Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Camelia Elena Luchian | -- | 8528 | 2023-09-11 09:24:19 | | | |
| 2 | Camila Xu | Meta information modification | 8528 | 2023-09-11 09:39:19 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Buican, B.; Colibaba, L.C.; Luchian, C.E.; Kallithraka, S.; Cotea, V.V. “Orange” Wine. Encyclopedia. Available online: https://encyclopedia.pub/entry/49008 (accessed on 07 February 2026).
Buican B, Colibaba LC, Luchian CE, Kallithraka S, Cotea VV. “Orange” Wine. Encyclopedia. Available at: https://encyclopedia.pub/entry/49008. Accessed February 07, 2026.
Buican, Bettina-Cristina, Lucia Cintia Colibaba, Camelia Elena Luchian, Stamatina Kallithraka, Valeriu V. Cotea. "“Orange” Wine" Encyclopedia, https://encyclopedia.pub/entry/49008 (accessed February 07, 2026).
Buican, B., Colibaba, L.C., Luchian, C.E., Kallithraka, S., & Cotea, V.V. (2023, September 11). “Orange” Wine. In Encyclopedia. https://encyclopedia.pub/entry/49008
Buican, Bettina-Cristina, et al. "“Orange” Wine." Encyclopedia. Web. 11 September, 2023.
Copy Citation
“Orange” wine, a product derived from white grapes, encapsulates the intriguing allure of ancient winemaking methods that trace their roots back to Georgia. The method enables an elevated presence of phenolic compounds, which can have a favorable influence on the sensory characteristics of the wines or their behavior during oxidative processes.
orange
qvevri
Georgia
ancient
winemaking
technology
1. Introduction
Originating from the cultural crossroads between Western Asia and Eastern Europe, specifically in Georgia, an ancient winemaking tradition thrives and continues to resonate in the modern wine world. This deep-rooted history traces back to the Neolithic period, around 6000–5000 BC, when wines were crafted in pottery vessels that served as multipurpose containers for both fermentation and aging of the wine. The preservation and revival of this ancient winemaking heritage highlight its enduring significance and its contribution to the rich tapestry of the wine industry [1]. In recent times, there has been a rebirth and restructuring of the Georgian technique for producing skin-macerated wines. This method has re-emerged in the wine world and has gained recognition within the industry. Notably, the International Organisation of Vine and Wine (OIV) stepped in and regulated this field in 2020, providing a framework and guidelines for the production of these unique wines. The acknowledgment from the OIV highlights the growing significance and acceptance of this Georgian winemaking technique on a global scale [2].
The shifting consumer trends towards conscious consumption have led to a heightened awareness of the ingredients and processes involved in everyday products. This has sparked a particular interest in understanding the inputs utilized in various technological processes, including winemaking. In recent years, there has been a significant rise in the popularity of wines produced using traditional methods, driven by a growing demand for sustainability and preservation.
While the skin maceration technique is commonly associated with red wine production, it is also employed in white wine production as pre-fermentative skin maceration. This technique aims to enhance the extraction of varietal aromas and improve the overall quality of the wine. By embracing such methods, winemakers can create unique and aromatic white wines that captivate the palates of discerning consumers seeking distinctive and high-quality products [3]. In the winemaking process, the grapes are typically destemmed and crushed before undergoing maceration together with the must. This maceration step takes place under carefully controlled conditions, with factors such as time and temperature playing vital roles. During maceration, phenolic compounds are extracted from the grape skins, which can contribute to increased bitterness, astringency, and the browning of white wines. These factors influence the overall flavor profile and characteristics of the final product [4][5]. In this matter, extensive research has been carried out to determine the optimal conditions and maceration techniques so that the quality of the wines is at its highest [3][6][7].
The informal labeling of “orange” wine, amber wine, or qvevri wine may be misleading; however, the processing style is similar to the one of the red winemaking, resulting in a “distinctive dry and tannic white-grape wine style” as Jancis Robinson states [8]. “Orange” wines refer to wines produced from white wine grape varieties possessing elevated phenolic compound content, undergoing an extended maceration–fermentation phase within the technological process [9][10]. These wines possess distinct sensory characteristics that set them apart from traditional white wines. While they retain some of the flavor profiles commonly associated with white wines, their texture and astringency resemble those of red wines. The defining attributes of these wines are primarily enhanced through the skin contact fermentation method, which involves fermenting the grapes with or without destemming, followed by a prolonged post-fermentation period that can last for weeks, months, or even years. This extended contact with the grape skins contributes to the unique sensory experience and complexity found in these wines [11]. The purpose of prolonged skin contact is to extract phenolic compounds from grape skins, seeds, and potentially even stems. These compounds play a crucial role in creating the distinctive stylistic differences observed in these wines. Additionally, the extended contact with the grape solids during fermentation can contribute to a potential oxidative characteristic, further adding to the unique qualities and flavors of these wines [12]. Every anatomical part of the grape cluster contributes to shaping the characteristics of “orange” wine in its own unique way. The significance of seeds in extracting phenolic compounds stands out, especially proanthocyanidins which comprise a considerable share—ranging from 60% to 70%—of the total extractable phenolics in grapes. These compounds showcase improved extraction with prolonged maceration, concurrently aiding in protein binding to prevent protein haze formation [13]. Additionally, they play a role in enhancing the wine’s flavor profile and aromatic complexity. Meanwhile, the grape skins are responsible for extracting varietal-specific and softer aroma compounds, enhancing the overall aromatic profile of the wine. In contrast, the stems have a clarifying effect, helping to remove unwanted solids and contribute to the wine’s clarity. Each component of the grape cluster plays a distinct role in the production of “orange” wine, contributing to its nuanced and multi-faceted character [14][15]. The extraction of aroma compounds is an initial and rapid process that typically occurs within the first few days of skin contact during maceration. However, the long-term maceration technique introduces changes to some of these compounds over time. These changes are influenced by various factors, and the specific effects are still being explored through ongoing research. As such, the understanding of how these compounds evolve during extended maceration is still in its early stages, necessitating further investigation and study [16][17].
In the absence of anthocyanins, the attention in “orange” wines is primarily centered around the extraction of tannins. Tannins, alongside various other polyphenolic compounds, are a focal point of comprehensive research within the realm of red wines [18][19]. This attention is attributed to their well-established impact due to their positive bioactive influence on the well-being of the consumer through moderate wine consumption. These effects encompass risk reduction for diabetes, atherogenesis, and coronary heart disease, as well as encompass anti-inflammatory, anti-cancer, and antiallergenic properties, among others [20][21][22][23]. The exploration of tannins and polyphenols in “orange” wines endeavors to elucidate their influence on wine attributes, while also aiming to forge distinct characteristics that set it apart from other products [24]. Albeit there is no extensive knowledge about these compounds in the prolonged maceration of white grape varieties, which opens a wide array of questions towards this topic, starting from whether there is any difference between the amount of tannins extracted compared to red wines or which ratio maceration time–skin amount is more suitable for the consumers taste up to which varieties are more suitable for this type of technique.
2. The History of an Old Wine in a New World
2.1. The Beginnings of the Wine Era
The process of domestication and the ancestral origins of Vitis vinifera ssp. vinifera are believed to have originated in the southern region of the Caucasus, which is located between the Black Sea and the Caspian Sea. This specific area is now known as the country of Georgia. The basis for this claim is supported by a range of evidence, including archaeobotanical and archaeological findings, as well as the abundant presence of diverse wild grapevine varieties indigenous to the region. These factors collectively contribute to the argument supporting Georgia as a significant historical hub for the domestication and origin of Vitis vinifera ssp. vinifera [25]. The Georgian country is home to a rich tapestry of viticulture, with over 500 indigenous grape varieties flourishing across its land [26]. Through painstaking archaeological investigations conducted over the past few decades, significant evidence has emerged to support the existence and production of Neolithic wine. This evidence stems from the analysis of pottery fragments discovered during an excavation in 1960 at Shulaveris Gora, located in the province of Kvemo Kartli (Figure 1), approximately 50 km south of the capital city, Tbilisi, in Georgia. The presence of tartaric acid/tartrate on the surface of these fragments, considered a biomarker of grapes and wines, sheds light on the intriguing world of the Shulaveri–Shomutepe culture, which dates back to 6000–4000 BCE.
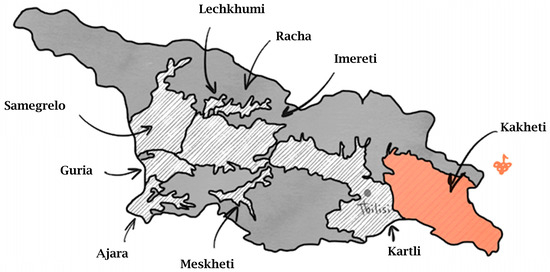
Figure 1. Wine regions of Georgia.
This discovery provides a fascinating glimpse into the ancient roots of winemaking and its historical significance in the region [1]. The subsequent culture known as the “Kura–Araxes culture,” which emerged between 4000 and 2000 BCE, played a pivotal role in propagating the wine tradition across the Southern Caucasus region [27]. Historical records indicate that during that period, vessels similar to the qvevri were commonly utilized for the production and storage of wines. These vessels, which share similarities with the qvevri, were employed to ferment and age the wines [28].
2.2. Symbolic State of the Georgian Wine during the Christian Period
Viticulture and winemaking continued to hold significant importance even during the Christian Period (4th–5th CE) in Georgia. A pivotal figure in the spread of Christianity in the region was Saint Nino, who played a significant role in introducing the faith to Georgian lands. Symbolically, Saint Nino used grapevine canes to create the “Grapevine Cross”, which became an enduring symbol of the Georgian Orthodox Church. This connection between the introduction of Christianity and the grapevine highlights the deep intertwining of religious and viticultural traditions in Georgian history. Saint Nino’s contribution further solidifies the enduring cultural significance of viticulture and winemaking in Georgia throughout different historical periods [29]. The cross he was spreading the Word of God with was given to him by itself, the Holy Virgin Mary, with the following blessing: “With this you will defeat the evil, insidiousness, and your sermons will be fruitful, I will be for you a shield” [30]. From an economic standpoint, wine production gradually became a central activity within the church, with the clergy incorporating it into their religious services. This integration of wine into religious practices led to the development of viticultural tools and winery equipment. Notably, the clay vessels known as “Qvevri” emerged during this period and have since become an iconic winemaking method in the country [31].
2.3. Challenges and Resilience under the Soviet Union’s Occupation
Despite facing numerous challenges and setbacks, the viticulture and winemaking industry in Georgia has shown remarkable resilience and perseverance, enabling it to maintain a truly distinctive product in the market. Over the years, the industry has encountered significant obstacles that have impacted both the quality and economic aspects of winemaking.
