Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Shulan Shi | -- | 2404 | 2023-09-14 11:24:02 | | | |
| 2 | Lindsay Dong | -1 word(s) | 2403 | 2023-09-15 03:52:21 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Shi, S.; Pan, J.; Dong, B.; Zhou, W.; Zhou, C. Primary Rare Earth Elements Resources. Encyclopedia. Available online: https://encyclopedia.pub/entry/49163 (accessed on 13 January 2026).
Shi S, Pan J, Dong B, Zhou W, Zhou C. Primary Rare Earth Elements Resources. Encyclopedia. Available at: https://encyclopedia.pub/entry/49163. Accessed January 13, 2026.
Shi, Shulan, Jinhe Pan, Bin Dong, Weiguang Zhou, Changchun Zhou. "Primary Rare Earth Elements Resources" Encyclopedia, https://encyclopedia.pub/entry/49163 (accessed January 13, 2026).
Shi, S., Pan, J., Dong, B., Zhou, W., & Zhou, C. (2023, September 14). Primary Rare Earth Elements Resources. In Encyclopedia. https://encyclopedia.pub/entry/49163
Shi, Shulan, et al. "Primary Rare Earth Elements Resources." Encyclopedia. Web. 14 September, 2023.
Copy Citation
Rare earth elements (REEs) are national strategic resources widely applied in industries such as petrochemicals, electronics, national defense and new energy. The primary REE resources are monazite, bastnaesite, xenotime and ion-adsorption type rare earth ore.
Rare earth elements
REEs
petrochemicals
electronics
1. Introduction
Rare earth elements (REEs) are national strategic resources widely applied in industries such as petrochemicals, electronics, national defense and new energy [1]. The primary REE resources are monazite, bastnaesite, xenotime and ion-adsorption type rare earth ore [2]. With the rapid development of the economy and the consumption of REE resources, unconventional resources, such as phosphogypsum, coal fly ash, red mud, and NdFeB magnets [3][4], show increasing exploitation value. Despite being considered industrial wastes which cause severe environmental problems and landfill issues, these resources contain a substantial amount of REEs. Therefore, recovering REEs from these secondary resources not only helps to