and proven below, a great volume of literature investigates the link between epileptic seizures and perforce disturbances in language and speech fluency—including stuttering, in particular. Yet, a significant low number of studies looks at how stuttering may trigger epileptic seizures. The ultimate objective of this research is to observe any trends in the perspectives through which published articles investigate the topic, and to shed light on the relevant diagnostic dilemma.
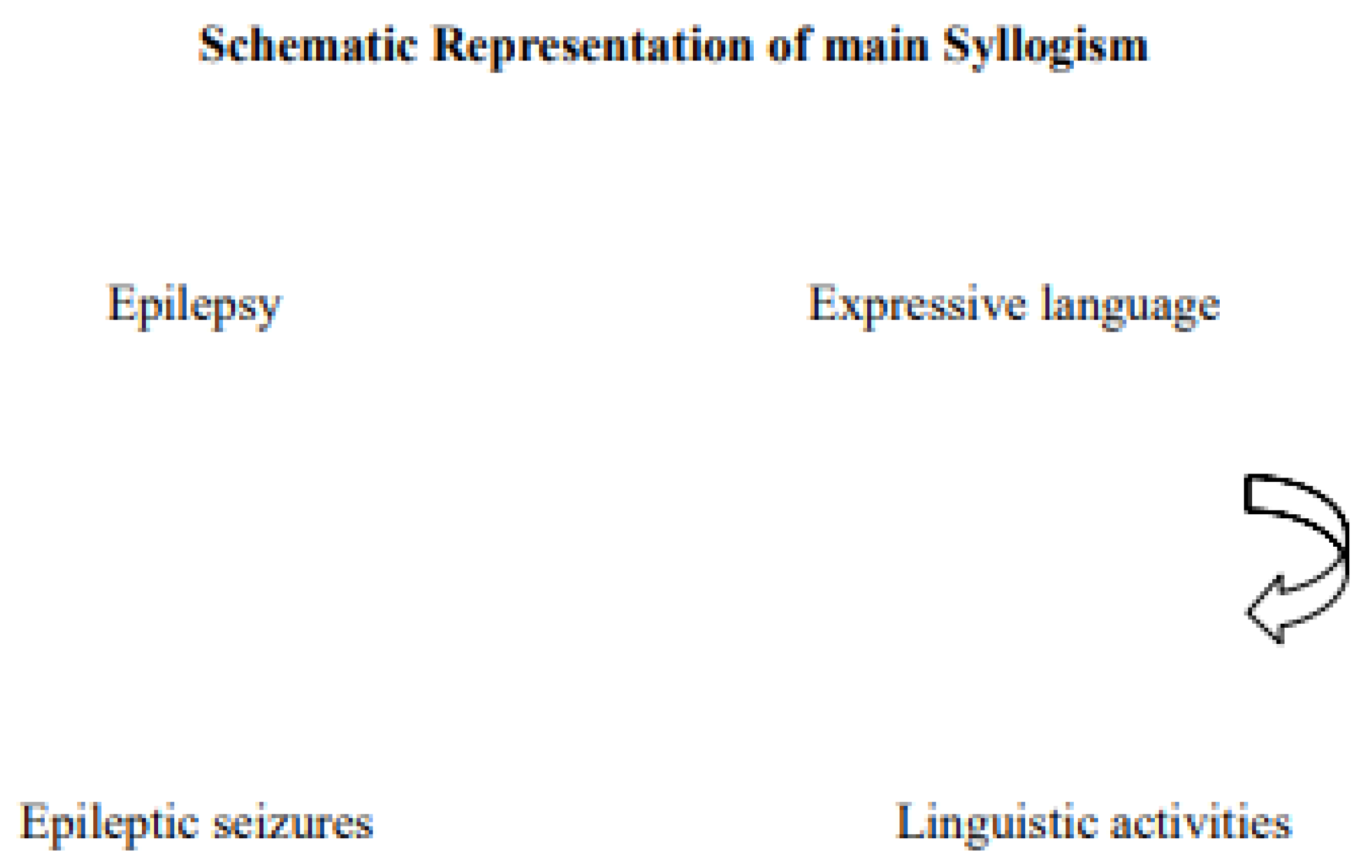
Figure 1. Schematic Representation of main Syllogism.