One such setback was the outbreak of phylloxera in the early 1880s, which devastated vineyard areas, leading to a substantial decline in production throughout the 20th century. Additionally, the country experienced a period of occupation by the Soviet Union from 1922 to 1991, which further influenced the wine industry and necessitated reforms. Despite these challenges, the unwavering determination of the Georgian population has played a crucial role in preserving their unique winemaking traditions and ensuring the continuation of their exceptional product in the market [32]. During the Soviet Union’s occupation of Georgia between 1922 and 1991, significant changes were implemented in the vineyards and winemaking practices. As part of the reforms, vineyards were uprooted, and there was an emphasis on planting autochthonous varieties such as Saperavi and Rkatsiteli. This strategic decision proved beneficial for the country and the Republic in the long run as it positioned Georgia prominently on the map of wine producers. The wines gained rapid popularity within the Union of Soviet Socialist Republics and found their place in the export market. However, the primary focus during this period was on quantity rather than quality, leading to a shift from small-scale farm properties to collective production. The emphasis on mass production impacted the overall quality of Georgian wines, prioritizing volume over craftsmanship [33]. However, households were still allowed to produce their own wine but only for the purpose of their own consumption [34]. During the same period, Georgia faced another significant challenge in the form of the anti-alcohol campaign (sukhoy zakon) initiated by Mikhail Gorbachev from 1985 to 1987. This campaign had a profound impact on the country’s vineyards, resulting in the destruction of more than 50% of the total planted areas. The vineyard land surfaces dwindled from 160,000 hectares in the 1980s to just 62,000 hectares by the time Georgia regained its independence. The anti-alcohol campaign aimed to reduce alcohol consumption but had unintended consequences for the Georgian wine industry, leading to a drastic reduction in vineyard areas and a subsequent decline in wine production [35].
2.4. Post-Soviet Georgian wine Industry Revival
The post-Soviet period brought prominent obstacles of considerable importance to the Georgian wine industry as it underwent a transition. The process was particularly difficult due to the lack of knowledge among farmers, stemming from the previous land privatization policies. This posed hurdles for the integration of the concept of geographical indication (GI) among small and medium-scale producers. However, in 1999, Georgia took a significant step by enacting a law to regulate, protect, and utilize GIs, known as “The Law on Appellations of Origin of Goods and Geographical Indication in Georgia.” This law recognized and registered 18 different GIs in the wine sector, providing a framework for the quality assurance and promotion of distinct regional wine production. Despite the initial challenges, the establishment of this law marked an important milestone in protecting and promoting the unique identity of Georgian wines [34][36]. Following a period of recovery efforts, the wine markets of Georgia and Moldova were dealt a significant blow when Russia imposed an embargo on their wines in 2006. This embargo had a devastating impact, resulting in a substantial loss of approximately 87% in terms of the export market. Russia had been the primary export destination for Georgian wines, making the embargo a severe setback for the industry. The sudden restriction placed on wine exports to Russia disrupted the established trade relationships and forced Georgian winemakers to seek new markets and diversify their export strategies [35]. Issues in the economic sector arose and the largest wineries tried to shift the exports towards more promising countries such as Germany, Ukraine, Poland, Estonia, Latvia, and Lithuania [37]. The informal gatherings of villagers and their unorthodox approach to winemaking played a crucial role in the preservation and promotion of rare grape varieties, as well as the unique production techniques associated with them. These community-driven efforts aimed to safeguard traditional winemaking practices from being lost and to highlight the value of lesser-known grape varieties. Through their shared knowledge and collective experiences, the villagers fostered a sense of protection and appreciation for their local winemaking heritage, ensuring its continuity for future generations [34].
2.5. The Travel of the Long-Term Macerated White Wine Style
The dissemination of this valuable knowledge faced initial challenges in reaching beyond borders. However, a significant breakthrough occurred when the traditional winemaking method of fermenting grapes in egg-shaped earthenware vessels, known as qvevri, was recognized and included in the UNESCO World Heritage List alongside renowned wine regions such as Tokaj and St. Emilion in 2013. This prestigious recognition catapulted Georgia into the spotlight of the global wine market, particularly for its distinctive amber wines. These wines, made from white grapes fermented together with their skins and sometimes stems in the traditional qvevri, became a hallmark of Georgia’s winemaking identity, representing the country’s unique contribution to the world of wine [38][39].
In the region of Collio, located in the northeastern area of the Friuli Venezia Giulia region, the practice of using long maceration in winemaking as a protection against oxidation has been employed for a long time. Oxidation has been known for many years to have an impact on the characteristics of wines, mainly in the browning phenomenon triggered by the reactions of oxidation of the polyphenolic compounds, reactions catalyzed by metals [40][41]. However, with the introduction of newer techniques in the 1960s and 1970s, such as stainless steel tanks equipped with cooling systems, there was a shift towards extended production styles, leaving the traditional macerated white wines mostly associated with rustic farmhouse production [42]. In contemporary winemaking, a multitude of techniques are employed to manage or encourage the oxidation phenomenon [43][44][45]. Notably prominent among these methods are the oxidative and reductive approaches. These two distinct winemaking styles characterize divergent philosophies and scientific principles, each exerting substantial influence over the aromatic, flavor, and textural dimensions of the resulting wine. Oxygenation plays a crucial role in the maturation process of wines, facilitating the emergence of matured (tertiary) notes in both flavor and aroma. Wines crafted using reductive methods typically boast a dynamic and revitalizing profile, marked by a dominant expression of varietal grape characteristics in both odor and taste [46][47]. In instances where sulfur dioxide is omitted, the processing of white grape juice triggers enzymatic oxidation, leading to the creation of insoluble brown pigments as phenolic compounds precipitate. Wines originating from oxidized must exhibit increased resilience against the quality deterioration brought about by oxidation during aging. Hyperoxidation, an intriguing technique employed for specific wines such as Sherry wines, intentionally promotes oxidation before fermentation, thereby enhancing the wines’ shelf life [48][49]. The sensory effects of this method primarily stem from the removal of flavonoids [50]. This technique could present an intriguing possibility for prolonged macerated white wines, particularly in the context of shelf life, which currently remains an area of limited research.
Josko Gravner, a highly esteemed oenologist from the Friuli region, experimented with different methods such as fermenting in barriques or small oak barrels but eventually decided to embrace the ancient traditions. In 1996, he conducted his initial trial, although it was not specifically carried out using amphorae. This first attempt proved to be successful, utilizing the Ribolla Gialla variety. Subsequently, after a visit to Georgia in 2000, Gravner made significant changes to his entire cellar, replacing the old containers with qvevri vessels that resemble the traditional technology used in the birthplace of long-macerated white wines [51].
Another region where the amphorae method has been implemented is in the southern region of Portugal, specifically within the Alentejo area, a tradition wherein wine is crafted using ancient methods involving clay vessels. In this distinctive approach, destemmed and crushed grapes undergo fermentation within sizable clay containers, and the process involves no incorporation of any additives. In contrast to the Kakhetian method, the Alentejo wines do not undergo the aging process within the clay vessels [52]. This technology, with its origins implemented in various other countries, has taken root in Slovenia, Slovakia, and Austria. Furthermore, it has recently experienced widespread adoption throughout Europe, but is taking up notable prominence in regions such as Australia and New Zealand as well [42][53].
3. “Orange” Wine: The Technology or How to Produce a Different Wine
3.1. The Technology for Producing Long-Term Macerated White Wines
Traditional white winemaking techniques typically do not involve prolonged contact between the solid parts of the grapes and the grape must during fermentation and post-fermentation stages. In order to extract specific aromatic or phenolic compounds, winemakers often employ pre-fermentation maceration, a method commonly utilized for aromatic grape varieties. This technique allows for the enhanced extraction of desired compounds prior to the actual fermentation process [54]. The ancient winemaking technique utilizing clay vessels known as qvevri has sparked a trend for long-macerated white wines both in Europe and beyond, often referred to as “orange” wine, “amber” wine, or qvevri wine. This unique technique involves an extended period of maceration, starting from one month and lasting up to years, where the wine remains in contact with the grape skins, seeds, and stems [55][56]. The method is used in almost all of the winemaking Georgian regions. However, the most renowned and the place of its origins is the Kakheti region (Figure 1), where the wine was and is still produced after the most archaic method, in clay vessels, qvevris [57]. The grape selection process is meticulous, focusing on choosing grapes that possess optimal characteristics for the winemaking method. Generally, grapes with higher sugar content are preferred, as this contributes to achieving phenolic maturity and ripe tannins. As a result, the harvest period is typically delayed to ensure the desired qualities in the grapes [58]. However, the concentration of the phenolic compounds is highly dependent on several factors, such as grape variety, viticultural area as well as viticultural practices [59]. Simultaneously, there is a preference for using grapes that exhibit excellent sanitary conditions. This is particularly important due to the prolonged skin contact method, as any potential flaws or issues with the grapes can have an impact on the sensory characteristics and overall integrity of the resulting wine. Therefore, careful attention is given to selecting grapes that are in a healthy and pristine condition [60].
In the traditional Kakhetian method, grapes were initially pressed using old wooden presses known as sacnaheli. However, in modern winemaking practices, grapes are not pressed but instead crushed using modern crushers. The resulting juice, along with the pulp, skins, and sometimes stems, is then transferred directly to the qvevri vessel, typically through a gravity-fed process. The cellars where these wines are produced are traditionally called marani, which are unique as they are built underground rather than the conventional above-ground cellars. The qvevris themselves are buried in the ground, allowing them to benefit from the consistent temperature and surrounding conditions that help regulate the fermentation process [61]. The qvevri vessel is typically filled to about three-quarters of its capacity to prevent overflow during fermentation. Similar to red wines, the chacha (seeds, grapes, and stems) undergoes a process known as punch down twice a day during fermentation. This step is crucial for extracting desired compounds such as phenols and also helps regulate the temperature to prevent overheating, which can lead to fermentation issues and yeast death. In most cases, fermentation occurs spontaneously without the addition of commercial yeast. However, some winemakers opt for inoculation to ensure a more controlled fermentation process.
Once fermentation is complete and yeast activity ceases (indicated by the fall of the cap), the vessel is sealed with two layers: one of clay and another of sand, placed on top of a stone. This sealing method protects the wine from impurities and limits oxygen diffusion during the maturation phase. Maturation can range from one month to several months, depending on the winemaker’s vision (Figure 2). In many cases, these wines are further aged in another clean qvevri until they are ready for bottling, often in the spring [58][62].