alleviate environmental problems but also turns waste into wealth. Physical and chemical approaches, including flotation; magnetic separation; gravity separation; roasting (with or without additives); and alkaline and acid leaching (e.g., H2SO4, HCl and HNO3) [5][6][7] are usually used to extract REEs from these resources. The rare earth elements in the leachate are then separated and recovered using chelating extractants, organophosphorus compounds, ionic liquids, etc., [8][9][10][11]. In the extraction process, high temperatures are often required to enhance extraction efficiency, which is energy-consuming [12][13][14].
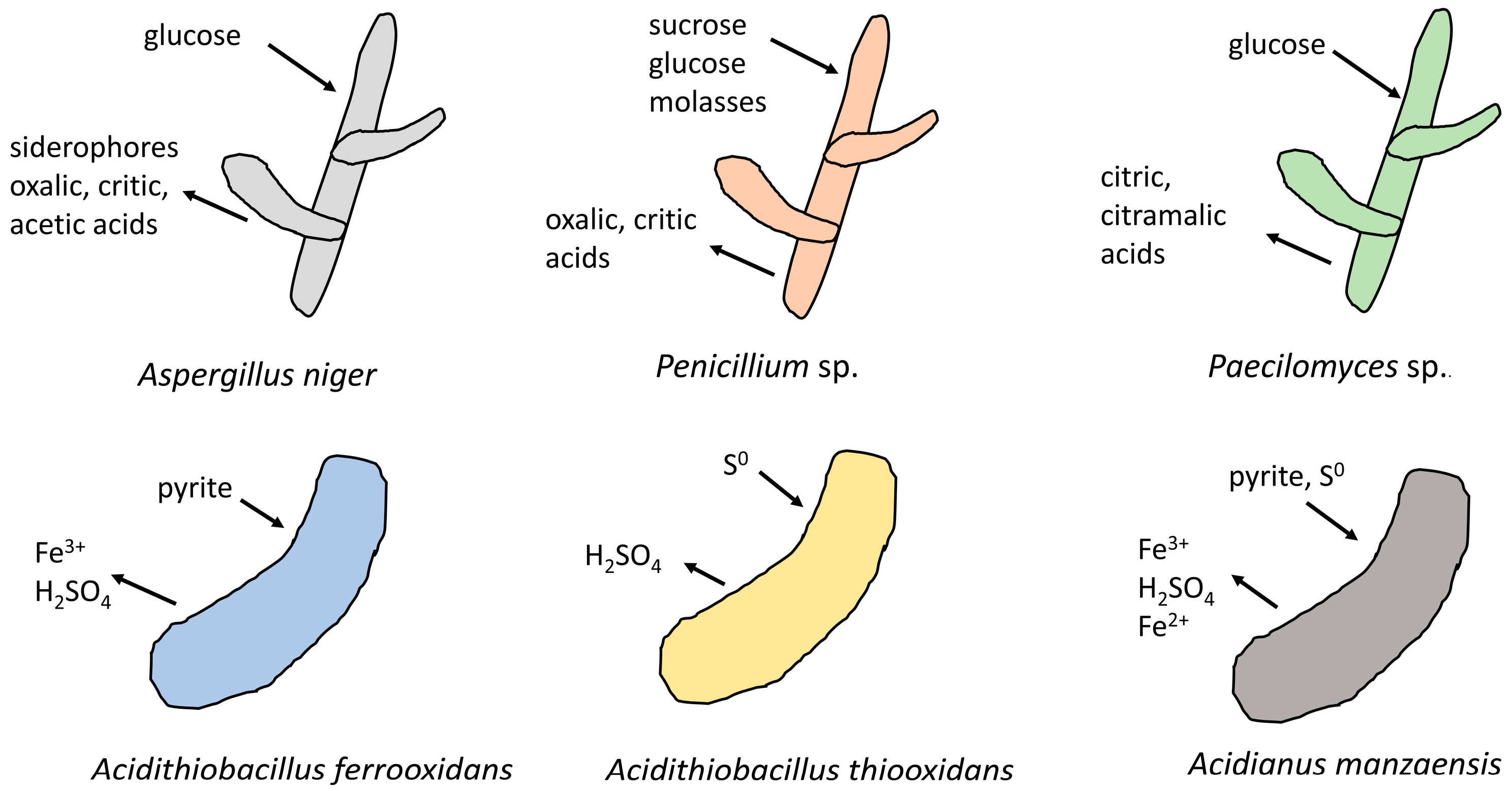
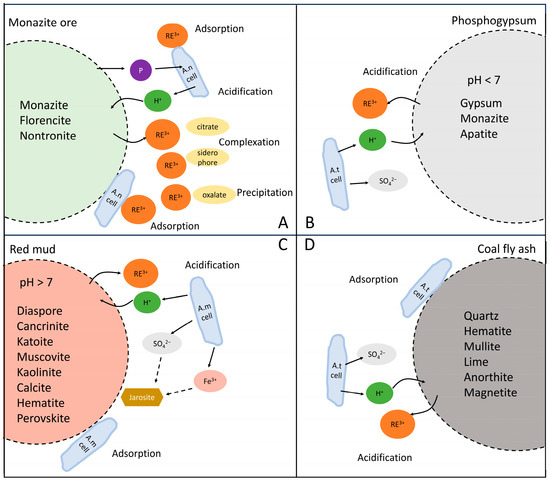
2. Primary REE Resources
2.1. Monazite Ore
Monazite, bastnaesite, xenotime and ion-adsorption rare earth ore (IRE-ore) are primary REE resources. Currently, the application of bioleaching approaches is mainly focused on monazite, while studies on the bioleaching of bastnaesite and IRE-ore are scarce, and there are no relevant studies on xenotime. Of these, monazite ((Ce,La,Nd,Th)PO4) is a kind of REE phosphate mineral, and an important industrial light REE resource. It is mainly composed of P2O5, Ce2O3, La2O3, Nd2O3 and ThO2 [15]. Low-grade monazite ores also contain ilmenite, silicate and zircon with both crystal and amorphous structures [16], making them complex in composition and difficult to dissolve. For example, a monazite-bearing rock contains monazite (51%), florencite (41%), and nontronite (8%) [17]. A sequential extraction procedure in the study showed that the majority (85%) of REEs were present in the residual fraction, and around 12% of REEs were found in the acid-soluble fraction. Conventionally, concentrated sodium hydroxide or sulfuric acid is employed to extract REEs from monazite, resulting in the production of toxic waste [18][19]. Organic acids are able to selectively release REEs from monazite, including oxalic acid, citric acid and ethylene diamine tetra-acetic acid (EDTA), but they have a lower recovery rate than inorganic acids [20][21].
