Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Undurti N Das | -- | 1329 | 2023-09-12 02:42:13 | | | |
| 2 | Fanny Huang | Meta information modification | 1329 | 2023-09-12 07:38:48 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Das, U.N. Toll-like Receptors and Eicosanoids in Sepsis. Encyclopedia. Available online: https://encyclopedia.pub/entry/49041 (accessed on 07 February 2026).
Das UN. Toll-like Receptors and Eicosanoids in Sepsis. Encyclopedia. Available at: https://encyclopedia.pub/entry/49041. Accessed February 07, 2026.
Das, Undurti N.. "Toll-like Receptors and Eicosanoids in Sepsis" Encyclopedia, https://encyclopedia.pub/entry/49041 (accessed February 07, 2026).
Das, U.N. (2023, September 12). Toll-like Receptors and Eicosanoids in Sepsis. In Encyclopedia. https://encyclopedia.pub/entry/49041
Das, Undurti N.. "Toll-like Receptors and Eicosanoids in Sepsis." Encyclopedia. Web. 12 September, 2023.
Copy Citation
Sepsis is the leading cause of death from infection. Its incidence is on the rise. Sepsis is characterized by life-threatening organ dysfunction caused by a dysregulated host response to infection, and it can occur after major surgery and injury. TLRs (toll-like receptors) regulate free radical generation, macrophage and leukocyte function, and modulate eicosanoid synthesis, and thus have a critical role in inflammation, immune response, and development and/or recovery from sepsis.
sepsis
eicosanoids
toll-like receptors
1. Introduction
Sepsis is the leading cause of death from infection. Its incidence is on the rise. In the United States alone, sepsis accounted for more than 20 billion dollars in hospital costs in 2011. Sepsis is defined as life-threatening organ dysfunction due to a dysregulated host response to infection. Septic shock is defined as a subset of sepsis, in which particularly profound circulatory, cellular, and metabolic abnormalities substantially increase mortality. The new diagnostic tool quickSOFA, or qSOFA, can be used to detect, at the bedside, patients who are at risk for sepsis. These are those who are experiencing:
-
An alteration in mental status
-
A decrease in systolic blood pressure of less than 100 mm Hg
-
A respiration rate greater than 22 breaths/min
Studies suggest that patients with two or more of these conditions are at a significantly greater risk of having a prolonged ICU stay (3 or more days) or dying in the hospital. Hence, these patients need to be investigated further for organ dysfunction, therapy should be initiated or escalated, as appropriate, and the frequency of monitoring should be increased [1]. Despite these clinical indices in the evaluation of patients with sepsis, it will be worthwhile to have and/or establish specific laboratory indices that could serve as indicators of prognosis and response to therapy. Such laboratory indices could include: plasma cytokines, nitric oxide (NO), antioxidants (such as SOD, catalase, glutathione), lipid peroxides, ROS (reactive oxygen species), and pro- and anti-inflammatory eicosanoids. Several studies did look at these indices in sepsis and other critical illnesses, but a comprehensive study of these parameters was not done. In addition, there were no studies that looked at the potential interaction(s) among the various indices enumerated above with specific reference to sepsis.
Sepsis is characterized by life-threatening organ dysfunction caused by a dysregulated host response to infection, and it can occur after major surgery and injury. Dysregulated innate and adaptive immunity, as seen in those with sepsis, can result in sustained immunosuppression, predisposing them to secondary opportunistic infections. Paradoxically, autopsy studies revealed only minimal signs of inflammation or necrosis [2][3]. It is likely that the initial hyperinflammatory response(s) may result in the development of subsequent immunosuppression [4]. It is likely that the duration of the initial hyperinflammatory response and subsequent immunosuppression are variable. The heterogeneous presentation(s) and responses seen in sepsis may result in failure to recover from tissue injury, wound healing, and restoration of homeostasis. When dynamic changes in the innate and adaptive immune responses in sepsis was correlated with patient outcomes, it was found that absolute lymphocyte counts and lymphocyte subsets levels were reduced in those with sepsis, and there was an increase in the proportion of Tregs correlated with disease progression and immunosuppression, suggesting that downregulated adaptive immunity is responsible for the prolonged immune suppression seen in sepsis. In contrast, though cellular immunity reverted to near normal within 2 weeks of admission, humoral and innate immunity recovery lags. These findings suggest that appropriate therapeutic approaches need to be developed to improve the immune responses in those with sepsis.