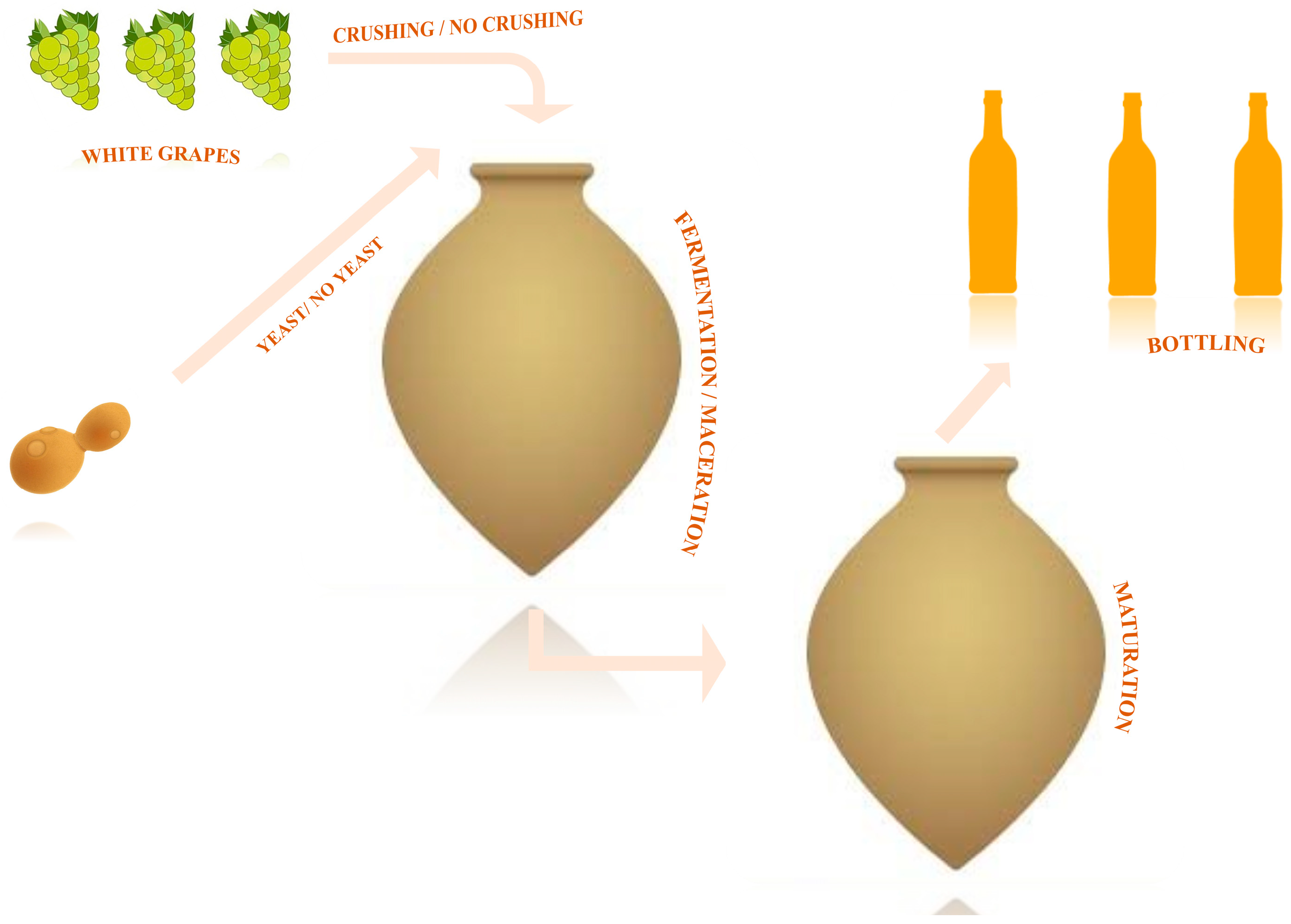
Figure 2. Basic representation of long-macerated white wine technology.
In western Georgia, specifically in the Imereti region, there is a slight variation in the winemaking technique with regard to the amount of chacha fractions used. Only around one-tenth of the chacha is utilized in the Imeretian method, and the use of stems is completely omitted. As a result, the resulting wine has lower tannin levels. However, the longer maturation period imparts the wine with distinctive Georgian characteristics. Moving to central Georgia, in Kartli, the winemaking technique differs in terms of the amount of chacha used. The quantity is similar to that in Imereti, but it distinguishes itself by the inclusion of stems in the fermentation process [61][63].
3.2. Characterisation of the Long-Term Macerated White Wines
3.2.1. Basic Oenological Characteristics
One notable effect observed in macerated wines, including those undergoing prolonged maceration, is a reduction in total acidity. This decrease can be attributed to the precipitation of potassium bitartrate, which occurs due to an elevated potassium content from the grape skins or the accumulation of buffering substances that neutralize the acids [16][63][64][65][66].
Additionally, the transformation of malic acid into lactic acid during extended maceration in amphorae takes place at a faster rate compared to fermentation without skin contact in barrels and barriques. This accelerated conversion is likely due to the close proximity between the wine and the pomace during fermentation in amphorae. The pomace contains various wild strains of lactic acid bacteria, which contribute to the completion of the malolactic fermentation process [65]. Several studies have also observed an increase in volatile acidity, possibly resulting from the oxidative conditions experienced by the wines during maceration and maturation stages [17][67]. Lukic et al. further noted an elevated dry extract as a consequence of prolonged contact with the skins and pomace, leading to a greater extraction of solid matter into the juice [64].
3.2.2. Phenolic Composition
Despite the lack of in-depth scientific resources related to the processes of this technique, several broad traits associated with maceration have been observed. These encompass an elevation in the concentration of specific phenolic compounds, enriched aromatic profiles, elevated dry extract, and the possibility of decreasing acidity [54][55]. Noteworthy observations indicate that, in various instances, wines subjected to prolonged maceration periods, often referred to as those obtained by the Kakhetian method, exhibit an increased concentration of phenolic compounds when compared to conventional European winemaking approaches (Table 1).
Besides winemaking techniques, the vineyard environment as well as the different types of products used during the winemaking steps (for example: enzymes) play a crucial role in the extraction of desired compounds in wine production [68]. Recently, the ancient technique of “orange” wine, which involves prolonged maceration times, has gained attention for its ability to extract specific compounds.
Studies on prolonged maceration techniques have reported similar but more pronounced results. For example, the fermentation of Zeta grapes using the Kakhetian method showed a significantly higher increase in total polyphenols (approximately 78%) compared to a typical fermentation of the same variety [67]. Comparative studies have also been conducted between Hungarian Zeta wines and Georgian wines produced using the traditional qvevri method, revealing intriguing similarities in polyphenol and catechin concentrations. This suggests that “orange” winemaking techniques can be applied to indigenous grape varieties from other countries, expanding its scope beyond Georgian borders [67][69].
Prior to the adoption of the law regarding white wine maceration by the International Organisation of Vine and Wine (OIV) in November 2020, the duration of maceration was not considered mandatory. Consequently, some research on the topic has been conducted with maceration periods of less than one month. For example, a study in New Zealand investigated the combination of Pinot Gris juice and “Thompson seedless” grape skins, showing that the tannin concentration varied depending on the skin ratio used. However, the addition of SO2 limited the extraction and also affected the polymerization of phenols, making it difficult to differentiate the mean degree of polymerization (mDP) in the wines [70].
The impact of prolonged maceration on phenolic content was further demonstrated in a study on Bianchetta Trevigiana wine in Italy. This study highlighted the importance of flavonol localization and its solubilization capacity in both aqueous and hydroalcoholic environments. Flavan-3-ols exhibited relatively consistent behavior during an average maceration period of 40 days. However, beyond this point, there was a rapid increase in flavan-3-ol concentration in the macerated wines, peaking at around 100 days. In contrast, traditional winemaking techniques showed a continual increase up to 60 days. The presence of abundant polyphenol oxidase (PPO) enzymes and elevated phenolic concentrations led to noticeable color changes and browning in the macerated wines [7]. Similar findings on color intensity have been observed by other researchers [67][69][70][71].
A comparison of various maceration and non-maceration treatments on Malvazija Istrarska grapes further supported the impact of prolonged maceration on phenolic content. Wines subjected to prolonged maceration exhibited the highest concentrations of flavan-3-ols, while the control and tannin addition treatments had the lowest concentrations. Maceration with pomace facilitated the migration of (+)-catechin and (−)-epicatechin into the fermenting must, explaining the significantly higher levels of these monomeric flavan-3-ols in macerated wines. The concentrations of procyanidin B1, procyanidin B3, and (+)-catechin showed a decrease or stabilization when maceration time increased from 7 to 21 days. In contrast, the concentrations of procyanidin B2, (−)-epicatechin, and procyanidin C1 increased during the extended maceration period [72]. Gallic acid, a hydroxybenzoic acid, exhibited a significant increase and is thought to be released via hydrolysis from specific esters or complex molecules such as epicatechin gallate units found in proanthocyanidins, primarily concentrated in the seeds. Therefore, a longer maceration duration may facilitate the extraction of gallic acid, explaining its higher concentration in prolonged macerated treatments [72][73][74].
Table 1. Overview of the different phenolic compounds extracted by applying long-term maceration white wine techniques.
| Technological Method | Variety | Subclass | Compounds | Yield/Released (mg/L) | Analysis Method | Reference |
|---|---|---|---|---|---|---|
| Prolonged maceration during and after fermentation |
Malvasija istarska | Hydroxycinnamic acids | Caffeic acid | 11.8 ± 5.0 | HPLC—UV-VIS | [17] |
| p-Coumaric acid | 2.3 ± 1.0 | |||||
| Ferulic acid | 2.2 ± 1.2 | |||||
| Hydroxybenzoic acids | Protocatechuic acid | 14.9 ± 6.9 | ||||
| Vanillic acid | 12.3 ± 11.8 | |||||
| Syringic acid | 2.3 ± 1.3 | |||||
| Flavan-3-ols | (−)-Epicatechin | 17.3 ± 14.2 | ||||
| (+)-Catechin | 31.6 ± 20.7 | |||||
| Qvevri method | Zeta | Flavan-3-ols | Catechin | 1136 | HPLC-UV/DAD | [67] |
| Stilbenoid | t-Resveratrol | 1.79 | ||||
| Mtsvane | Stilbenoid | t-Resveratrol | 0.38 | |||
| Flavonol | Quercitin | 0.34 | ||||
| Rkatsiteli Mtsvane | Stilbenoid | t-Resveratrol | 1.27 | |||
| Qvevri method | Zeta | Flavan-3-ols | Catechin | 379 ± 14 | HPLC—UV-VIS | [69] |
| Mtsvane | Flavan-3-ols | Catechin | 550 ± 7 | |||
| Rkatsiteli Mtsvane | Flavan-3-ols | Catechin | 512 ± 4 | |||
| Prolonged post-fermentative maceration | Malvasija istarska | Hydroxycinnamic acids | Cis-Caftaric | 0.83 ± 0.03 | HPLC-DAD-FLD | [72] |
| Caffeic aid | 8.86 ± 0.04 | |||||
| p-Coumaric acid | 1.25 ± 0.01 | |||||
| Ferulic acid | 1.53 ± 0.02 | |||||
| Hydroxybenzoic acids | Protocatechuic acid | 3.65 ± 0.02 | ||||
| p-hydroxybenzoic acid | 0.70 ± 0.02 | |||||
| Syringic acid | 2.47 ± 0.06 | |||||
| Flavan-3-ols | (+)-Catechin | 13.89 ± 0.31 | ||||
| (−)-Epicatechin | 8.75 ± 0.21 | |||||
| Procyanidin B1 | 7.26 ± 0.36 | |||||
| Procyanidin B3 | 1.13 ± 0.09 | |||||
| Extended maceration -with/without SO2)- |
Pinot Gris (juice) × Thompson seedless (skins) -without SO2- |
Hydroxybenzoic acids | Procatechuic acid | 3.16 | HPLC-UV-VIS | [70] |
| Vanillic acid | 2.98 | |||||
| Syringic acid | 1.51 | |||||
| Flavan-3-ols | Gallocatechin | 29.24 | ||||
| Epigallocatechin | 3.07 | |||||
| Catechin | 4.42 | |||||
| Epicatechin | 1.45 | |||||
| Hydroxycinnamic acids | Caffeic acid | 0.35 | ||||
| Caftaric acid | 2.57 | |||||
| Ferulic acid | 0.53 | |||||
| p-Coumaric acid | 0.48 | |||||
| Pinot Gris (juice) × Thompson seedless (skins) -with SO2- |
Hydroxybenzoic acids | Procatechuic acid | 3.62 | |||
| Vanillic acid | 3.32 | |||||
| Syringic acid | 1.33 | |||||
| Flavan-3-ols | Gallocatechin | 21.62 | ||||
| Epigallocatechin | 26.92 | |||||
| Catechin | 6.60 | |||||
| Epicatechin | 1.76 | |||||
| Hydroxycinnamic acids | Caffeic acid | 0.06 | ||||
| Caftaric acid | 4.86 | |||||
| p-Coumaric acid | 0.52 | |||||
| Kakhethian method | Rkatsiteli | Flavan-3-ols | Catechins | 640 | HPLC-UV-VIS | [15] |
| Proanthocyanidins | 690 | |||||
| Khikhvi | Flavan-3-ols | Catechins | 453 | |||
| Proanthocyanidins | 1097 | |||||
| Ribolla | Flavan-3-ols | Catechins | 509 | |||
| Proanthocyanidins | 392 | |||||
| European method | Rkatsiteli | Flavan-3-ols | Catechins | 39 | ||
| Proanthocyanidins | 47.8 | |||||
| Kakhuri Mtsvane | Flavan-3-ols | Catechins | 27 | |||
| Proanthocyanidins | 43.2 | |||||
| Tsulukidzis Tetra | Flavan-3-ols | Catechins | 77 | |||
| Proanthocyanidins | 165 | |||||
| Rebula | Flavan-3-ols | Catechins | 8 | |||
| Earthware amphora maturation method (different types) | Falanghina | HPLC-DAD | [75] | |||