2. Language Induced Epilepsy
Language-induced epilepsy is a subcategory of reflex epilepsy during which specific language stimuli appear to be the triggering mechanism. Specifically, higher mental activities, such as reading, speaking, writing, calculating, concentrating, playing chess, reading music, and playing a musical instrument, among others, have been reported as triggering focal or generalized seizures, under certain circumstances. To avoid misconceptions, it is deemed important here to exclude seizures triggered by non-verbal higher brain activities related to spatial processing and ideation or movements from the category of language-induced epilepsy, as such are considered praxis-induced seizures.
Language in any of the three modalities—reading, writing and speaking—has been reported in the research as a seizure-provoking stimulus [5]. This type of epilepsy is used to describe seizures provoked by failed attempts to speak, read, or write, while the phenomenon–although associated with inextricable facets in patients’ daily routine–is only partly investigated in published scientific works [3]. The literature review conducted for the purposes of the present work has also revealed that there is limited investigation on graphogenic or writing epilepsy as another variant of language induced epilepsy [6]. Yet, the existence of such studies points to the need of further investigating how some tasks involving complex mental involvement for activities performed by the hands confirm the precipitation of myoclonic jerks in patients with juvenile myoclonic epilepsy [6][7]. Admittedly, writing is an intensive mental activity which involves praxis sub-activity, an observation which not only explains the different categorizations between general praxis-induced epilepsy and graphogenic epilepsy, but also emphasizes the causal relationship between stimulus and the emergence of epileptic seizures.
3. Pathophysiology and Genetics of Stuttering—A Bridge to Epilepsy
As previously mentioned, a link between stuttering and epilepsy is suggested by bibliography, but the nature of the link is yet to be clarified. Given the high prevalence and the severity of the disease, the pathophysiological mechanisms of human epilepsy are well-studied, while the pathophysiology of stuttering remains still obscure. Modern brain-activity recording methods and neuroimaging techniques attempt to provide insight into the mechanisms underlying the clinical manifestation of stuttering.
Some of the first studies suggested that there is incomplete lateralization or abnormal cerebral dominance in people who stutter, with the cerebral hemispheres holding opposing roles. The left hemisphere is considered related to the production of stuttered speech, while the right one may act in a compensatory manner to the symptom [8]. In 2000, Salmelin and colleagues used whole-head magnetoencephalography (MEG) in developmental stutterers (DS) and fluent speakers in an attempt to record the sequence of cortical activation, while subjects read aloud and vocalized single words. DS presented cortical activation first in the motor cortex and premotor area (associated with motor programming), and immediately after to the left inferior frontal region (associated with articulation and language processes). Fluent speakers exhibited the reverse pattern, and thus it seemed that DS initiates motor programs before articular code is prepared [9].
A neuroimaging study from Michigan State University in 2015 measured the fractional anisotropy derived from cerebral white matter using Magnetic Resonance Imaging (MRI) in children who stutter, and compared the respective measurements from fluent age-matched controls in an attempt to detect neuroanatomical differences. Scientists observed reduced fractional anisotropy in stuttering children relative to controls in white matter tracts that interconnect auditory and motor structures, in the corpus callosum, and in tracts interconnecting cortical and subcortical areas, which suggests possible structural connectivity deficits in this study group, a finding consistent with those of previous studies [10][11]. Another brain MRI study detected and compared regional Cerebral Blood Flow (rCBF) in a group of stutterers and a control group of fluent speakers. The study revealed decreased rCBF in Broca’s area (key component to speech production) and increased rCBF in cerebellar nuclei and parietal cortex (a possible compensatory mechanism) in the stuttering group compared to controls [11].
A most recent study of a large family with inherited stuttering, using T1-weighted and diffusion-weighted MRI, demonstrated a disruption in the cortico-basal ganglia-thalamo-cortical network (fundamental brain network in many activities, including initiating speech motor programs), an increase in globus pallidi bilaterally, and structural differences in Broca’s area between the study group and control group [12][13].
