
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Yakindra Prasad Timilsena | -- | 2859 | 2023-09-11 00:43:24 | | | |
| 2 | Jason Zhu | Meta information modification | 2859 | 2023-09-11 07:17:03 | | |
Video Upload Options
Saponins are a diverse group of naturally occurring plant secondary metabolites present in a wide range of foods ranging from grains, pulses, and green leaves to sea creatures. They consist of a hydrophilic sugar moiety linked to a lipophilic aglycone, resulting in an amphiphilic nature and unique functional properties. Their amphiphilic structures enable saponins to exhibit surface-active properties, resulting in stable foams and complexes with various molecules. In the context of food applications, saponins are utilized as natural emulsifiers, foaming agents, and stabilizers. They contribute to texture and stability in food products and have potential health benefits, including cholesterol-lowering and anticancer effects. Saponins possess additional bioactivities that make them valuable in the pharmaceutical industry as anti-inflammatory, antimicrobial, antiviral, and antiparasitic agents to name a few. Saponins can demonstrate cytotoxic activity against cancer cell lines and can also act as adjuvants, enhancing the immune response to vaccines.
1. Introduction
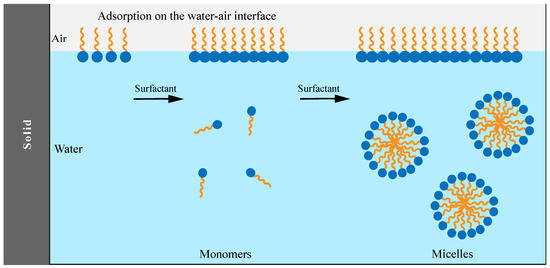
2. Saponins as Natural Surfactants and Emulsifiers
3. Saponins as Natural Foaming Agents
4. Saponins as Natural Antioxidants
5. Medicinal Applications of Saponins
References
- San Martin, R.; Briones, R. Quality control of commercial quillaja (Quillaja saponaria Molina) extracts by reverse phase HPLC. J. Sci. Food Agric. 2000, 80, 2063–2068.
- Karnwal, A.; Shrivastava, S.; Al-Tawaha, A.R.M.S.; Kumar, G.; Singh, R.; Kumar, A.; Mohan, A.; Yogita; Malik, T. Microbial Biosurfactant as an Alternate to Chemical Surfactants for Application in Cosmetics Industries in Personal and Skin Care Products: A Critical Review. BioMed Res. Int. 2023, 2023, 2375223.
- Güçlü-Üstündağ, Ö.; Mazza, G. Saponins: Properties, applications and processing. Crit. Rev. Food Sci. Nutr. 2007, 47, 231–258.
- San Martin, R.; Briones, R. Industrial Uses and Sustainable Supply of Quillaja saponaria (Rosaceae) Saponins. Econ. Bot. 1999, 53, 302–311.
- Thompson, L.U. Potential health benefits and problems associated with antinutrients in foods. Food Res. Int. 1993, 26, 131–149.
- Ridout, C.L.; Price, K.R.; Dupont, M.S.; Parker, M.L.; Fenwick, G.R. Quinoa saponins—Analysis and preliminary investigations into the effects of reduction by processing. J. Sci. Food Agric. 1991, 54, 165–176.
- Kim, S.-W.; Park, S.-K.; Kang, S.-l.; Kang, H.-C.; Oh, H.-J.; Bae, C.-Y.; Bae, D.-H. Hypocholesterolemic property ofYucca schidigera andQuillaja saponaria extracts in human body. Arch. Pharm. Res. 2003, 26, 1042–1046.
- Gurfinkel, D.M.; Rao, A.V. Soybeansaponins: The relationship between chemical structure and colon anticarcinogenic activity. Nutr. Cancer 2003, 47, 24–33.
- Alice, C.; Vargas, V.; Silva, G.; De Siqueira, N.; Schapoval, E.; Gleye, J.; Henriques, J.; Henriques, A. Screening of plants used in south Brazilian folk medicine. J. Ethnopharmacol. 1991, 35, 165–171.
- Liu, J.; Henkel, T. Traditional Chinese medicine (TCM): Are polyphenols and saponins the key in-gredients triggering biological activities? Curr. Med. Chem. 2002, 9, 1483–1485.
- Kerwin, S. Soy saponins and the anticancer effects of soybeans and soy-based foods. Curr. Med. Chem. Anti Cancer Agents 2004, 4, 263–272.
- Oakenfull, D. Soy protein, saponins and plasma cholesterol. J. Nutr. 2001, 131, 2971.
- Matsuura, H. Saponins in garlic as modifiers of the risk of cardiovascular disease. J. Nutr. 2001, 131, 1000S–1005S.