| Raw amphora | Flavan-3-ols | Epigallocatechin | 41.1–44.8 | |||
| Catechin | 36.6–48.8 | |||||
| Hydroxycinnamic acids | Caffeic acid | 3.1–3.8 | ||||
| Flavan-3-ols | Epicatechin gallate | 4.4–5.4 | ||||
| Glazed amphora | Flavan-3-ols | Epigallocatechin | 32.9–35.6 | |||
| Catechin | 39.9–48 | |||||
| Hydroxycinnamic acids | Caffeic acid | 2.7–3.5 | ||||
| Flavan-3-ols | Epicatechin gallate | 5.2–6.2 | ||||
| Engobe amphora | Flavan-3-ols | Epigallocatechin | 25.7–39.5 | |||
| Catechin | 40–45.9 | |||||
| Hydroxycinnamic acids | Caffeic acid | 2.3–3.2 | ||||
| Flavan-3-ols | Epicatechin gallate | 5.2–5.9 | ||||
| Earthware amphora maturation method (different types) |
Minutolo | HPLC-DAD | [76] | |||
| Raw amphora | Flavan-3-ols | Caftaric acid | 100.5 ± 1.9 | |||
| Catechin | 16.4 ± 1.1 | |||||
| Hydroxycinnamic acids | Ferulic acid | 11.5 ± 0.8 | ||||
| Flavan-3-ols | Epicatechin | 1.5 ± 0.1 | ||||
| Coumaric acid | 38 ± 2.4 | |||||
| Glazed amphora | Flavan-3-ols | Caftaric acid | 97.7 ± 1.3 | |||
| Catechin | 17.1 ± 2.3 | |||||
| Hydroxycinnamic acids | Ferulic acid | 12.2 ± 1.5 | ||||
| Flavan-3-ols | Epicatechin | 1.4 ± 0.2 | ||||
| Coumaric acid | 36.1 ± 0.5 | |||||
| Engobe amphora | Flavan-3-ols | Caftaric acid | 98.4 ± 1.3 | |||
| Catechin | 16.8 ± 0.7 | |||||
| Hydroxycinnamic acids | Ferulic acid | 11.6 ± 0.2 | ||||
| Flavan-3-ols | Epicatechin | 1.4 | ||||
| Coumaric acid | 39.5 ± 1.5 | |||||
A more comprehensive examination of the phenolic compound types and grape varieties investigated in existing literature distinctly uncovers noticeable differences. These variations are influenced by a range of factors, including the type of grape, the duration of extended maceration, the technology employed, and the specific kind of container used. These aspects are clearly outlined in Table 1. Flavonoids primarily appear as catechins, epicatechins, or proanthocyanidins, resulting from the prolonged contact between grape must, wine, and the skins and seeds [77]. The presence of these elements is greatly impacted by grape processing methods, encompassing harvest techniques and press cycles (both initial and maximum pressure). This is because these compounds are more concentrated in the skins and seeds compared to the pulp. Abundant documentation underscores that upon oxidation, these compounds display high reactivity with oxygen and can form irreversible bonds with thiols produced by yeast, particularly in varieties rich in thiols [78]. The concentration of these compounds exhibits noteworthy disparities between European varieties and indigenous Georgian ones. The Georgian varieties exhibit significantly higher concentrations, even under traditional winemaking conditions. However, when employing the Kakhetian winemaking approach, concentrations can increase by up to tenfold [15]. This heightened variation in concentration is also evident across different vessel coatings. The impact of container type is complex due to factors such as porosity and material composition. However, this influence becomes more pronounced with extended aging [76]. A similar concentration increase pattern is observable in other analyzed phenolic compounds (hydroxycinnamic acids, hydroxybenzoic acids, and stilbenoids) in Georgian varieties, although there are resemblances in these characteristics with other varieties such as Hungary’s Zeta variety [67].
3.2.3. Antioxidant Capacity
In recent years, there has been a growing interest in the antioxidant activity of wines as a means to protect against oxidative stress, alongside the focus on polyphenols [79]. In a comparative study that examined wines from various European countries and Georgian wines, a significant difference in antioxidant activity was observed. Wines produced using the Kakhetian method in Georgia displayed notably higher antioxidant activity compared to wines produced using European methods. Additionally, the study analyzed the total sulfite content and found that wines with higher antioxidant activity had substantially lower sulfite concentrations [80]. These findings support the results of a previous study conducted in 2007, which compared Georgian wines to European wines and reported an approximately 2.8-fold increase in antiradical efficiency in Georgian wines [15][81].
3.2.4. Sensorial Characteristics
The research dedicated to exploring the evolution of aromatic compounds in extended maceration wines is rather limited. However, the existing studies [64][82] underscore noteworthy distinctions in the behavior of these compounds throughout this particular process (Table 2). For instance, in the fermentation of the Palomino Fino variety in Spain, it was observed that maceration involving 20% skin contact resulted in the highest fruity and floral characteristics [16]. Similar results were obtained in Italy with the Garganea variety [82] and in extended skin contact fermented wines from the Chenin Blanc variety in China [83]. However, the same research also noted a negative impact on esters due to skin contact, which could be attributed to higher fermentation-maceration temperatures or interactions with wine lees that absorb and release esterases involved in ester hydrolysis [17].
Monoterpenes and C13-isoprenoids do not appear to be strongly affected by prolonged maceration. Generally, maceration extracts monoterpenes, which then hydrolyze and release volatile forms during maturation [84]. Lukic et al. found that prolonged maceration of white wines led to an increase in monoterpenes, C13-norisoprenoids, methanol, higher alcohols, ethyl acetate, esters from hydroxyl and dicarboxylic acids, furans, ethyl phenols, and acetals, while straight-chain ethyl and acetate esters experienced a significant decrease [17]. In another study, Lukic et al. observed the inhibition of esters, β-damascone, and hotrienol in a trial with Muscat blanc grapes under prolonged maceration, which, combined with intense phenol extraction and oxidation reactions, posed a risk in terms of sensory evaluation [64].
Sulfur compounds are also of interest in this winemaking technique as multiple factors contribute to their formation. Fedrizzi et al. confirmed an increase in sulfur compounds, particularly dimethyl sulfide (DMS), during the fermentation of Garganea grapes, which is derived from S-methyl methionine [82].
From a sensory perspective, wines with prolonged maceration exhibit increased astringency and bitterness compared to other treatments. The higher concentration of total flavan-3-ols in these wines contributes to intensified astringency and bitterness, as flavan-3-ols are known to affect these sensory attributes [85][86]. Bitterness is primarily associated with lower molecular weight phenols, such as flavan-3-ol monomers, dimers, and trimers, while astringency arises from higher molecular weight phenols or flavan-3-ol polymers. Extended post-fermentative maceration and late-harvest vinification treatments have a significant impact on the mouthfeel and aftertaste of wines, particularly due to tannin attributes [72].
In wines crafted through extended maceration and maturation, the influence of straight-chain esters and β-damascenone is notably significant. However, the most prominent contributors to the aromatic profile encompass compounds commonly associated with fermentative maceration, such as higher alcohols and linalool. Additionally, certain compounds, such as ethyl acetate, 4-ethylguaiacol, and ethyl lactate, predominantly or exclusively arise from microorganisms other than yeasts. These compounds tend to emerge in (semi-)aerobic conditions and at higher temperatures, including ethyl acetate, ethyl 3-methylbutyrate, and isoamyl alcohol. Moreover, the aromatic composition is influenced by compounds whose levels typically intensify with the wine’s aging process, including ethyl acetate, ethyl 3-methylbutyrate, isoamyl alcohol, TDN, and ethyl lactate [17][87]. These compounds exhibit a range of aromas, transitioning from floral notes such as roses and sweet sensations to fruity elements. As the maturation progresses, tertiary aromas become perceptible, lowering the perception of aforementioned compounds as being affected by different maturation conditions. Kerosene or petrol odors, typical in general for Riesling produced by TDN (1,1,6-Trimethyl-1,2-dihydronaphthalene), were also detected as a key compound in the prolonged macerated white wines produced from Malvasia istraka, however, having a concentration under the rejection threshold reported in Riesling varieties under standard winemaking (71–82 µg/L) [88]. Aroma attributes such as honey, dried figs, and tobacco experienced significant enhancement as a result of prolonged skin contact, and this intensification was found to be linked to the presence of benzenoid compounds [82][89]. The pattern exhibited by acetates and linear ethyl esters, as extensively documented in the literature, entails a swift reduction over the course of aging, particularly in instances where the wine is not stored in appropriate conditions [90]. This decline in aromas, such as fruity and floral notes, has also been noted by panelists, contributing to the characterization of prolonged macerated white wines as possessing a more rounded, spicier profile while displaying fewer green characteristics [65]. The potential negative impacts of evaporation caused by elevated temperatures, cap manipulation, and barrel porosity during fermentation and maceration cannot be disregarded. Additionally, the wines resulting from extended maceration came into contact with lees, which possess the capacity to absorb esters and also release esterases capable of hydrolyzing them [91]. When considering the contrast between wines crafted in amphorae and those aged in barrels/barriques, it becomes evident that wines produced in amphorae exhibit a mature bouquet and diminished “green” characteristics compared to their counterparts aged in barrels and barriques. However, an interesting observation reveals that wines created in amphorae showcase relatively subdued varietal aromas, which might be attributed to potential excessive maceration during the winemaking process [65].