This heterogenous presentation of sepsis could be attributed to mutations in Toll-like receptor-4 (TLR-4) [5][6][7][8], though this has been disputed [9]. Mutations in TLR-4 are associated with differences in lipopolysaccharide (LPS) responsiveness in humans, implying that host response to infections may be variable, rendering some to develop sepsis, while others may be resistant [5][6]. This indicates that an imbalance in the synthesis and release of pro-inflammatory and inflammation-resolving molecules determines the degree of infection, injury, and recovery from sepsis. Thus, a disparity in the timing and generation of adequate amounts of pro- and anti-inflammatory molecules determines the degree of the inflammatory process, inflammation resolution, and tissue repair in a sequential and coordinated fashion. TLRs regulate free radical generation, macrophage and leukocyte function, and modulate eicosanoid synthesis, and thus have a critical role in inflammation, immune response, and development and/or recovery from sepsis [10][11][12]. Hence, efforts need to be made to revert the initial hyperinflammatory response and subsequent immunosuppression to facilitate recovery from sepsis to one in which there could be a role for essential fatty acids (EFAs) and their metabolites. This is further supported by the observation that EFAs and their metabolites have a regulatory role in the elaboration of various cytokines, reactive oxygen species (ROS), TLR and NF-kB expression, and the cGAS-STING pathway.
2. TLRs and Eicosanoids in Sepsis
Polyunsaturated fatty acids (PUFAs) form an important constituent of all cell membranes and regulate cell membrane fluidity and the expression of receptors on their surface and serve as mechanotransducers to convey external stimuli to the cytoplasm and DNA [13]. This cross-communication between the cell membrane PUFAs and the nucleus (and, consequently, genes) enables the cell to tailor its responses to external stimuli and produce adequate changes in cell shape and control dynamic cell behavior [13][14][15][16]. Thus, cells sense their physical environment through their plasma membrane, which harbors mechanosensitive ion channels and adhesion molecules. It is likely that cells sense their environment though the cell membrane that induces stretch in the nuclear membrane, leading to the activation of the enzyme cytosolic phospholipase A2 (cPLA2), which induces the release of cell membrane PUFAs (especially arachidonic acid, AA). The released AA, in turn, initiates cell blebbing and movements, as well as other cell functions [13]. It is also known that cPLA2 senses nuclear swelling upon osmotic shock to initiate rapid immune cell chemotaxis [17][18]. AA can be converted to form various eicosanoids, which attract immune cells and control cell differentiation and survival, among various other functions (see Figure 1 for metabolism of essential fatty acids). These studies suggest that the fluidity of plasma and nuclear membranes sense physical clues from the cell environment and converts them into chemical signals that drive inflammation. It is noteworthy that cPLA(2)(−/−) (cPLA2 knockout) mice recover from allergen-induced bronchoconstriction and show no airway hyperresponsiveness. Their peritoneal macrophages [cPLA(2)(−/− mice] do not produce prostaglandins (PGs), leukotriene B4 (LTB4), and cysteinyl leukotrienes after stimulation. Moreover, cPLA(2)(−/−) mice bone marrow-derived mast cells also do not produce eicosanoids. Thus, cPLA2 has a critical role in inflammation and tissue injury that is relevant to the pathobiology of sepsis.
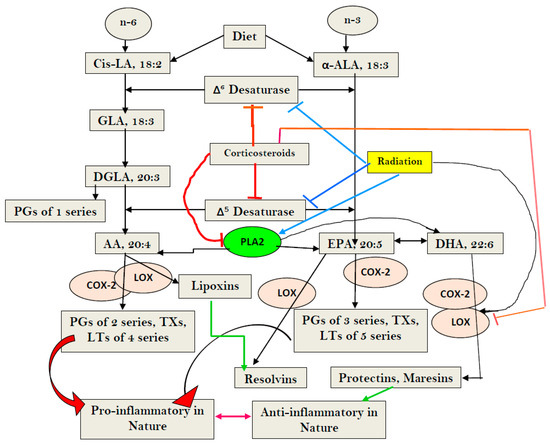
Figure 1. Scheme showing metabolism of essential fatty acids (EFAs) and the effect of corticosteroids on the same. Note the differences in the actions of corticosteroids (dexamethasone) and radiation. Corticosteroids inhibit desaturases, and PLA2, COX-2, and LOX enzymes, whereas radiation blocks only desaturases, but activates PLA2, COX-2, and LOX enzymes. Radiation enhances the formation of PGs, LTs, and TXs, but blocks the formation of LXA4, resolvins, protectins, and maresins by inducing deficiency of AA/EPA/DHA, whereas corticosteroids block the formation of PGS, LTs, TXs, and LXA4, resolvins, protectins, and maresins by inhibiting PLA2, COX-2, and LOX enzymes, and induces deficiency of AA/EPA/DHA by inhibiting desaturases. Thus, corticosteroids are potent inhibitors of inflammation but also block resolution of inflammation, whereas radiation is a potent inducer of inflammation and interferes with the inflammation resolution process.