The implication of neurotransmitters was also considered. Maguire et al. studied a small group of DS before and after their treatment with risperidone (a D2/5-HT2 antagonist) using positron emission tomography (PET). In the risperidone-treated group, increased metabolism in the left striatum (caudate and putamen) and Broca’s area was observed, a finding that strengthens previous research that implicated the role of increased dopamine and striatal hypometabolism in stuttering [14].
Functional MRI (fMRI) became available after PET and nowadays dominates neuroimaging of stuttering due to its high spatial resolution. Speech production and resting state fMRI studies have reported several abnormalities in widely distributed brain regions, as well as in connectivity between regions of critical importance for speech organization and production [15]. However, fMRI studies appeared to have several limitations until nowadays [11][15].
The genetic basis of stuttering is still to be defined. Twin studies suggest that monozygotic twins display stuttering in higher rates compared to dizygotic twins, indicating a relatively strong genetic component, while studies in families with stuttering members attempt to identify a mode of inheritance [12][16]. Despite limitations carried by genomic studies, mutations in GNPTAB gene (encodes the enzyme N-acetylglucosamine-1-phosphotransferase) found in Pakistani families with stuttering, mutations in AP4E1 gene (encoding adaptor protein complex 4) in a large Cameroonian study, and loci on chromosomes 1 and 4, determined by genetic mapping, in a large family with inherited stuttering, suggest an autosomal dominant pattern. Nevertheless, stuttering seems to be a complex trait, and more in-depth genetic research will improve current understanding of this clinical manifestation [12][16][17].
As indicated in previous sections of the research and by relevant bibliography, the rates of stuttering among patients with epilepsy are higher than in the general population. The interrelation between epilepsy and stuttering is not straightforward, especially when stuttering is considered the stimuli for an epileptic seizure to occur, and not the clinical symptom of an epileptic seizure [18][19][20]. From a genetic perspective, intragenic deletions of the contactin-associated protein-like 2 gene (CNTNAP2) have been found in patients with epileptic syndrome and stuttering. Normally, the gene products are responsible for bridging the intercellular space between neurons. The CNTNAP2 alleles that express the aforementioned deletions interfere with the physiological process of connecting neuronal cells and present a molecular basis for several neurodevelopmental disorders, including epilepsy and stuttering. Other conditions that are associated with intragenic deletions of the CNTNAP2 are Gilles de la Tourette syndrome, intellectual disability, obsessive-compulsive disorder, language impairments, and attention deficit hyperactivity disorder [21].
Despite the limited available bibliography on epileptic seizures induced by language, there have been case reports and studies that used video-EEG with electromyogram (EMG) recordings in an attempt investigate stuttering patients. In a case of language-induced epilepsy, the patient exhibited facial myoclonus while reading aloud, and dysfluent language, mimicking stuttering. Paroxysmal discharges in EEG recordings of the left frontal region were consistently associated with a brief interruption of language. In this particular patient, silent reading did not induce any epileptic discharge, and as a result, articulatory movements during phonation were assumed to be the triggering factor. The patient was treated with antiepileptic medication [18]. Michel et al. conducted video-polygraphic EEG recordings in four patients with a diagnosis of juvenile myoclonic epilepsy (JME) in whom coexisted praxis- and language-induced jerks. Complex stimuli-reading and praxis-induced reflex seizures in these patients, characterized by facial myoclonias and stuttering. EEG recordings were indicative of brief paroxysms of very fast spikes followed by a slow wave, mainly in the frontocentroparietal areas [22]. In a neurophysiological study of nine members of a family with history of idiopathic generalized epilepsy (IGE) with interictal stuttering, spontaneous language was the main triggering factor for the occurrence of myoclonic jerks in five members. They also reported acquired stuttering (which was proved to be of epileptogenic origin). Stuttering was also present while reading, and EEG recordings exhibited abnormalities (e.g., spikes followed by slow waves) [23]. The above observations suggest that some forms of acquired stuttering could be linked to epileptic seizure, and electrophysiological studies may prove useful in investigating them. Although it was not possible to identify specific pathophysiological mechanisms shared by epilepsy and language as a trigger for epileptic seizure from the current bibliographical review, nor from the systematic literature that investigates both of them using modern neuroimaging techniques, it is apparent that there is ground for research to be covered.