Table 2. Aroma compounds and their possible odors identified in prolonged macerated white wines.
| Grape Variety | Particular Examined Parameters | Volatile Compounds | Conc (µg/l) | Aroma Descriptors ** |
|---|---|---|---|---|
| Malvazija istarska (in oak) [17] |
Monoterpenes | α-Terpinolene | 8.4 ± 2.8 | Pine, turpentine [92] |
| α-Terpineol | 114.7 ± 38.2 | Flowers, lilies, sweet | ||
| Linalool | 37.4 ± 23.5 | Ginger, flowers [88], grape-like, sweet, citrus [89] | ||
| Geraniol | 8.1 ± 8.6 | Citrus, floral, geranium [93] | ||
| C13-norisoprenoids | Vitispirane | 37.1 ± 36.3 | Camphor, spicy, wood [94] | |
| TDN | 3.3 ± 3.3 | Kerosene [92], petrol [93] | ||
| C6-alcohols | 1-Hexanol | 1046.7 ± 622.5 | Herbaceous, grass [88], woody, resin [94] | |
| Cis-3-hexen-1-ol | 49.4 ± 36.1 | Green, grassy, earthy [95] | ||
| Methanol * | 218.8 ± 23.0 | Alcohol [88][96] | ||
| Higher alcohols | Isobutanol * | 48.3 ± 15.8 | Alcohol, nail polish, fusel [96] | |
| Isoamyl alcohol * | 243.5 ± 37.6 | Herbaceous, whiskey, malt, burnt [97] | ||
| Fatty acids | Octanoic acid * | 0.7 ± 0.6 | Fatty, waxy, rancid oily, vegetable, cheesy, moss [93] | |
| Decanoic acid * | 0.1 ± 0.0 | Fatty, rancid [96] | ||
| Ethyl acetate * | 75.1 ± 8.6 | Pineapple, fruity, solvent, balsamic [96] | ||
| Ethyl esters of fatty acids | Ethyl butyrate | 141.7 ± 62.6 | Blueberry [96], strawberry, apple, fruity [98] | |
| Ethyl hexanoate | 145.7 ± 84.3 | Anise, caramel, fruit, wine [94] | ||
| Ethyl octanoate | 128.1 ± 74.2 | Fruit, must, soap, sweet, waxy [94] | ||
| Ethyl decanoate | 76.9 ± 56.3 | Brandy, fruity, grape [96] | ||
| Esters of hydroxy and dicarboxylic acids | Ethyl lactate * | 143.9 ± 44.3 | Butter, acidic, ethereal [91], fruit, sweet [98] | |
| Isoamyl lactate | 461.4 ± 89.9 | Banana, pear [98] | ||
| Acetaldehyde * | 45.5 ± 32.0 | Unripe walnut, bruised fruit [99] | ||
| Volatile phenols | 4-Ethylguaiacol | 445.7 ± 392.2 | Phenolic, sweet [99] | |
| 4-Ethylphenol | 119.0 ± 127.8 | Medicinal, stable [99] | ||
| 4-Vinylguaiacol | 117.1 ± 73.4 | Paint, watercolor [100] | ||
| Benzenoids | Benzaldehyde | 4.2 ± 2.7 | Almond, fragrant [96] | |
| Acetals | 2,4,5-Trimethyl-1,3-dioxolane | 8.0 ± 19.5 | Honey like, woody, fruity [101] | |
| 1,1-Diethoxy-3-methylbutane | 21.8 ± 24.1 | Fruity, fatty aroma [102] | ||
| Garganega [82] |
Esters | Isoamyl acetate | 182.5–278.9 | Banana, pear [98] |
| β-phenylethyl acetate | 23.5–28.0 | Rose, flower [100] | ||
| Ethyl octanoate | 305.7–413.0 | Fruit, must, soap, sweet, waxy [94] | ||
| Ethyl decanoate | 90.7–90.9 | Brandy, fruity, grape [96] | ||
| Monoterpenes | Linalool | 5.3–7.5 | Ginger, flowers, grape-like [88], sweet, citrus [89] | |
| α-Terpineol | 2.2–3.9 | Flowers, lilies, sweet [96] | ||
| Citronellol | 2.4–6.7 | Citrus, clove, floral, fresh, green, rose, sour, sweet [94] | ||
| Geraniol | 4.7–5.7 | Citrus, floral, geranium [93] | ||
| Norisoprenoids | 3-oxo-α-ionol | 81.2–90.9 | Burnt, spicy [92] | |
| Benzenoids | Benzaldehyde | 8.9–16.4 | Almond, fragrant [96] | |
| Vanillin | 12.8–51.3 | Sweet, vanilla [94] | ||
| Verdicchio [82] |
Esters | Isoamyl acetate | 572.8 | Banana, pear [98] |
| β-phenylethyl acetate | 54.7 | Rose, flower [100] | ||
| Ethyl octanoate | 649.4 | Fruit, must, soap, sweet, waxy [94] | ||
| Ethyl decanoate | 144.9 | Brandy, fruity, grape [96] | ||
| Monoterpenes | Linalool | 5.4 | Ginger, flowers, grape-like [88], sweet, citrus [89] | |
| α-Terpineol | 8.6 | Flowers, lilies, sweet [96] | ||
| Citronellol | 3.1 | Citrus, clove, floral, fresh, green, rose, sour, sweet [94] | ||
| Geraniol | 2.2 | Citrus, floral, geranium [93] | ||
| Norisoprenoids | 3-oxo-α-ionol | 119.5 | Burnt, spicy [92] | |
| Benzenoids | Benzaldehyde | 296.2 | Almond, fragrant [96] | |
| Vanillin | 117.6 | Sweet, vanilla [94] | ||
| Chenin Blanc [83] |
Terpenes | Linalool | 1.94 ± 0.58 | Ginger, flowers, grape-like [88], sweet, citrus [89] |
| Citronellol | 1.86 ± 0.39 | Citrus, clove, floral, fresh, green, rose, sour, sweet [94] | ||
| Higher alcohols | Isopentanol | 353.3 ± 9.72 | Fusel, alcoholic, fermented, pungent, bready, yeasty [103] | |
| 2-Methyl-1-butanol | 125.5 ± 11.52 | Fermented, malt, wine [104] | ||
| Esters | Isoamyl acetate | 183.2 ± 9.44 | Banana, pear [98] | |
| Ethyl hexanoate | 295.5 ± 19.66 | Anise, caramel, fruit, wine [94] | ||
| Fatty acids | Decanoic acid | 0.81 ± 0.03 | Fatty, rancid [96] | |
| Octanoic acid | 32.88 ± 7.26 | Fatty, rancid [96] |
* Concentrations expressed in mg/L; ** Potential aroma descriptors discovered in other research pertaining to the aroma compounds identified in wines produced using the extended maceration white wine technology. TDN—1,1,6-Trimethyl-1,2-dihydronaphthalene.
4. Qvevri—The Ancient Winemaking Vessel
4.1. Manufacturing
The qvevri vessels hold a truly extraordinary place within the cultural identity of Georgia. These unique earthenware amphorae embody centuries of tradition, craftsmanship, and a deep connection to the land. The knowledge and experience of qvevri manufacture and winemaking are deeply ingrained within the fabric of Georgian society. Within Georgian households, the secrets of qvevri manufacture are carefully guarded and transmitted from elders to the younger members of the family [105]. The craftsmanship of creating these magnificent earthenware vessels is a cherished tradition, where skills are shared through hands-on learning, mentorship, and storytelling. This intergenerational transfer of knowledge not only ensures the preservation of ancient techniques but also strengthens familial bonds and a sense of shared identity [106]. Georgia’s traditional winemaking method of fermenting grapes in earthenware, egg-shaped vessels has gained recognition on the UNESCO World Heritage List in 2013, which is a significant milestone for Georgian culture. This esteemed honor not only recognizes the craftsmanship and cultural importance of the qvevri vessels but also acknowledges the invaluable contribution of Georgian winemaking to the global heritage of viticulture [107].
Currently, the production of qvevris is predominantly centered in three distinct regions of Georgia: Guria, Kakheti, and Imereti [107]. The prevailing dimensions for qvevris are typically 800 L and 1000–1500 L, enabling substantial receptacles that effectively manage temperature control during the fermentation process. Nonetheless, these vessels are also crafted in diminutive sizes, ranging from 2 L to colossal variants harkening back to antiquity, boasting voluminous capacities of 10,000 to 15,000 L [107][108].
The quality of qvevri relies on various factors, which include the clay type, by accurate removal of the stones from the mass of the clean clay by creating a homogeneous, pliable clay dough. The few qvevri makers that remained have a distinct choice when it comes to the material and production method they prefer. The clay utilized in the production of qvevris incorporates limestone and traces of valuable metals such as gold, silver, and copper. Lime, which interacts with tartaric acid, serves a dual purpose in the qvevri-making process: it reinforces the vessel’s walls while also acting as a natural antiseptic [109]. The qvevri crafting process must be divided into multiple phases (Figure 3), as it involves the assembling of circular layers of clay, each having a thickness of approximately 30–40 cm. It requires careful attention to preserve their uniform shape and methodically connect them [110]. The process of constructing the vessel is gradual and time consuming, as only a small amount of clay, typically 15–20 cm, can be added at a time. Each addition is followed by a full 24 h pause to allow the preceding layer to adequately solidify [111]. Once completed, the vessels undergo a thorough drying and firing process, both internally and externally, which typically spans over 40 days for complete drying. It is crucial to carry out the drying process over an extended period of time and avoid exposing the qvevri to direct sunlight. Direct exposure to the sun increases the risk of micro-cracks forming in the clay walls, which means that drying should be carried out in shaded rooms. The dried qvevries are delicately arranged within a furnace and enclosed with bricks to retain the heat effectively. The firing process must occur gradually and at extremely high temperatures, as the risk of wall cracking remains a significant concern [107][112]. The blue–white flickers serve as a captivating sign that the operation has reached its end. In preparation for its inaugural use and subsequent burial, the outer surface of the qvevri undergoes a treatment involving the application of quicklime. Quicklime, renowned for its antiseptic and thermoregulatory properties, is applied to enhance the vessel’s durability and prolong its service life. In the case of larger qvevri, a generous coating of lime mortar is typically employed to ensure comprehensive coverage of the surface [111]. The walls of the qvevri possess permeability to water and air, necessitating a specific treatment. To address this, melted beeswax is applied to the inner surface of the qvevri, effectively sealing the larger pores. The beeswax, with its water-repellent properties, not only aids in the ease of cleaning but also discourages the adherence of bacteria to the surface. As a result, the inner portion of the vessel becomes impermeable to external water, ensuring a secure barrier. However, the smaller pores are left unobstructed, allowing the wines to breathe and maintain their desired characteristics [111]. Emphasizing the significance of wax quality is vital. It is crucial to avoid beeswax that contains paraffin or any other foreign substances. Additionally, the use of artificial honeycombs by beekeepers should be approached with caution, as they may contain paraffin, stearin, or other additives that can potentially contaminate the beeswax [113].
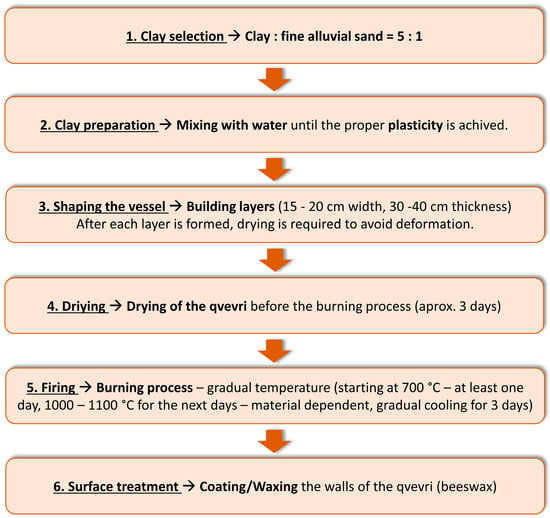
Figure 3. Steps for manufacturing qvevri vessels.
4.2. Vessel Sanitation Technique
In the pursuit of producing high-quality wine, the cleaning of winery equipment holds paramount importance alongside various technological steps. However, when it comes to cleaning qvevri vessels, a distinctive process must be followed due to their unconventional material composition. The absence of a standardized cleaning protocol remains a significant challenge in Georgia, particularly in terms of maintaining proper hygiene. This challenge is further amplified by the unique characteristics of qvevri vessels, which are notoriously difficult to clean due to the porous nature of their clay construction and their immovable underground positioning [61]. The potential danger lies not only in visible coarse dirt on the walls, which can be readily identified and removed but also in the presence of fine sludge and tartar. These substances have the ability to penetrate deeply into the porous material of the qvevri, making their complete removal crucial to avoid contamination risks [107].