The fungus A. niger is a widely used microorganism in bioleaching. It is geoactive and extensively applied in industry, and also exhibits a high tolerance to REEs. With glucose as an energy source, the maximum concentration of Ce extracted from monazite was 0.7 mg/L after 30 days of leaching at 30 °C and a 1% pulp density [15]. The authors also noted that monazite can provide a phosphorus source for cells, and maybe other essential trace metals, illustrating the feasibility of monazite bioleaching. In another study, using a minimal medium, A. niger solubilized 1.37 mg/L of REEs at a 1% pulp density, which was significantly higher than that in a rich medium (0.97 mg/L) [22]. The results suggest that obtaining nutrients from monazite favors the bioleaching of REEs. Also, X-ray diffraction (XRD) analyses revealed that aluminum cerium phosphate hydroxide in monazite ore is decomposed by A. niger. Furthermore, the direct interaction mechanisms between A. niger and monazite were explored by scanning electron microscopy and energy-dispersive X-ray analysis in one research study [23]. The authors proposed that A. niger released REEs and phosphate by the tunnelling, splitting, and penetration of mineral particles. Moreover, A. niger can produce siderophores and various organic acids, including oxalic acid, acetic acid, and citric acid (Figure 1) [15]. Of these, citric acid plays a role in REE dissolution through acidolysis and chelation, as the citric acid concentration is positively correlated with the contents of the released REEs. Osman et al. found that siderophores produced by A. niger are trihydroxymate in nature, extracting the REEs from phosphorites via chelation [24].

Figure 1. Microorganisms frequently utilized in REEs bioleaching, as well as their main metabolic substrates and products, including Aspergillus niger, Penicillium sp., Paecilomyces sp., Acidithiobacillus ferrooxidans, Acidithiobacillus thiooxidans, and Acidianus manzaensis.
However, the leaching efficiency of REEs was low (<3%) in these studies, which may be attributed to the adsorption of REEs to cells and precipitation of the REEs induced by oxalic acid (Figure 2). Adsorption of the REEs onto cells was observed via SEM, and the presence of La and Ce oxalate were detected by XRD after bioleaching [22]. Furthermore, the REE oxalates were identified as Ce2(C2O4)3·10H2O and La2(C2O4)3·10H2O, with various morphologies such as sheets, needles, and tablets [25]. Considering that the leaching of monazite with microbial culture supernatants generates REE oxalates of high purity, it could serve as a novel method for the biorecovery of REEs.

A study on monazite bioleaching using heterotrophic bacteria Enterobacter aerogenes showed that microbial contact contributes to the leaching of REEs [26]. E. aerogenes secreted malic, acetic and gluconic acid, which decreased the pH and complexed with REE3+. Exopolysaccharides produced by the bacteria facilitated the attachment of cells to minerals. After 18 days of incubation at 30 °C and a 1% pulp density, the biotic-contact bioleaching approach showed a higher REE recovery (3.66 mg/L) than the non-contact approach (0.94 mg/L), indicating that microbial contact contributes to the leaching of REEs. Consistently, the attachment of cells onto a monazite surface was observed by atomic force microscopy (AFM) and confocal Raman microscopy (CRM). The adsorption of cells onto minerals creates a microenvironment where high concentrations of organic acids accumulate, thereby enhancing the solubilization of REEs.