4. Psychogenic Nonepileptic Seizures vs. Epileptic Seizures—The Role of Ictal Stuttering
Among patients who are evaluated for refractory epileptic seizures, approximately 25% are found to have psychogenic nonepileptic seizure-like events (PNES). This finding is of critical importance for clinicians who deal with refractory cases of seizures, because misdiagnosis leads to administration of unnecessary antiepileptic medication with subsequent side effects and a significant financial burden (up to 4 billion USD).
PNES and epileptic seizure exhibit multiple overlapping clinical features. Ictal stuttering (IS) can be used as a useful sign to help distinguish between PNES from epileptic seizure in adult patients. In 2004, a study conducted by Vossler et al. compared two groups of patients with PNES and epileptic seizure, and evaluated them for IS. Interestingly, IS was observed only among patients with PNES (8.5% of 117 patients). Other features that assist clinicians to distinguish between these two groups of disorders are the “yellow” clinical characteristics of PNES. Specifically, seizures in PNES are usually characterized by gradual onset, a longer ictal duration (>2 min), and stimuli is often an emotional stressful event. Patients with PNES exhibit higher rates of psychiatric conditions, such as cluster A or B personality disorders, compared to patients with epileptic seizure.
EEG-video monitoring is the gold standard in PNES diagnosis. No EEG changes during a clinical event, accompanied by clinical spells inconsistent with seizure types that should induce changes in EEG recording, almost rule out PNES diagnosis [24][25][26].
Overall, given the diagnostic challenges posed by the clinical manifestations of both PNES and epileptic seizure, clinicians should be very careful, especially when evaluating refractory cases of seizures, because eventually misdiagnosis prevents patients from receiving suitable treatment for their condition.
References
- Stafstrom, C.E.; Carmant, L. Seizures and epilepsy: An overview for neuroscientists. Cold Spring Harb. Perspect. Med. 2015, 5, a022426.
- Kaplan, P.W.; Stagg, R. Frontal lobe nonconclusive status epilepticus: A case of epileptic stuttering, aphemia and aphasia—Not a sign of psychogenic nonepileptic seizures. Epilepsy Behav. 2011, 21, 191–195.
- Sechi, G.P.; Cocco, G.A.; D’Onofrio, M.; Deriu, M.G.; Rosati, G. Disfluent speech in patients with partial epilepsy: Beneficial effect of levetiracetam. Epilepsy Behav. 2006, 9, 521–523.
- Geschwind, N.; Sherwin, I. Language-Induced Epilepsy. Arch. Neurol. 1967, 16, 25–31.
- Guaranha, M.S.; Filho, G.M.; Lin, K.; Guilhoto, L.M.; Caboclo, L.O.; Yacubian, E.M. Prognosis of juvenile myoclonic epilepsy is related to endophenotypes. Seizure 2011, 20, 42–48.
- Oshima, T.; Hirose, K.; Murakami, H.; Suzuki, S.; Kanemoto, K. Graphogenic epilepsy: A variant of language-induced epilepsy distinguished from reading- and praxis-induced epilepsy. Seizure 2003, 12, 56–59.
- Matsuoka, H.; Takahashi, T.; Sasaki, M.; Matsumoto, K.; Yoshida, S.; Numachi, Y.; Saito, H.; Ueno, T.; Sato, M. Neuropsychological EEG activation in patients with epilepsy. Brain 2000, 123, 318–330.
- Costa, D.; Kroll, R. Stuttering: An update for physicians. CMAJ 2000, 162, 1849–1855.
- Salmelin, R.; Schnitzler, A.; Schmitz, F.; Freund, H.J. Single word reading in developmental stutterers and fluent speakers. Brain 2000, 123, 1184–1202.
- Chang, S.E.; Zhu, D.C.; Choo, A.L.; Angstadt, M. White matter neuroanatomical differences in young children who stutter. Brain 2015, 138, 694–711.