In general, lime water is commonly used as the primary agent for washing qvevri. However, an alternative solution called “ash-wash,” which consists of sodium water, can also be employed in the cleaning process. Lime and ash serve as natural cleansers, ensuring the safety of the vessel walls as they do not cause any damage. This stands in contrast to soda (especially caustic soda) and other chemical agents that have the potential to harm the qvevri walls [111]. The initial step involves cleaning the inner walls using a scraper crafted from cherry bark. Following that, the walls are thoroughly rinsed with a solution of slaked lime and vigorously scrubbed with a specialized brush known as a “qvevri brush,” which is made from St. John’s wort roots [107]. Although no precise proportions have been officially established for qvevri-washing mixtures, current practices indicate that using a ratio of 3–5 kg of lime dissolved in 10–15 L of water, or alternatively, 1–2 kg of ash in 5 L of water, yields satisfactory results in terms of effective cleaning [111]. To conclude the process, the qvevri is thoroughly rinsed with a combination of hot (60 °C) and cold water until the draining water becomes completely colorless and free of any lingering odors [114]. Additionally, for the purpose of disinfecting qvevri walls, sulfur can be burned inside the vessel using the recommended proportions of 3 g of sulfur per 100 L of volume. This practice helps ensure effective sanitization. Furthermore, it is crucial to note that when using sulfur to disinfect qvevri walls, the vessel must be completely dry. Failure to do so may result in the sulfur smoke reacting with any residual water, leading to the formation of a crystal coating that can impart a bitter taste to the wine [111].
4.3. Influences of Qvevri Vessels on Long-Term Macerated White Wine Quality
4.3.1. Influence of the Vessel Coating on the Long-Term Macerated White Wine Quality
The selection of vessels used for fermentation and maceration plays a crucial role in shaping the characteristics and qualities of wines, alongside the influence of long-term maceration technology. However, there is a lack of comprehensive research specifically focusing on the direct impact of qvevri vessels on wines undergoing fermentation or maturation within them. To address this gap, a study conducted in Italy explored the effects of storage on lees for 12 months using three types of earthware amphoras (raw, glazed, and engobe) in relation to wines produced from the Falanghina grape variety. A comparison was made with conventionally produced wines stored in stainless steel tanks. Notably, differences between the wines stored in amphoras and those in stainless steel tanks were already noticeable after 2 months of storage. One significant difference was observed in terms of alcohol content, which appeared to be lower in the wines stored in amphoras due to potential evaporation and diffusion through the vessel walls. Additionally, a slight increase in pH values, particularly in the raw amphoras, was observed. The author concluded that this might be attributed to the reaction of the clay material with acids and alkalis present in the wine [75]. Another research study investigated the direct interaction between Armenian clay-based ceramics and a model wine during the storage phase. It was found that the wine in direct contact with the ceramic vessels exhibited mineral dissolution, potentially leading to an increase in pH values. To mitigate this effect, different coating materials have been utilized [115].
The type of container used also impacted the phenolic compounds present in the wines. The engobe amphoras and stainless steel tanks showed increased retention of phenolic compounds compared to other types of amphoras. Flavonoids experienced a significant reduction after 12 months of storage in all containers, while flavans underwent a complete reduction in raw and glazed amphoras and a reduction of up to 59% in other containers. These findings align with similar research conducted on Minutolo grapes under reductive conditions [76].
Despite reductions in specific compounds, the concentration of total phenolic content remained constant in steel tanks and raw amphoras, while other containers displayed fluctuations, with reductions ranging from 4% to 10% [75]. Regarding mineral characteristics, raw and glazed amphorae exhibited higher values, but a decrease in the aromatic intensity of the wines was observed in this case. However, there was no significant change in persistence during aging. In comparison to stainless steel tanks, glazed, and engobe amphorae, the uncoated vessels were found to be more harmonious in terms of intensity, persistence, and minerality [76].
4.3.2. Influence of the Vessel Type on the Long-Term Macerated White Wine Quality
A recent study examined the fermentation of Fiano grapes using four different vessels: stainless steel, earthenware amphora, mulberry wood barrel, and cherry wood barrel. The research revealed notable variations in basic analyses, including residual sugar and volatile acidity. Specifically, the study found distinct patterns in stainless steel and amphora fermentations. The stainless steel fermentation resulted in lower residual sugar levels and consequently higher alcohol content, whereas both vessels exhibited lower volatile acidity values. The aforementioned study also found that the characteristic volatile compounds of Fiano grapes were detected in the wood and clay containers. This observation can be attributed to micro-oxygenation, which aids in the retention of yeast within the pores of the container walls. Consequently, this process enhances the production of alcohols and esters [116]. Extensive research has been conducted over the years to examine the impact of oxygenation or micro-oxygenation on white wines. While the detrimental effects of oxygen exposure are widely known, it has also been revealed that there are positive effects associated with controlled oxygenation. These positive effects encompass the reduction of undesirable aromas, improved long-term oxidative stability, the creation of a more intricate wine profile, and the provision of essential nutrients for the yeast. However, it is crucial to recognize that these effects are significantly influenced by various factors, including substrates, grape variety, winemaking conditions, and notably, the choice of vessel for fermentation or maturation [117]. It was shown that claystone containers are conducive to oxidative maturation due to their controllable oxygen permeability. In contrast, earthenware containers rely on coatings to prevent liquid loss through the pores and regulate the ingress of atmospheric oxygen. However, an essential factor to consider in the case of claystone containers is their size, as it significantly influences the amount of oxygen absorbed [118].
The color of the amphora wines was highlighted to be more intense in the case of Fiano grapes fermented in clay containers, a fact which was observed also in Hungary in research carried out on the Kakethian style of fermentation [67]. From an olfactory point of view, the wines expressed a higher freshness, with a tertiary aroma profile, but also a slight vegetal impregnation [116]. The findings are consistent with those of Rosetti et al. (2017), in which the panelists reported observations regarding maturation notes. It is worth mentioning that the research did not directly assess the quantity of oxygen absorbed by the different vessels, which can potentially have negative implications for various wine parameters, as discussed earlier.
References
- McGovern, P.; Jalabadze, M.; Batiuk, S.; Callahan, M.P.; Smith, K.E.; Hall, G.R.; Kvavadze, E.; Maghradze, D.; Rusishvili, N.; Bouby, L.; et al. Early Neolithic wine of Georgia in the South Caucasus. Proc. Natl. Acad. Sci. USA 2017, 114, E10309–E10318.
- OIV. International Code of Oenological Practices. Paris, France. 2021. Available online: https://www.oiv.int/public/medias/7713/en-oiv-code-2021.pdf (accessed on 18 July 2023).
- Gómez-Míguez, M.J.; González-Miret, M.L.; Hernanz, D.; Fernández, M.Á.; Vicario, I.M.; Heredia, F.J. Effects of prefermentative skin contact conditions on colour and phenolic content of white wines. J. Food Eng. 2007, 78, 238–245.
- Arenas, I.; Ribeiro, M.; Filipe-Ribeiro, L.; Vilamarim, R.; Costa, E.; Siopa, J.; Cosme, F.; Nunes, F.M. Effect of Pre-Fermentative Maceration and Fining Agents on Protein Stability, Macromolecular, and Phenolic Composition of Albarino White Wines: Comparative Efficiency of Chitosan, k-Carrageenan and Bentonite as Heat Stabilisers. Foods 2021, 10, 608.
- Ramey, D.; Bertrand, A.; Ough, C.S.; Singleton, V.L.; Sanders, E. Effects of Skin Contact Temperature on Chardonnay Must and Wine Composition. Am. J. Enol. Vitic. 1986, 37, 99–106.
- McRae, J.M.; Kennedy, J.A. Wine and grape tannin interactions with salivary proteins and their impact on astringency: A review of current research. Molecules 2011, 16, 2348–2364.
- Hernanz, D.; Recamales, Á.F.; González-Miret, M.L.; Gómez-Míguez, M.J.; Vicario, I.M.; Heredia, F.J. Phenolic composition of white wines with a prefermentative maceration at experimental and industrial scale. J. Food Eng. 2007, 80, 327–335.
- Robinson, J.; Harding, J. The Oxford Companion to Wine; Oxford University Press: Oxford, UK, 2015; 859p.
- Howard, C. Skin contact whites: Perhaps amber is the new “orange”. Wine Vitic. J. 2017, 32, 21–25.
- Hodakov, O.L.; Sarkisyan, G.O.; Sugachenko, T.S.; Melnyk, I.V.; Miroshnychenko, O.M.; Taranenko, O.G.; Tkachenko, L.O. Improvement of technological modes of production of Amber Wine in the conditions of the Odesa region. Tavrian Sci. Bull. Ser. Tech. Sci. 2023, 2, 228–238.
- Schneider, V.; Chichua, D. Orange wines: Tannin extraction kinetics during maceration of white grapes. J. Vitic. Enol. 2021, 7, 1–9.
- Maggu, M.; Winz, R.; Kilmartin, P.A.; Trought, M.C.; Nicolau, L. Effect of skin contact and pressure on the composition of Sauvignon Blanc must. J. Agric. Food Chem. 2007, 55, 10281–10288.
- Romanini, E.; McRae, J.M.; Bilogrevic, E.; Colangelo, D.; Gabrielli, M.; Lambri, M. Use of grape seeds to reduce haze formation in white wines. Food Chem. 2021, 341, 128250.
- Kosinska-Cagnazzo, A.; Heeger, A.; Udrisard, I.; Mathieu, M.; Bach, B.; Andlauer, W. Phenolic compounds of grape stems and their capacity to precipitate proteins from model wine. J. Food Sci. Technol. 2020, 57, 435–443.
- Shalashvili, A.; Ugrekhelidze, D.; Targamadze, I.; Zambakhidze, N.; Tsereteli, L. Phenolic Compounds and Antiradical Efficiency of Georgian (Kakhethian) Wines. J. Food Sci. Eng. 2011, 1, 361.
- Sancho-Galán, P.; Amores-Arrocha, A.; Jiménez-Cantizano, A.; Palacios, V. Influence of the Presence of Grape Skins during White Wine Alcoholic Fermentation. Agronomy 2021, 11, 452.
- Lukic, I.; Jedrejcic, N.; Ganic, K.K.; Staver, M.; Persuric, D. Phenolic and Aroma Composition of White Wines Produced by Prolonged Maceration and Maturation in Wooden Barrels. Food Technol. Biotechnol. 2015, 53, 407–418.
- Ertan Anli, R.; Vural, N. Antioxidant phenolic substances of Turkish red wines from different wine regions. Molecules 2009, 14, 289–297.
- Kıralp, S.; Toppare, L. Polyphenol content in selected Turkish wines, an alternative method of detection of phenolics. Process Biochem. 2006, 41, 236–239.
- Chung, K.T.; Wong, T.Y.; Wei, C.I.; Huang, Y.W.; Lin, Y. Tannins and human health: A review. Crit. Rev. Food Sci. Nutr. 1998, 38, 421–464.
- Buljeta, I.; Pichler, A.; Simunovic, J.; Kopjar, M. Beneficial Effects of Red Wine Polyphenols on Human Health: Comprehensive Review. Curr. Issues Mol. Biol. 2023, 45, 782–798.