In addition, Penicillium sp. released 12.32 mg/L of REEs from weathered monazite [31], which is higher than that observed in A. niger and E. aerogenes, but lower than that of Paecilomyces sp. The fungus Paecilomyces sp. was able to leach REEs from monazite using monazite as the sole phosphorous source [32]. The consumption of phosphorous by microbes in the leachate induces further solubilization of monazite. After six days of incubation at room temperature and a 1% pulp density, the maximum REE concentration in the leachate was 112 mg/L, which is 1.7–3.8 times higher than that of HCl leaching (with a comparable pH). It is worth noting that the radioactive element Th remains undissolved in the process, demonstrating the high selectivity of the bioleaching approach. Furthermore, a metabolomic analysis of Paecilomyces sp. detected 210 metabolites, in which citric and citramalic acids contributed largely to the monazite bioleaching (Figure 1) [33]. Hence, increasing the production of citric and citramalic acids could potentially enhance the bioleaching efficiency of Paecilomyces sp.
A roasting pretreatment was proven to increase the bioleaching efficiency of monazite rock. Maes et al. found that after roasting monazite with Na2CO3 and NaCl at 800 °C, the leaching efficiency of La and Nd increased significantly [34]. During the roasting, REE phosphates were transformed into more soluble REE oxides. As a result, 279 mg/L of Nd and 287 mg/L of La were obtained after seven cycles of leaching with the fungal supernatant (Paeciliomyces sp.), which were significantly higher than results obtained in the other studies mentioned above. Acetic, succinic, and gluconic acids in the fermentation supernatant were the main factors that solubilized the REEs. It is the first time that roasting was combined with bioleaching to process monazite rock, and it is innovative to adopt a multiple-cycle leaching approach. The results are enlightening for researchers seeking to promote monazite bioleaching efficiency.
Compared to single strain microorganisms, cooperation among different microorganisms often demonstrates higher bioleaching efficiency. In one study, heterotrophic E. aerogenes and autotrophic At. ferrooxidans were co-cultured to leach REEs from a high-grade monazite ore [17]. At. ferrooxidans is able to oxidize inorganic ferrous ions or reduced sulfur compounds (e.g., elemental S, pyrite) to obtain energy, while E. aerogenes needs organic carbon and an energy source (glucose) to survive. After 12 days of incubation at 30 °C and a 1% pulp density, REE concentrations reached 40 mg/L in the co-culture bioleaching system, significantly higher than that of the single culture of E. aerogenes (5.8 mg/L) or At. ferrooxidans (23.6 mg/L). The synergic effect is attributed to the cooperation of organic acids and sulfuric acid. Additionally, At. ferrooxidans may help E. aerogenes to obtain a phosphorus source. A synergic effect could also occur between indigenous and inoculated microorganisms. The bioleaching of non-sterile monazite using Penicillum sp. resulted in a total REE concentration of 23.7 mg/L in eight days, significantly higher than that of sterile monazite (12.32 mg/L) [35]. Further investigations showed that Firmicutes accounted for the majority (78%) of the indigenous microbial community and contributed to the increased REE recovery rate. The authors also claimed that the inoculated microorganisms secreted secondary metabolites that simulated the growth of the indigenous microbial community, but experimental evidence was not supplied. The research is meaningful for industrial applications, as the actual bioleaching system operates in open air rather than in a sterile environment. Therefore, an investigation of the effect of indigenous microorganisms on inoculated microbes is imperative. Overall, the interactions between different microbes could be leveraged to enhance bioleaching, yet the interaction mechanisms remain to be further studied.
In summary, among the microbial species mentioned above, the fungus Paecilomyces sp. leached the highest concentration of REEs from monazite (Table 1), and deserve to be investigated further. Meanwhile, more microbes with the potential to dissolve monazite ores can be employed for bioleaching in the future. One such example is phosphate-solubilizing microorganisms, which can make P soluble and promote plant growth; thus, they are widely applied in agriculture. Phosphate-solubilizing microorganisms involve many microbial taxa which are distantly related [36], such as Pseudomonas, Bacillus, and Azotobacter. Monazite is a kind of phosphate mineral, thus phosphate-solubilizing microorganisms must destroy the structure of monazite in order to release P [37], and may release REE cations in the meantime.