PUFAs modulate the expression of TLRs. COX-2 (cyclooxygenase-2) mediated high production of PGE2 following LPS stimulation, whereas LPS down-regulated COX-1, and COX-1 deficiency enhanced PGE2 production. Thus, coordinated down-regulation of COX-1 facilitates PGE2 production after TLR activation [19][20][21]. On the other hand, the supplementation of AA and docosahexaenoic acid (DHA) inhibited intestinal TLR-4 gene expression and ameliorated NEC (necrotizing enterocolitis) [22][23]. Resolvins and protectin D1, which are derived from DHA, inhibited the number of infiltrating leukocytes, blocked TLR-mediated activation of macrophages, and suppressed ischemia-reperfusion-induced kidney injury [24][25]. These results emphasize the fact that the coordinated synthesis, release, and action of pro- and anti-inflammatory eicosanoids can control inflammation and its resolution, including in sepsis [23][24][25][26][27][28].
References
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.-D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810.
- Toti, P.; De Felice, C.; Occhini, R.; Schuerfeld, K.; Stumpo, M.; Epistolato, M.C.; Vatti, R.; Buonocore, G. Spleen depletion in neonatal sepsis and chorioamnionitis. Am. J. Clin. Pathol. 2004, 122, 765–771.
- Monneret, G. How to identify systemic sepsis-induced immunoparalysis. Adv. Sepsis 2005, 4, 42–49.
- Monneret, G.; Venet, F.; Pachot, A.; Lepape, A. Monitoring Immune Dysfunctions in the Septic Patient: A New Skin for the Old Ceremony. Mol. Med. 2008, 14, 64–78.
- Arbour, N.C.; Lorenz, E.; Schutte, B.C.; Zabner, J.; Kline, J.N.; Jones, M.; Frees, K.; Watt, J.L.; Schwartz, D.A. TLR4 mutations are associated with endotoxin hyporesponsiveness in humans. Nat. Genet. 2000, 25, 187–191.
- Schwartz, D.A. The role of TLR4 in endotoxin responsiveness in humans. J. Endotoxin Res. 2001, 7, 389–493.
- Albert Vega, C.; Karakike, E.; Bartolo, F.; Mouton, W.; Cerrato, E.; Brengel-Pesce, K.; Giamarellos-Bourboulis, E.J.; Mallet, F.; Trouillet-Assant, S. Differential response induced by LPS and MPLA in immunocompetent and septic individuals. Clin. Immunol. 2021, 226, 108714.
- Holmes, C.L.; Russell, J.A.; Walley, K.R. Genetic polymorphisms in sepsis and septic shock: Role in prognosis and potential for therapy. Chest 2003, 124, 1103–1115.
- Imahara, S.D.; Jelacic, S.; Junker, C.E.; O’Keefe, G.E. The TLR4 +896 polymorphism is not associated with lipopolysaccharide hypo-responsiveness in leukocytes. Genes Immun. 2005, 6, 37–43.
- Shishido, T.; Nozaki, N.; Takahashi, H.; Arimoto, T.; Niizeki, T.; Koyama, Y.; Abe, J.-I.; Takeishi, Y.; Kubota, I. Central role of endogenous Toll-like receptor-2 activation in regulating inflammation, reactive oxygen species production, and subsequent neointimal formation after vascular injury. Biochem. Biophys. Res. Commun. 2006, 345, 1446–1453.
- Norris, P.C.; Reichart, D.; Dumlao, D.S.; Glass, C.K.; Dennis, E.A. Specificity of eicosanoid production depends on the TLR-4-stimulated macrophage phenotype. J. Leukoc. Biol. 2011, 90, 563–574.
- Lefebvre, J.S.; Marleau, S.; Milot, V.; Lévesque, T.; Picard, S.; Flamand, N.; Borgeat, P. Toll-like receptor ligands induce polymorphonuclear leukocyte migration: Key roles for leukotriene B4 and platelet-activating factor. FASEB J. 2010, 24, 637–647.
- Das, U.N. Arachidonic Acid as Mechanotransducer of Renin Cell Baroreceptor. Nutrients 2022, 14, 749.