- Lucarini, M.; Durazzo, A.; Lombardi-Boccia, G.; Souto, E.B.; Cecchini, F.; Santini, A. Wine Polyphenols and Health: Quantitative Research Literature Analysis. Appl. Sci. 2021, 11, 4762.
- Nash, V.; Ranadheera, C.S.; Georgousopoulou, E.N.; Mellor, D.D.; Panagiotakos, D.B.; McKune, A.J.; Kellett, J.; Naumovski, N. The effects of grape and red wine polyphenols on gut microbiota—A systematic review. Food Res. Int. 2018, 113, 277–287.
- Sharma, K.; Kumar, V.; Kaur, J.; Tanwar, B.; Goyal, A.; Sharma, R.; Gat, Y.; Kumar, A. Health effects, sources, utilization and safety of tannins: A critical review. Toxin Rev. 2019, 40, 432–444.
- Aurand, J.-M.; Maghradze, D.; Aslanishvili, A.; Mdinaradze, I.; Tkemaladze, D.; Mekhuzla, L.; Lordkipanidze, D.; Jalabadze, M.; Kvavadze, E.; Rusishvili, N.; et al. Progress for research of grape and wine culture in Georgia, the South Caucasus. BIO Web Conf. 2019, 12, 03003.
- Bouby, L.; Wales, N.; Jalabadze, M.; Rusishvili, N.; Bonhomme, V.; Ramos-Madrigal, J.; Evin, A.; Ivorra, S.; Lacombe, T.; Pagnoux, C.; et al. Tracking the history of grapevine cultivation in Georgia by combining geometric morphometrics and ancient DNA. Veg. Hist. Archaeobotany 2021, 30, 63–76.
- Alizadeh, K.; Eghbal, H.; Samei, S. Approaches to social complexity in Kura-Araxes culture: A view from Köhne Shahar (Ravaz) in Chaldran, Iranian Azerbaijan. Paléorient 2015, 41, 37–54.
- McGovern, P. Ancient Wine: The Search for the Origins of Viniculture; Princeton University Press: Princeton, NJ, USA, 2003.
- Aurand, J.-M.; Maghradze, D.; Samanishvili, G.; Mekhuzla, L.; Mdinaradze, I.; Tevzadze, G.; Aslanishvili, A.; Chavchanidze, P.; Lordkipanidze, D.; Jalabadze, M.; et al. Grape and wine culture in Georgia, the South Caucasus. BIO Web Conf. 2016, 7, 03027.
- Ghambashidze, N.J.S.S. Vine and Woman—One of the Cardinal Symbols of Georgian Identity (Ethnological Research). Sociology 2017, 7, 285–291.
- Chkhartishvili, N.; Maghradze, D. Viticulture and winemaking in Georgia. VITIS J. Grapevine Res. 2012, 169–176.
- Bittner, S.V. American Roots, French Varietals, Russian Science: A Transnational History of the Great Wine Blight in Late-Tsarist Bessarabia. Past Present 2015, 227, 151–177.
- Anderson, K. Is Georgia the Next “New” Wine-Exporting Country? J. Wine Econ. 2013, 8, 1–28.
- Shtaltovna, A.; Feuer, H. Modern Resilience of Georgian Wine: Geographical Indications and International Exposure; Routledge: London, UK, 2019; pp. 134–153.
- Ghvanidze, S.; Bitsch, L.; Hanf, J.; Svanidze, M. “The Cradle of Wine Civilization”—Current Developments in the Wine Industry of the Caucasus. Cauc. Anal. Dig. 2020, 117, 9–15.
- Kemashvili, E. Uncorking Georgia’s Winemaking Potential. Wipo Magazine, April 2012.
- Gilinsky, P.; Trela, B. Shavteli Winery: Where to go from here? Emerald Emerg. Mark. Case Stud. 2011, 1, 1–27.
- Ieri, F.; Campo, M.; Cassiani, C.; Urciuoli, S.; Jurkhadze, K.; Romani, A. Analysis of aroma and polyphenolic compounds in Saperavi red wine vinified in Qvevri. Food Sci. Nutr. 2021, 9, 6492–6500.
- Meladze, M. Wine Tourism as a Great Opportunity for Georgia. Eur. Sci. J. ESJ 2016, 12.
- Luchian, C.E.; Cotea, V.V.; Sandu, I.; Copcia, V.E. Removal of Mn(II), Ni(II) and Cu(II) Ions from White Wine through Ion Exchange in Microporous Mordenite and Mesoporous Al-MCM-41. Rev. Chim. 2011, 62, 782–786.
- Dumitriu, G.D.; Lopez de Lerma, N.; Luchian, C.E.; Cotea, V.V.; Peinado, R.A. Study of the potential use of mesoporous nanomaterials as fining agent to prevent protein haze in white wines and its impact in major volatile aroma compounds and polyols. Food Chem. 2018, 240, 751–758.
- Woolf, S. The Amber Revolution; Interlink Publishing Group, Inc.: Northampton, MA, USA, 2021.
- Lisanti, M.T.; Blaiotta, G.; Nioi, C.; Moio, L. Alternative Methods to SO(2) for Microbiological Stabilization of Wine. Compr. Rev. Food Sci. Food Saf. 2019, 18, 455–479.
- Santos, M.C.; Nunes, C.; Saraiva, J.A.; Coimbra, M.A. Chemical and physical methodologies for the replacement/reduction of sulfur dioxide use during winemaking: Review of their potentialities and limitations. Eur. Food Res. Technol. 2011, 234, 1–12.
- Barril, C.; Clark, A.C.; Scollary, G.R. Chemistry of ascorbic acid and sulfur dioxide as an antioxidant system relevant to white wine. Anal Chim. Acta 2012, 732, 186–193.
- Antonelli, A.; Arfelli, G.; Masino, F.; Sartini, E. Comparison of traditional and reductive winemaking: Influence on some fixed components and sensorial characteristics. Eur. Food Res. Technol. 2010, 231, 85–91.
- Mattivi, F.; Fedrizzi, B.; Zenato, A.; Tiefenthaler, P.; Tempesta, S.; Perenzoni, D.; Cantarella, P.; Simeoni, F.; Vrhovsek, U. Development of reliable analytical tools for evaluating the influence of reductive winemaking on the quality of Lugana wines. Anal. Chim. Acta 2012, 732, 194–202.
- Castro, R.; Barroso, C.G. Behavior of a Hyperoxidized Must During Biological Aging of Fino Sherry Wine. Am. J. Enol. Vitic. 2000, 51, 98–102.
- Mayén, M.; Mérida, J.; Medina, M. Influence of the addition of sulphur dioxide and must hyperoxidation on the phenolic fractions during vinification of Sherry wines. Food Chem. 1996, 56, 7–13.
- Schneider, V. Must Hyperoxidation: A Review. Am. J. Enol. Vitic. 1998, 49, 65–73.
- Maker, M. The Gravner Notebook. Terroir Review. 2022. Available online: https://terroirreview.com/2022/09/05/the-gravner-notebook/ (accessed on 18 July 2023).
- Cabrita, M.J.; Martins, N.; Barrulas, P.; Garcia, R.; Dias, C.B.; Pérez-Álvarez, E.P.; Freitas, A.M.C. Multi-element composition of red, white and palhete amphora wines from Alentejo by ICPMS. Food Control 2018, 92, 80–85.
- Scollary, G.R. Grapevine: Amber, Not Orange, Wine. Available online: https://search.informit.org/doi/abs/10.3316/IELAPA.555547742879819 (accessed on 18 July 2023).
- Darias-Martín, J.J.; Rodríguez, O.; Díaz, E.; Lamuela-Raventós, R.M. Effect of skin contact on the antioxidant phenolics in white wine. Food Chem. 2000, 71, 483–487.
- Glonti, T. Traditional technologies and history of Georgian wine. Bull. L’oiv 2010, 83, 335–343.
- Mirvelashvili, M.; Maghradze, D. Grape and Wine Culture in Georgia; National Wine Agency of Georgia: Tbilisi, Georgia, 2015; 220p.
- Capece, A.; Siesto, G.; Poeta, C.; Pietrafesa, R.; Romano, P. Indigenous yeast population from Georgian aged wines produced by traditional “Kakhetian” method. Food Microbiol. 2013, 36, 447–455.
- Baghaturia, N.S. Georgian Winemaking. Theory and Practice, 1st ed.; private ed.: Tibilisi, Georgia, 2010. Available online: https://dspace.nplg.gov.ge/bitstream/1234/8260/1/Gruzinskoe_Vinodelie.pdf (accessed on 18 July 2023).
- Popîrdă, A.; Luchian, C.E.; Colibaba, L.C.; Focea, E.C.; Nicolas, S.; Noret, L.; Cioroiu, I.B. Carbon-Isotope Ratio (δ13C) and Phenolic-Compounds Analysis in Authenticity Studies of Wines from Dealu Mare and Cotnari Regions (Romania). Agronomy 2022, 12, 2286.
- Steel, C.C.; Blackman, J.W.; Schmidtke, L.M. Grapevine bunch rots: Impacts on wine composition, quality, and potential procedures for the removal of wine faults. J. Agric. Food Chem. 2013, 61, 5189–5206.
- Granik, L. The Wines of Georgia. In Infinite Ideas, 1st ed.; Infinite Ideas Ltd.: London, UK, 2019; 288p.
- Feiring, A. For the Love of Wine: My Odyssey through the World’s Most Ancient Wine Culture; Potomac Books: Sterling, VA, USA, 2016; 208p.
- Olejar, K.J.; Fedrizzi, B.; Kilmartin, P.A. Antioxidant activity and phenolic profiles of Sauvignon Blanc wines made by various maceration techniques. Aust. J. Grape Wine Res. 2015, 21, 57–68.
- Lukic, I.; Lotti, C.; Vrhovsek, U. Evolution of free and bound volatile aroma compounds and phenols during fermentation of Muscat blanc grape juice with and without skins. Food Chem. 2017, 232, 25–35.
- Rossetti, F.; Boselli, E. Effects of In-Amphorae Winemaking on the Chemical and Sensory Profile of Chardonnay Wine. Sci. Agric. Bohem. 2017, 48, 39–46.
- Salemnia, S.; Garcia-Torres, R.; Herman, D.; Fajardo-Lira, C. Red, White, And…Orange? A New Look into an Old Wine (P20-007-19). Curr. Dev. Nutr. 2019, 3, nzz040-P20.
- Bene, Z.; Kállay, M. Polyphenol contents of skin-contact fermented white wines. Acta Aliment. 2019, 48, 515–524.
- Scutarasu, E.C.; Luchian, C.E.; Vlase, L.; Colibaba, L.C.; Gheldiu, A.M.; Cotea, V.V. Evolution of phenolic profile of white wines treated with enzymes. Food Chem. 2021, 340, 127910.
- Bene, Z.; Kallay, M.; Horvath, B.O.; Nyitrai-Sardy, D. Comparison of selected phenolic components of white qvevri wines. Mitteilungen Klosterneubg. 2019, 69, 76–82.
- Townshend, E.R.; Harrison, R.; Tian, B. The phenolic composition of orange wine—Effects of skin contact and sulfur dioxide addition on white wine tannin. Wine Vitic. J. 2019, 34, 25–32.
- Lomolino, G.; Zocca, F.; Spettoli, P.; Zanin, G.; Lante, A. A preliminary study on changes in phenolic content during Bianchetta Trevigiana winemaking. J. Food Compos. Anal. 2010, 23, 575–579.