Table 1. A summary of studies on the bioleaching of rare earth elements (REEs) from monazite, bastnaesite and ion-adsorption rare earth ore. The number indicates the weighted mean or a range of listed REEs unless otherwise stated. NA, not provided by the authors.
| Publications | REE Resources | Main REEs | REE Content | Microbial Species | Recovery Rate (%)/Concentration (ppm) |
|---|---|---|---|---|---|
| [32] | monazite sand | Ce, La, Nd, Pr | NA | Paecilomyces sp. | 112 ppm |
| Aspergillus terreus | 101 ppm | ||||
| Aspergillus niger | 86 ppm | ||||
| [37] | monazite-bearing ore | Ce, La, Nd, Pr | 6.55% | Acetobacter aceti | 0.13% Ce, 0.11% La |
| [31] | weathered monazite | Ce, La, Nd, Pr | 31% | Penicillium sp. | 12.32 ppm |
| monazite concentrate | Ce, La, Nd | 30% | Penicillium sp. | <0.06 ppm | |
| [15] | monazite | Ce, La, Nd, Pr, Y | NA | Aspergillus niger | 0.7 ppm Ce |
| [34] | monazite | Ce, La, Nd, Pr | NA | Paecilomyces sp. | 279 ppm Nd, 287 ppm La |
| [35] | monazite concentrate | Ce, La, Nd, Pr | 31% | Penicillium sp. | 42.3 ppm |
| [17] | monazite | Ce, La, Nd, Pr, Y | 31% | Acidithiobacillus ferrooxidans, Enterobacter aerogenes | 3.1%, 40 ppm |
| [26] | weathered monazite | Ce, La, Nd, Pr, Y | 31% | Enterobacter aerogenes | 3.66 ppm |
| [23] | monazite | Ce, La, Nd | NA | Aspergillus niger | 1.1 ppm |
| [38] | bastnaesite-bearing rock | Ce, La, Nd, Pr, Sm, Eu, Gd, Tb, Dy, Ho, Er, Tm, Yb, Lu, Y | 13.4% | Streptomyces sp. | 0.08% |
| [39] | ion-adsorption clay | La, Ce, Dy, Lu | 2039.6 ppm | Aspergillus sp. | 65% La, Dy, Lu; 15.3% Ce |
| Bacillus sp. | 55% La, Dy, Lu; 14% Ce | ||||
| [40] | ion-adsorption rare earth ore | La, Nd, Y, Pr, Ce, Sm, Eu, Cd, Tb, Dy, Ho, Er, Tm, Yb | 719.2 ppm | Aspergillus niger | 48%–60% |
| Yarrowia lipolytica | 40%–55% |
However, the biorecovery rate of monazite ores is extremely low (<10%) so far compared to that of chemical leaching [18]. It demonstrates that the bioleaching approach is not competitive for processing monazite with high REE contents. Nevertheless, monazite is a main occurrence phase of REEs, so investigating the bioleaching of monazite will provide theoretical foundations for the bioleaching of other REE resources containing monazite, such as phosphogypsum and red mud.
2.2. Others
Bastnaesite is a kind of fluorocarbonate mineral, and the main light rare earth (Ce and La) resource. Typically, REEs are extracted from bastnaesite via alkaline/acid roasting and leaching at high temperatures [41][42]. Bioleaching approaches are rarely applied. In one study, four actinobacteria were isolated from REE mines and the surrounding soil, and utilized for the bioleaching of bastnaesite-bearing rock, which was mainly composed of quartz, barite, bastnaesite, and aegirine [38]. After incubation at room temperature and a 0.5% pulp density for 20 days, the most efficient strain, Streptomyces sp., leached only 0.08% of REEs (548 μg/L) from bastnaesite-bearing rock, showing poor efficiency. Possible reasons for the inefficiency are the precipitation of REE minerals, resorption of REE ions to cells, and an inadequate nutrient supply. High-performance liquid chromatography (HPLC) and a chrome azurol sulfonate (CAS) assay detected organic acids (lactic, oxalic, and pyruvic acids) and siderophores in the leachate, which contributed to the solubilization of REEs via complexation.