- Muir, A.D.; Paton, D.; Ballantyne, K.; Aubin, A.A. Process for Recovery and Purification of Saponins and Sapogenins from Quinoa. U.S. Patent 6,355,249, 12 March 2002.
- Rickert, D.A.; Meyer, M.A.; Hu, J.; Murphy, P.A. Effect of Extraction pH and Temperature on Isoflavone and Saponin Partitioning and Profile During Soy Protein Isolate Production. J. Food Sci. 2004, 69, C623–C631.
- Akbari, S.; Abdurahman, N.H.; Kudrashou, V. Surface Activity and Emulsification Properties of Saponins as Biosurfactants. In Advancements in Biosurfactants Research; Aslam, R., Mobin, M., Aslam, J., Zehra, S., Eds.; Springer International Publishing: Cham, Switzerland, 2023; pp. 137–153.
- Zhou, W.; Yang, J.; Lou, L.; Zhu, L. Solubilization properties of polycyclic aromatic hydrocarbons by saponin, a plant-derived biosurfactant. Environ. Pollut. 2011, 159, 1198–1204.
- Kjellin, M.; Johansson, I. (Eds.) Saponin-Based Surfactants; John Wiley & Sons: Hoboken, NJ, USA, 2010.
- Zana, R. Dynamics of Surfactant Self-Assemblies: Micelles, Microemulsions, Vesicles, and Lyotropic Phases; Routledge: Abingdon on Thames, UK, 2005.
- Mitra, S.; Dungan, S.R. Cholesterol Solubilization in Aqueous Micellar Solutions of Quillaja Saponin, Bile Salts, or Nonionic Surfactants. J. Agric. Food Chem. 2001, 49, 384–394.
- Bachran, C.; Bachran, S.; Sutherland, M.; Bachran, D.; Fuchs, H. Chapter 9—Preclinical Studies of Saponins for Tumor Therapy. In Recent Advances in Medicinal Chemistry; Attaur, R., Choudhary, M.I., Perry, G., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 272–302.
- McClements, D.J.; Gumus, C.E. Natural emulsifiers—Biosurfactants, phospholipids, biopolymers, and colloidal particles: Molecular and physicochemical basis of functional performance. Adv. Colloid Interface Sci. 2016, 234, 3–26.
- Kezwon, A.; Wojciechowski, K. Interaction of Quillaja bark saponins with food-relevant proteins. Adv. Colloid Interface Sci. 2014, 209, 185–195.
- Pekdemir, T.; Çopur, M.; Urum, K. Emulsification of Crude Oil–Water Systems Using Biosurfactants. Process Saf. Environ. Prot. 2005, 83, 38–46.
- Chung, C.; Sher, A.; Rousset, P.; Decker, E.A.; McClements, D.J. Formulation of food emulsions using natural emulsifiers: Utilization of quillaja saponin and soy lecithin to fabricate liquid coffee whiteners. J. Food Eng. 2017, 209, 1–11.
- Yang, Y.; Leser, M.E.; Sher, A.A.; McClements, D.J. Formation and stability of emulsions using a natural small molecule surfactant: Quillaja saponin (Q-Naturale®). Food Hydrocoll. 2013, 30, 589–596.
- Zhang, J.; Bing, L.; Reineccius, G.A. Formation, optical property and stability of orange oil nanoemulsions stabilized by Quallija saponins. LWT Food Sci. Technol. 2015, 64, 1063–1070.
- Böttcher, S.; Keppler, J.K.; Drusch, S. Mixtures of Quillaja saponin and beta-lactoglobulin at the oil/water-interface: Adsorption, interfacial rheology and emulsion properties. Colloids Surf. A Physicochem. Eng. Asp. 2017, 518, 46–56.
- Reichert, C.L.; Salminen, H.; Badolato Bönisch, G.; Schäfer, C.; Weiss, J. Concentration effect of Quillaja saponin—Co-surfactant mixtures on emulsifying properties. J. Colloid Interface Sci. 2018, 519, 71–80.
- de Faria, J.T.; de Oliveira, E.B.; Minim, V.P.R.; Minim, L.A. Emulsifying properties of β-lactoglobulin and Quillaja bark saponin mixtures: Effects of number of homogenization passes, pH, and NaCl concentration. Int. J. Food Prop. 2017, 20, 1643–1654.
- Drenckhan, W.; Saint-Jalmes, A. The science of foaming. Adv. Colloid Interface Sci. 2015, 222, 228–259.
- Reichert, C.L.; Salminen, H.; Weiss, J. Quillaja Saponin Characteristics and Functional Properties. Annu. Rev. Food Sci. Technol. 2019, 10, 43–73.
- Rio, E.; Drenckhan, W.; Salonen, A.; Langevin, D. Unusually stable liquid foams. Adv. Colloid Interface Sci. 2014, 205, 74–86.