- Bestulić, E.; Rossi, S.; Plavša, T.; Horvat, I.; Lukić, I.; Bubola, M.; Peršurić, A.S.I.; Jeromel, A.; Radeka, S. Comparison of different maceration and non-maceration treatments for enhancement of phenolic composition, colour intensity, and taste attributes of Malvazija istarska (Vitis vinifera L.) white wines. J. Food Compos. Anal. 2022, 109, 104472.
- Koyama, K.; Goto-Yamamoto, N.; Hashizume, K. Influence of maceration temperature in red wine vinification on extraction of phenolics from berry skins and seeds of grape (Vitis vinifera). Biosci. Biotechnol. Biochem. 2007, 71, 958–965.
- Yilmaz, Y.; Toledo, R.T. Major flavonoids in grape seeds and skins: Antioxidant capacity of catechin, epicatechin, and gallic acid. J. Agric. Food Chem. 2004, 52, 255–260.
- Baiano, A.; Varva, G.; De Gianni, A.; Viggiani, I.; Terracone, C.; Del Nobile, M.A. Influence of type of amphora on physico-chemical properties and antioxidant capacity of ‘Falanghina’ white wines. Food Chem. 2014, 146, 226–233.
- Baiano, A.; Varva, G. Evolution of physico-chemical and sensory characteristics of Minutolo white wines during aging in amphorae: A comparison with stainless steel tanks. LWT 2019, 103, 78–87.
- Clarke, S.; Bosman, G.; du Toit, W.; Aleixandre-Tudo, J.L. White wine phenolics: Current methods of analysis. J. Sci. Food Agric. 2023, 103, 7–25.
- du Toit, W.J.; Marais, J.; Pretorius, I.S.; du Toit, M. Oxygen in Must and Wine: A review. S. Afr. J. Enol. Vitic. 2017, 27, 76–94.
- Kallithraka, S.; Salacha, M.I.; Tzourou, I. Changes in phenolic composition and antioxidant activity of white wine during bottle storage: Accelerated browning test versus bottle storage. Food Chem. 2009, 113, 500–505.
- Tauchen, J.; Marsik, P.; Kvasnicova, M.; Maghradze, D.; Kokoska, L.; Vanek, T.; Landa, P. In vitro antioxidant activity and phenolic composition of Georgian, Central and West European wines. J. Food Compos. Anal. 2015, 41, 113–121.
- Shalashvili, A.; Targamadze, I.; Zambakhidze, N.; Chichua, D.; Nareklishvili, V.; Ugrekhelidze, D. Comparison of wines of Kakhetian and European types according to quantitative content of flavonoids and antiradical efficiency. Bull. Georgian Natl. Acad. Sci. 2007, 175, 102–104.
- Fedrizzi, B.; Versini, G.; Finato, F.; Casarotti, E.; Nicolis, E.; Ferrarini, R. Evaluation of the Impact of an Archaic Protocol on White Wine Free Aroma Compounds. Flavor Chem. Wine Other Alcohol. Beverages 2012, 8, 117–131.
- Wang, J.; Huo, S.; Zhang, Y.; Liu, Y.; Fan, W. Impact of various maceration techniques on the phenolic and volatile composition of Chenin Blanc wines. Int. J. Food Sci. Technol. 2016, 51, 2360–2366.
- Redeka, S.; Lukic, I.; Persuric, D. Influence of Different Maceration Treatments on the Aroma Profile of Rosé and Red Wines from Croatian Aromatic cv. Muškat Ruža Porečki (Vitis vinifera L.). Food Technol. Biotechnol. 2012, 4, 50.
- Kennedy, J.A.; Saucier, C.; Glories, Y. Grape and Wine Phenolics: History and Perspective. Am. J. Enol. Vitic. 2006, 57, 239–248.
- Ferrer-Gallego, R.; Hernández-Hierro, J.M.; Rivas-Gonzalo, J.C.; Escribano-Bailón, M.T. Sensory evaluation of bitterness and astringency sub-qualities of wine phenolic compounds: Synergistic effect and modulation by aromas. Food Res. Int. 2014, 62, 1100–1107.
- Cordente, A.G.; Espinase Nandorfy, D.; Solomon, M.; Schulkin, A.; Kolouchova, R.; Francis, I.L.; Schmidt, S.A. Aromatic Higher Alcohols in Wine: Implication on Aroma and Palate Attributes during Chardonnay Aging. Molecules 2021, 26, 4979.
- Tarasov, A.; Giuliani, N.; Dobrydnev, A.; Schuessler, C.; Volovenko, Y.; Rauhut, D.; Jung, R. 1,1,6-Trimethyl-1,2-dihydronaphthalene (TDN) Sensory Thresholds in Riesling Wine. Foods 2020, 9, 606.
- Francis, I.L.; Kassara, S.; Noble, A.C.; Williams, P.J. (Eds.) The Contribution of Glycoside Precursors to Cabernet Sauvignon and Merlot Aroma: Sensory and Compositional Studies; ACS Publications: Washington, DC, USA, 1998.
- Carlin, S.; Lotti, C.; Correggi, L.; Mattivi, F.; Arapitsas, P.; Vrhovsek, U. Measurement of the Effect of Accelerated Aging on the Aromatic Compounds of Gewurztraminer and Teroldego Wines, Using a SPE-GC-MS/MS Protocol. Metabolites 2022, 12, 180.
- Liberatore, M.T.; Pati, S.; Nobile, M.A.D.; Notte, E.L. Aroma quality improvement of Chardonnay white wine by fermentation and ageing in barrique on lees. Food Res. Int. 2010, 43, 996–1002.
- Slaghenaufi, D.; Ugliano, M. Norisoprenoids, Sesquiterpenes and Terpenoids Content of Valpolicella Wines During Aging: Investigating Aroma Potential in Relationship to Evolution of Tobacco and Balsamic Aroma in Aged Wine. Front Chem. 2018, 6, 66.
- Vilela, A. Modulating Wine Pleasantness Throughout Wine-Yeast Co-Inoculation or Sequential Inoculation. Fermentation 2020, 6, 22.
- Perestrelo, R.; Silva, C.L.; Câmara, J.S.J.M. Madeira Wine Volatile Profile. A Platform to Establish Madeira Wine Aroma Descriptors. Molecules 2019, 24, 3028.
- Nicolini, G.; Versini, G.; Amadei, E.; Marchio, M. 3-hexen-1-ol isomers in Müller-Thurgau wines: A "varietal" characteristic affected by must sulfiting time. Vitis –Geilweilerhof 1996, 35, 147–148.
- Peinado, R.A.; Moreno, J.; Bueno, J.E.; Moreno, J.A.; Mauricio, J.C. Comparative study of aromatic compounds in two young white wines subjected to pre-fermentative cryomaceration. Food Chem. 2004, 84, 585–590.
- Francis, I.L.; Newton, J.L. Determining wine aroma from compositional data. Aust. J. Grape Wine Res. 2005, 11, 114–126.
- Swiegers, J.H.; Bartowsky, E.J.; Henschke, P.A.; Pretorius, I.S. Yeast and bacterial modulation of wine aroma and flavour. Aust. J. Grape Wine Res. 2005, 11, 139–173.
- Romano, P.; Braschi, G.; Siesto, G.; Patrignani, F.; Lanciotti, R. Role of Yeasts on the Sensory Component of Wines. Foods 2022, 11, 1921.
- Vazquez-Pateiro, I.; Arias-Gonzalez, U.; Miras-Avalos, J.M.; Falque, E. Evolution of the Aroma of Treixadura Wines during Bottle Aging. Foods 2020, 9, 1419.
- Machamangalath, R.; Arekar, C.; Lele, S.S. Exotic tropical fruit wines from Garcinia indica and Musa acuminate. J. Inst. Brew. 2016, 122, 745–753.
- Wei, Z.; Liu, X.; Huang, Y.; Lu, J.; Zhang, Y. Volatile aroma compounds in wines from Chinese wild/hybrid species. J. Food Biochem. 2019, 43, e12684.
- du Plessis, H.; du Toit, M.; Nieuwoudt, H.; van der Rijst, M.; Kidd, M.; Jolly, N. Effect of Saccharomyces, Non-Saccharomyces Yeasts and Malolactic Fermentation Strategies on Fermentation Kinetics and Flavor of Shiraz Wines. Fermentation 2017, 3, 64.
- Park, H.J.; Lee, S.M.; Song, S.H.; Kim, Y.S. Characterization of volatile components in makgeolli, a traditional Korean rice wine, with or without pasteurization, during storage. Molecules 2013, 18, 5317–5325.
- Kharbedia, M. The history of Georgian Wine Georgian Wine Guide. Vinoge Magazin. 3 April 2014. Available online: http://vinoge.com/Books/qarTuli-Rvinis-gzamkvlevi-2014 (accessed on 18 July 2023).
- UNESCO. Ancient Georgian traditional Qvevri wine-making method. 2013. Available online: https://ich.unesco.org/en/RL/ancient-georgian-traditional-qvevri-wine-making-method-00870 (accessed on 18 July 2023).
- Reblova, M. Orange wine and its production, kvevri and the Kakheti method—2nd part. Encycl. Wine 2018.
- Harutyunyan, M.; Malfeito-Ferreira, M. Historical and Heritage Sustainability for the Revival of Ancient Wine-Making Techniques and Wine Styles. Beverages 2022, 8, 10.
- White, P. Qvevri culture. World Fine Wine 2016, 118–122.
- Dzagnidze, B. Georgian Qvevri Craft: The Secret of Georgian Wine. 2023. Available online: https://www.neff-home.com/theingredient/story/quevery (accessed on 18 July 2023).
- Barisashvili, B. Making Wine in Qvevri—A Unique Georgian Tradition; Biological Farming Association “Elkana”: Tbilisi, Georgia, 2011.
- Petzold, J. Fundamental ceramic investigations of 3 clay quarries to prepare Qvevri Pots. GIZ 2016, 65.
- Gamtkitsulashvili, G.V. The Research of Constituent Clay-Minerals of Qvevri and Its Influence on Wine. Anc. Art Wine Mak. 2018.
- Mertz, U.; Bitarishvili, I. Identity of Kvevri Wine—On the Example of the Practice of the Members of the Kvevari Wine Cluster of Georgia; German International Cooperation Society (GIZ): Tbilisi, Georgia, 2017; ISBN 978-9941-9491-5-9.
- Esoyan, S.; Loupiac, C.; Hovhannisyan, N.; Gougeon, R.; Karbowiak, T.; Michalke, B.; Schmitt-Kopplin, P.; Fontaine, C.; Valange, S.; Bodart, P. Interaction between Armenian clay-based ceramics and model wine during storage. OENO One 2023, 57, 101–113.
- Di Renzo, M.; Letizia, F.; Di Martino, C.; Karaulli, J.; Kongoli, R.; Testa, B.; Avino, P.; Guerriero, E.; Albanese, G.; Monaco, M.; et al. Natural Fiano Wines Fermented in Stainless Steel Tanks, Oak Barrels, and Earthenware Amphora. Processes 2023, 11, 1273.
- Prusova, B.; Baron, M. Effect of controlled micro-oxygenation on white wine. Ciência Técnica Vitivinícola 2018, 33, 78–89.
- Nevares, I.; Alamo-Sanza, M.D. Characterization of the Oxygen Transmission Rate of New-Ancient Natural Materials for Wine Maturation Containers. Foods 2021, 10, 140.
More
Information
Subjects:
Food Science & Technology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.2K
Revisions:
2 times
(View History)
Update Date:
11 Sep 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