Another type of primary REE resource is ion-adsorption rare earth ore (IRE-ore), which is the main heavy REE reserve around the world. There is quartz, feldspar and kaolinite in IRE-ore, and REEs are mainly adsorbed on the surface of kaolinite. Therefore, REEs are commonly extracted by the ion-exchange method with ammonium sulfate [43]. As for bioleaching, Aspergillus sp. and Bacillus sp. showed potential for bioleaching ion-adsorption deposits [39] but with lower efficiency (~70%) than that of salt leaching (~80%). Compared to salt leaching, bioleaching was also more time-consuming and released more impurities, such as Si and Fe. These issues need to be addressed to apply the technology in practice. However, bioleaching has a higher heavy-to-light REE ratio compared to salt leaching because the organic acids (gluconic acid, citric acid, oxalic acid) produced by the cells have stronger complexation for HREEs than LREEs. This is advantageous, as HREEs are more valuable. In 2022, Meng et al. reported that both A. niger and Yarrowia lipolytica could recover REEs from IRE-ore at 30 °C and a 10% pulp density, with a similar leaching rate (~50%) [40]. Citrate is the main metabolite of the two microbial species, thus ammonium citrate (NH4)3Cit and citric acid obtained from the fermentation broth were used to leach REEs from IRE-ore to explore the bioleaching mechanism. The authors found that 3.3 mmol/L of (NH4)3Cit leached 90% of the REEs from IRE-ore, which was higher than the leaching efficiency achieved by citric acid or (NH4)2SO4 at the same concentration. The results suggest that complexation and ion-exchange reactions synergistically lead to the release of REEs. In addition, since REEs are mainly adsorbed on the surface of kaolinite, the leaching process did not alter the mineral phase of IRE-ore. Overall, the study provides a clean and efficient approach to extracting REEs from IRE-ore.
References
- Hossain, M.K.; Raihan, G.A.; Akbar, M.A.; Kabir Rubel, M.H.; Ahmed, M.H.; Khan, M.I.; Hossain, S.; Sen, S.K.; Jalal, M.I.E.; El-Denglawey, A. Current Applications and Future Potential of Rare Earth Oxides in Sustainable Nuclear, Radiation, and Energy Devices: A Review. ACS Appl. Electron. Mater. 2022, 4, 3327–3353.
- Erust, C.; Karacahan, M.K.; Uysal, T. Hydrometallurgical Roadmaps and Future Strategies for Recovery of Rare Earth Elements. Miner. Process. Extr. Metall. Rev. 2023, 44, 436–450.
- Rasoulnia, P.; Barthen, R.; Lakaniemi, A.-M.; Ali-Löytty, H.; Puhakka, J.A. Low residual dissolved phosphate in spent medium bioleaching enables rapid and enhanced solubilization of rare earth elements from end-of-life NiMH batteries. Miner. Eng. 2022, 176, 107361.
- Nguyen, T.T.H.; Lee, M.S. A simple process for the recovery of rare earth elements and iron from sulfuric acid leaching solution of ndfeb magnets by double salt precipitation. Miner. Process. Extr. Metall. Rev. 2023, 8, 1–5.
- Zhang, L.; Guo, X.-Y.; Tian, Q.-H.; Li, D.; Zhong, S.-P.; Qin, H. Improved thiourea leaching of gold with additives from calcine by mechanical activation and its mechanism. Miner. Eng. 2022, 178, 107403.
- Pan, J.; Nie, T.; Vaziri Hassas, B.; Rezaee, M.; Wen, Z.; Zhou, C. Recovery of rare earth elements from coal fly ash by integrated physical separation and acid leaching. Chemosphere 2020, 248, 126112.
- Pan, J.H.; Hassas, B.V.; Rezaee, M.; Zhou, C.C.; Pisupati, S.V. Recovery of rare earth elements from coal fly ash through sequential chemical roasting, water leaching, and acid leaching processes. J. Clean. Prod. 2021, 284, 124725.
- Yudaev, P.; Chistyakov, E. Chelating extractants for metals. Metals 2022, 12, 1275.
- Yudaev, P.A.; Kolpinskaya, N.A.; Chistyakov, E.M. Organophosphorous extractants for metals. Hydrometallurgy 2021, 201, 105558.
- Yudaev, P.A.; Chistyakov, E.M. Ionic liquids as components of systems for metal extraction. ChemEngineering 2022, 6, 6.
- Rychkov, V.; Kirillov, E.; Kirillov, S.; Bunkov, G.; Botalov, M.; Semenishchev, V.; Smyshlyaev, D.; Malyshev, A.; Taukin, A.; Akcil, A. Rare earth element preconcentration from various primary and secondary sources by polymeric ion exchange resins. Sep. Purif. Rev. 2022, 51, 468–483.