- Desai, J.; Huo, Y.; Wang, Z.; Bansal, R.; Williams, S.C.; Lythgoe, D.; Zelaya, F.O.; Peterson, B.S. Reduced perfusion in Broca’s Area in Developmental Stuttering. Hum. Brain Mapp. 2017, 38, 1865–1874.
- Thompson-Lake, D.G.Y.; Scerri, T.S.; Block, S.; Turner, S.J.; Reilly, S.; Kefalianos, E.; Bonthrone, A.F.; Helbig, I.; Bahlo, M.; Scheffer, I.E.; et al. Atypical development of Broca’s area in a large family with inherited stuttering. Brain 2021, awab364.
- Chang, S.E.; Erickson, K.I.; Ambrose, N.G.; Hasegawa-Johnson, M.A.; Ludlow, C.L. Brain anatomy differences in childhood stuttering. Neuroimage 2008, 39, 1333–1344.
- Maguire, G.A.; Yoo, B.R.; SheikhBahaei, S. Investigation of Risperidone Treatment Associated with Enhanced Brain Activity in Patients Who Stutter. Front. Neurosci. 2021, 15, 598949.
- Etchell, A.C.; Civier, O.; Ballard, K.J.; Sowman, P.F. A systematic literature review of neuroimaging research on developmental stuttering between 1995 and 2016. J. Fluen. Disord. 2018, 55, 6–45.
- Frigerio-Domingues, C.; Drayna, D. Genetic contributions to stuttering: The current evidence. Mol. Genet. Genom. Med. 2017, 5, 95–102.
- Kraft, S.J.; Yairi, E. Genetic bases of stuttering: The state of the art, 2011. Folia Phoniatr. Logop. 2012, 64, 34–47.
- Michel, V.; Burduad, P.; Tailard, J.; Gaida, T.; Joseph, P.A.; Duché, B.; Bioulac, B. Stuttering or reflex seizure? A case report. Epileptic Disord. 2004, 6, 181–185.
- Rejnö-Habte Selassie, G.; Hedström, A.; Viggedal, G.; Jennische, M.; Kyllerman, M. Speech, language, and cognitive dysfunction in children with focal epilepticum activity: A follow-up study. Epilepsy Behav. 2010, 18, 267–275.
- Lebrun, Y.; Fabbro, F. (Eds.) Stuttering and Epilepsy. In Language and Epilepsy; Whurr Publishers: London, UK, 2002; pp. 57–61.
- Poot, M. Intragenic CNTNAP2 Deletions: A Bridge Too Far? Mol. Syndromol. 2017, 8, 118–130.
- Da Silva Sousa, P.; Lin, K.; Garzon, E.; Ceiki Sakamoto, A.; Yacubian, E.M. Language- and praxis-induced jerks in patients with juvenile myoclonic epilepsy. Epileptic Disord. 2005, 7, 115–121.
- Valenti, M.P.; Rudolf, G.; Carré, S.; Vrielynck, P.; Thibault, A.; Szepetowski, P.; Hirsch, E. Language-induced epilepsy, acquired stuttering, and idiopathic generalized epilepsy: Phenotypic study of one family. Epilepsia 2006, 47, 766–772.
- Widyadharma, I.P.E.; Soejitno, A.; Samatra, D.P.G.P.; Sinardja, A.M.G. Clinical differentiation of psychogenic non-epileptic seizure: A practical diagnostic approach. Egypt J. Neurol. Psychiatry Neurosurg. 2021, 57, 19.
- Vossler, D.G.; Haltiner, A.M.; Schepp, S.K.; Friel, P.A.; Caylor, L.M.; Morgan, J.D.; Doherty, M.J. Ictal stuttering: A sign suggestive of psychogenic nonepileptic seizures. Neurology 2004, 63, 516–519.
- Benbadis, S.R. The EEG in Nonepileptic Seizures. J. Clin. Neurophysiol. 2006, 23, 340–352.
More
Information
Subjects:
Neurosciences
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
543
Revisions:
2 times
(View History)
Update Date:
15 Sep 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