- Hill, C.; Eastoe, J. Foams: From nature to industry. Adv. Colloid Interface Sci. 2017, 247, 496–513.
- Böttcher, S.; Drusch, S. Interfacial Properties of Saponin Extracts and Their Impact on Foam Characteristics. Food Biophys. 2016, 11, 91–100.
- Chen, Y.F.; Yang, C.H.; Chang, M.S.; Ciou, Y.P.; Huang, Y.C. Foam properties and detergent abilities of the saponins from Camellia oleifera. Int. J. Mol. Sci. 2010, 11, 4417–4425.
- Canto, G.S.d.; Treter, J.; Yang, S.; Borré, G.L.; Peixoto, M.P.G.; Ortega, G.G. Evaluation of foam properties of saponin from Ilex paraguariensis A. St. Hil. (Aquifoliaceae) fruits. Braz. J. Pharm. Sci. 2010, 46, 237–243.
- Uluata, S.; McClements, D.J.; Decker, E.A. Physical Stability, Autoxidation, and Photosensitized Oxidation of ω-3 Oils in Nanoemulsions Prepared with Natural and Synthetic Surfactants. J. Agric. Food Chem. 2015, 63, 9333–9340.
- Gülçin, İ.; Mshvildadze, V.; Gepdiremen, A.; Elias, R. Antioxidant Activity of Saponins Isolated from Ivy: α-Hederin, Hederasaponin-C, Hederacolchiside-E and Hederacolchiside-F. Planta Med. 2004, 70, 561–563.
- Nowicki, J.; Murray, M.T. Bronchitis and Pneumonia. In Textbook of Natural Medicine; Murray, M.T., Pizzorno, J.E., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; Volume e1, pp. 1196–1201.
- Gülçin, İ.; Mshvildadze, V.; Gepdiremen, A.; Elias, R. Screening of antiradical and antioxidant activity of monodesmosides and crude extract from Leontice smirnowii tuber. Phytomedicine 2006, 13, 343–351.
- Takada, Y.; Tayama, I.; Sayama, T.; Sasama, H.; Saruta, M.; Kikuchi, A.; Ishimoto, M.; Tsukamoto, C. Genetic analysis of variations in the sugar chain composition at the C-3 position of soybean seed saponins. Breed. Sci. 2012, 61, 639–645.
- Yoshiki, Y.; Kahara, T.; Okubo, K.; Sakabe, T.; Yamasaki, T. Superoxide- and 1,1-Diphenyl-2-picrylhydrazyl Radical-scavenging Activities of Soyasaponin β g Related to Gallic Acid. Biosci. Biotechnol. Biochem. 2001, 65, 2162–2165.
- Tippel, J.; Gies, K.; Harbaum-Piayda, B.; Steffen-Heins, A.; Drusch, S. Composition of Quillaja saponin extract affects lipid oxidation in oil-in-water emulsions. Food Chem. 2017, 221, 386–394.
- Han, Y.; Chi, J.; Zhang, M.; Zhang, R.; Fan, S.; Huang, F.; Xue, K.; Liu, L. Characterization of saponins and phenolic compounds: Antioxidant activity and inhibitory effects on α-glucosidase in different varieties of colored quinoa (Chenopodium quinoa Willd). Biosci. Biotechnol. Biochem. 2019, 83, 2128–2139.
- Lim, J.G.; Park, H.M.; Yoon, K.S. Analysis of saponin composition and comparison of the antioxidant activity of various parts of the quinoa plant (Chenopodium quinoa Willd.). Food Sci. Nutr. 2020, 8, 694–702.
- Li, H.; Zhai, B.; Sun, J.; Fan, Y.; Zou, J.; Cheng, J.; Zhang, X.; Shi, Y.; Guo, D. Antioxidant, Anti-Aging and Organ Protective Effects of Total Saponins from Aralia taibaiensis. Drug Des. Dev. Ther. 2021, 15, 4025–4042.
- Ashraf, M.F.; Abd Aziz, M.; Stanslas, J.; Ismail, I.; Abdul Kadir, M. Assessment of Antioxidant and Cytotoxicity Activities of Saponin and Crude Extracts of Chlorophytum borivilianum. Sci. World J. 2013, 2013, 216894.
- Waheed, A.; Barker, J.; Barton, S.J.; Owen, C.P.; Ahmed, S.; Carew, M.A. A novel steroidal saponin glycoside from Fagonia indica induces cell-selective apoptosis or necrosis in cancer cells. Eur. J. Pharm. Sci. 2012, 47, 464–473.
- Hassan, S.M.; Haq, A.U.; Byrd, J.A.; Berhow, M.A.; Cartwright, A.L.; Bailey, C.A. Haemolytic and antimicrobial activities of saponin-rich extracts from guar meal. Food Chem. 2010, 119, 600–605.