- Zhang, L.; Jiang, T.; Guo, X.-Y.; Tian, Q.-H.; Zhong, S.-P.; Dong, L.; Qin, H.; Liu, Z.-W.; Makuza, B. Sustainable processing of gold cyanide tailings: Reduction roasting, mechanical activation, non-cyanide leaching, and magnetic separation. Hydrometallurgy 2023, 217, 106028.
- Zhang, L.; Chen, H.; Pan, J.; Wen, Z.; Shi, S.; Long, X.; Zhou, C. The effect of physical separation and calcination on enrichment and recovery of critical elements from coal gangue. Minerals 2022, 12, 1371.
- Rafique, M.M.A. Pyrometallurgy and electrometallurgy of rare earths—Part A: Analysis of metallothermic reduction and its variants. Miner. Process. Extr. Metall. Rev. 2023, 1–8.
- Keekan, K.K.; Jalondhara, J.C. Abhilash Extraction of Ce and Th from monazite using REE tolerant Aspergillus niger. Miner. Process. Extr. Metall. Rev. 2017, 38, 312–320.
- Borai, E.H.; Hamed, M.M.; Shahr El-Din, A.M. A new method for processing of low-grade monazite concentrates. J. Geol. Soc. India 2017, 89, 600–604.
- Fathollahzadeh, H.; Hackett, M.J.; Khaleque, H.N.; Eksteen, J.J.; Kaksonen, A.H.; Watkin, E.L. Better together: Potential of co-culture microorganisms to enhance bioleaching of rare earth elements from monazite. Bioresour. Technol. Rep. 2018, 3, 109–118.
- Kumari, A.; Panda, R.; Jha, M.K.; Kumar, J.R.; Lee, J.Y. Process development to recover rare earth metals from monazite mineral: A review. Miner. Eng. 2015, 79, 102–115.
- Teixeira, L.A.V.; Silva, R.G.; Avelar, A.; Majuste, D.; Ciminelli, V.S.T. Selective extraction of rare earth elements from monazite ores with high iron content. Min. Metall. Explor. 2019, 36, 235–244.
- Lazo, D.E.; Dyer, L.G.; Alorro, R.D.; Browner, R. Treatment of monazite by organic acids I: Solution conversion of rare earths. Hydrometallurgy 2017, 174, 202–209.
- Lazo, D.E.; Dyer, L.G.; Alorro, R.D.; Browner, R. Treatment of monazite by organic acids II: Rare earth dissolution and recovery. Hydrometallurgy 2018, 179, 94–99.
- Castro, L.; Blázquez, M.L.; González, F.; Muñoz, J.A. Bioleaching of phosphate minerals using Aspergillus niger: Recovery of copper and rare earth elements. Metals 2020, 10, 978.
- Kang, X.; Csetenyi, L.; Gadd, G.M. Colonization and bioweathering of monazite by Aspergillus niger: Solubilization and precipitation of rare earth elements. Environ. Microbiol. 2021, 23, 3970–3986.
- Osman, Y.; Gebreil, A.; Mowafy, A.M.; Anan, T.I.; Hamed, S.M. Characterization of Aspergillus niger siderophore that mediates bioleaching of rare earth elements from phosphorites. World J. Microbiol. Biotechnol. 2019, 35, 93.
- Kang, X.; Csetenyi, L.; Gadd, G.M. Monazite transformation into Ce- and La-containing oxalates by Aspergillus niger. Environ. Microbiol. 2020, 22, 1635–1648.
- Fathollahzadeh, H.; Becker, T.; Eksteen, J.J.; Kaksonen, A.H.; Watkin, E.L. Microbial contact enhances bioleaching of rare earth elements. Bioresour. Technol. Rep. 2018, 3, 102–108.
- Tayar, S.P.; Palmieri, M.C.; Bevilaqua, D. Sulfuric acid bioproduction and its application in rare earth extraction from phosphogypsum. Miner. Eng. 2022, 185, 107662.
- Yang, X.; Makkonen, H.T.; Pakkanen, L. Rare earth occurrences in streams of processing a phosphate ore. Minerals 2019, 9, 262.