- Lomakin, A.J.; Cattin, C.J.; Cuvelier, D.; Alraies, Z.; Molina, M.; Nader, G.P.F.; Srivastava, N.; Sáez, P.J.; Garcia-Arcos, J.M.; Zhitnyak, I.Y.; et al. The nucleus acts as a ruler tailoring cell responses to spatial constraints. Science 2020, 370, eaba2894.
- Venturini, V.; Pezzano, F.; Castro, F.C.; Häkkinen, H.-M.; Jiménez-Delgado, S.; Colomer-Rosell, M.; Marro, M.; Tolosa-Ramon, Q.; Paz-López, S.; Valverde, M.A.; et al. The nucleus measures shape changes for cellular proprioception to control dynamic cell behavior. Science 2020, 370, eaba2644.
- Shen, Z.; Niethammer, P. A cellular sense of space and pressure. Science 2020, 370, 295–296.
- Enyedi, B.; Kala, S.; Nikolich-Zugich, T.; Niethammer, P. Tissue damage detection by osmotic surveillance. Nature 2013, 15, 1123–1130.
- Sapirstein, A.; Bonventre, J.V. Specific physiological roles of cytosolic phospholipase A2 as defined by gene knockouts. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2000, 1488, 139–148.
- Font-Nieves, M.; Sans-Fons, M.G.; Gorina, R.; Bonfill-Teixidor, E.; SalasPerdomo, A.; Marquez-Kisinousky, L. Induction of COX-2 Enzyme and Down-regulation of COX-1 Expression by Lipopolysaccharide (LPS) Control Prostaglandin E2 Production in Astrocytes. J. Biol. Chem. 2012, 287, 6454–6468.
- Gorina, R.; Font-Nieves, M.; Márquez-Kisinousky, L.; Santalucia, T.; Planas, A.M. Astrocyte TLR4 activation induces a proinflammatory environment through the interplay between MyD88-dependent NFκB signaling, MAPK, and Jak1/Stat1 pathways. Glia 2011, 59, 242–255.
- Balistreri, C.R.; Caruso, C.; Listì, F.; Colonna-Romano, G.; Lio, D.; Candore, G. LPS-mediated production of pro/anti-inflammatory cytokines and eicosanoids in whole blood samples: Biological effects of +896A/G TLR4 polymorphism in a Sicilian population of healthy subjects. Mech. Ageing Dev. 2011, 132, 86–92.
- Lu, J.; Jilling, T.; Li, D.; Caplan, M.S. Polyunsaturated Fatty Acid Supplementation Alters Proinflammatory Gene Expression and Reduces the Incidence of Necrotizing Enterocolitis in a Neonatal Rat Model. Pediatr. Res. 2007, 61, 427–432.
- Caplan, M.S.; Russell, T.; Xiao, Y.; Amer, M.; Kaup, S.; Jilling, T. Effect of Polyunsaturated Fatty Acid (PUFA) Supplementation on Intestinal Inflammation and Necrotizing Enterocolitis (NEC) in a Neonatal Rat Model. Pediatr. Res. 2001, 49, 647–652.
- Duffield, J.S.; Hong, S.; Vaidya, V.S.; Lu, Y.; Fredman, G.; Serhan, C.N.; Bonventre, J.V. Resolvin D Series and Protectin D1 Mitigate Acute Kidney Injury. J. Immunol. 2006, 177, 5902–5911.
- Hassan, I.R.; Gronert, K. Acute changes in dietary omega-3 and omega-6 polyunsaturated fatty acids have a pronounced impact on survival following ischemic renal injury and formation of renoprotective docosahexaenoic acid-derived protectin D1. J. Immunol. 2009, 182, 3223–3322.
- Spite, M.; Norling, L.V.; Summers, L.; Yang, R.; Cooper, D.; Petasis, N.A.; Flower, R.J.; Perretti, M.; Serhan, C.N. Resolvin D2 is a potent regulator of leukocytes and controls microbial sepsis. Nature 2009, 461, 1287–1292.
- Das, U. HLA-DR expression, cytokines and bioactive lipids in sepsis. Arch. Med. Sci. 2014, 10, 325–335.
- Das, U.N. Pro- and anti-inflammatory bioactive lipids imbalance contributes to the pathobiology of autoimmune diseases. Eur. J. Clin. Nutr. 2022, 77, 637–651.
More
Information
Subjects:
Critical Care Medicine
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
435
Revisions:
2 times
(View History)
Update Date:
12 Sep 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