- Just, M.J.; Recio, M.C.; Giner, R.M.; Cuéllar, M.J.; Máñez, S.; Bilia, A.R.; Ríos, J.-L. Anti-Inflammatory Activity of Unusual Lupane Saponins from Bupleurum fruticescens. Planta Med. 1998, 64, 404–407.
- Sparg, S.G.; Light, M.E.; van Staden, J. Biological activities and distribution of plant saponins. J. Ethnopharmacol. 2004, 94, 219–243.
- Sindambiwe, J.B.; Calomme, M.; Geerts, S.; Pieters, L.; Vlietinck, A.J.; Vanden Berghe, D.A. Evaluation of Biological Activities of Triterpenoid Saponins from Maesa lanceolata. J. Nat. Prod. 1998, 61, 585–590.
- Simões, C.M.O.; Amoros, M.; Girre, L. Mechanism of antiviral activity of triterpenoid saponins. Phytother. Res. 1999, 13, 323–328.
- Ellen, D.; Ellen, L.; Danny, G.; Guy, S. Novel advances with plant saponins as natural insecticides to control pest insects. Pest Technol. 2007, 1, 96–105.
- Cheng, T.-C.; Lu, J.-F.; Wang, J.-S.; Lin, L.-J.; Kuo, H.-I.; Chen, B.-H. Antiproliferation Effect and Apoptosis Mechanism of Prostate Cancer Cell PC-3 by Flavonoids and Saponins Prepared from Gynostemma pentaphyllum. J. Agric. Food Chem. 2011, 59, 11319–11329.
- Mbaveng, A.T.; Ndontsa, B.L.; Kuete, V.; Nguekeu, Y.M.M.; Çelik, İ.; Mbouangouere, R.; Tane, P.; Efferth, T. A naturally occuring triterpene saponin ardisiacrispin B displayed cytotoxic effects in multi-factorial drug resistant cancer cells via ferroptotic and apoptotic cell death. Phytomedicine 2018, 43, 78–85.
- Abdel-Gawad, M.M.; El-Sayed, M.M.; Abdel-Hameed, E.S. Molluscicidal steroidal saponins and lipid content of Agave decipiens. Fitoterapia 1999, 70, 371–381.
- Moghimipour, E.; Handali, S. Saponin: Properties, methods of evaluation and applications. Annu. Res. Rev. Biol. 2015, 5, 207–220.
- Oboh, H.A.; Omofoma, C.O. The effects of heat treated lima beans (Phaseolus lunatus) on plasma li-pids in hypercholesterolemic rats. Pak. J. Nutr. 2008, 7, 636–639.
- El Barky, A.R.; Ali, E.M.M.; Mohamed, T.M. Marine Sea Cucumber Saponins and Diabetes. Austin. Pancreat. Disord. 2017, 1, 1002.
- Marrelli, M.; Conforti, F.; Araniti, F.; Statti, G.A. Effects of Saponins on Lipid Metabolism: A Review of Potential Health Benefits in the Treatment of Obesity. Molecules 2016, 21, 1404.
- Zhao, D. Challenges associated with elucidating the mechanisms of the hypocholesterolaemic activity of saponins. J. Funct. Foods 2016, 23, 52–65.
- Mohan, V.R.; Tresina, P.S.; Daffodil, E.D. Antinutritional Factors in Legume Seeds: Characteristics and Determination. In Encyclopedia of Food and Health; Caballero, B., Finglas, P.M., Toldrá, F., Eds.; Academic Press: Oxford, UK, 2016; pp. 211–220.
- Segal, R.; Schlösser, E. Role of glycosidases in the membranlytic, antifungal action of saponins. Arch. Microbiol. 1975, 104, 147–150.
- Marciani, D.J.; Ptak, R.G.; Voss, T.G.; Reynolds, R.C.; Pathak, A.K.; Chamblin, T.L.; Scholl, D.R.; May, R.D. Degradation of Quillaja saponaria Molina saponins: Loss of the protective effects of a herpes simplex virus 1 subunit vaccine. Int. Immunopharmacol. 2002, 2, 1703–1711.
- Netala, V.R.; Ghosh, S.B.; Bobbu, P.; Anitha, D.; Tartte, V. Triterpenoid saponins: A review on biosynthesis, Applications and mechanism of their action. Int. J. Pharm. Pharm. Sci. 2015, 7, 24–28.
- Qi, L.-W.; Wang, C.-Z.; Yuan, C.-S. Ginsenosides from American ginseng: Chemical and pharmacological diversity. Phytochemistry 2011, 72, 689–699.
- Sheng, H.; Sun, H. Synthesis, biology and clinical significance of pentacyclic triterpenes: A multi-target approach to prevention and treatment of metabolic and vascular diseases. Nat. Prod. Rep. 2011, 28, 543–593.