- Zhang, D.-R.; Chen, H.-R.; Nie, Z.-Y.; Xia, J.-L.; Li, E.-P.; Fan, X.-L.; Zheng, L. Extraction of Al and rare earths (Ce, Gd, Sc, Y) from red mud by aerobic and anaerobic bi-stage bioleaching. Chem. Eng. J. 2020, 401, 125914.
- Su, H.; Tan, F.; Lin, J. An integrated approach combines hydrothermal chemical and biological treatment to enhance recycle of rare metals from coal fly ash. Chem. Eng. J. 2020, 395, 124640.
- Corbett, M.K.; Eksteen, J.J.; Niu, X.-Z.; Croue, J.-P.; Watkin, E.L.J. Interactions of phosphate solubilising microorganisms with natural rare-earth phosphate minerals: A study utilizing Western Australian monazite. Bioprocess Biosyst. Eng. 2017, 40, 929–942.
- Brisson, V.L.; Zhuang, W.Q.; Alvarez-Cohen, L. Bioleaching of rare earth elements from monazite sand. Biotechnol. Bioeng. 2016, 113, 339–348.
- Brisson, V.L.; Zhuang, W.-Q.; Alvarez-Cohen, L. Metabolomic analysis reveals contributions of citric and citramalic acids to rare earth bioleaching by a Paecilomyces fungus. Front. Microbiol. 2020, 10, 3008.
- Maes, S.; Zhuang, W.-Q.; Rabaey, K.; Alvarez-Cohen, L.; Hennebel, T. Concomitant leaching and electrochemical extraction of rare earth elements from monazite. Environ. Sci. Technol. 2017, 51, 1654–1661.
- Corbett, M.K.; Eksteen, J.J.; Niu, X.-Z.; Watkin, E.L.J. Syntrophic effect of indigenous and inoculated microorganisms in the leaching of rare earth elements from Western Australian monazite. Res. Microbiol. 2018, 169, 558–568.
- Billah, M.; Khan, M.; Bano, A.; Hassan, T.U.; Munir, A.; Gurmani, A.R. Phosphorus and phosphate solubilizing bacteria: Keys for sustainable agriculture. Geomicrobiol. J. 2019, 36, 904–916.
- Shin, D.; Kim, J.; Kim, B.-S.; Jeong, J.; Lee, J.-C. Use of phosphate solubilizing bacteria to leach rare earth elements from monazite-bearing ore. Minerals 2015, 5, 189–202.
- Zhang, L.; Dong, H.; Liu, Y.; Bian, L.; Wang, X.; Zhou, Z.; Huang, Y. Bioleaching of rare earth elements from bastnaesite-bearing rock by actinobacteria. Chem. Geol. 2018, 483, 544–557.
- Barnett, M.J.; Palumbo-Roe, B.; Gregory, S.P. Comparison of heterotrophic bioleaching and ammonium sulfate ion exchange leaching of rare earth elements from a Madagascan ion-adsorption clay. Minerals 2018, 8, 236.
- Meng, X.; Zhao, H.; Zhang, Y.; Shen, L.; Gu, G.; Qiu, G.; Zhang, X.; Yu, H.; He, X.; Liu, C. Simulated bioleaching of ion-adsorption rare earth ore using metabolites of biosynthetic citrate: An alternative to cation exchange leaching. Miner. Eng. 2022, 189, 107900.
- Cen, P.; Bian, X.; Liu, Z.; Gu, M.; Wu, W.; Li, B. Extraction of rare earths from bastnaesite concentrates: A critical review and perspective for the future. Miner. Eng. 2021, 171, 107081.
- Wang, L.; Huang, X.; Yu, Y.; Zhao, L.; Wang, C.; Feng, Z.; Cui, D.; Long, Z. Towards cleaner production of rare earth elements from bastnaesite in China. J. Clean. Prod. 2017, 165, 231–242.
- Zhang, Q.; Shu, W.; Li, F.; Li, M.; Zhou, J.; Tian, C.; Liu, S.; Ren, F.; Chen, G. Nitrate source apportionment and risk assessment: A study in the largest ion-adsorption rare earth mine in China. Environ. Pollut. 2022, 302, 119052.
More
Information
Subjects:
Mineralogy
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
695
Revisions:
2 times
(View History)
Update Date:
15 Sep 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




