
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Mohammed Hassanein | -- | 2341 | 2023-09-06 17:03:42 | | | |
| 2 | Alfred Zheng | Meta information modification | 2341 | 2023-09-07 04:17:43 | | |
Video Upload Options
Recent years have witnessed the emergence of growing evidence concerning vitamin D's potential role in women's health, specifically in postmenopausal women. This evidence also includes its connection to various genitourinary disorders and symptoms. Numerous clinical studies have observed improvements in vulvovaginal symptoms linked to the genitourinary syndrome of menopause (GSM) with vitamin D supplementation. These studies have reported positive effects on various aspects such as vaginal pH, dryness, sexual functioning, reduced libido, and a decrease in urinary tract infections. Many mechanisms underlying these pharmacological effects have since been proposed. Vitamin D receptors (VDRs) have been identified as a major contributor to its effects. It is now well known that VDRs are expressed in the superficial layers of the urogenital organs. Additionally, vitamin D plays a crucial role in supporting immune function and modulating the body's defense mechanisms. However, the characterization of these effects requires more investigation. Reviewing existing evidence regarding vitamin D's impact on post-menopausal women's vaginal, sexual, and urological health is the purpose of this article. As research in this area continues, there is a potential for vitamin D to support women's urogenital and sexual health during the menopausal transition and postmenopausal periods.
1. Introduction
2. Therapeutic Effects of Vitamin D on Urogenital Functions
2.1. Cellular Effects of Vitamin D on Urogenital Tissues
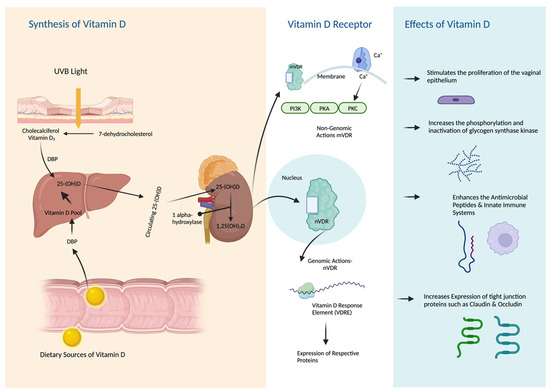
2.2. The Effects of Vitamin D on Vaginal Epithelium and pH
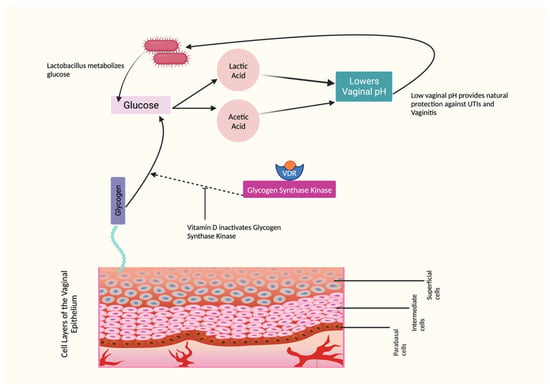
2.3. The Effects of Vitamin D on Vaginal Symptoms
2.4. The Effects of Vitamin D on Vaginal Infections
2.5. The Effects of Vitamin D on Sexual Functions
2.6. The Role of Vitamin D in UTIs: Effects on the Immune Function
2.7. The Role of Vitamin D in UTIs: Effects on Tight Junction Proteins
2.8. The Effects of Vitamin D on Pelvic Floor Disorders
Understanding the benefits of vitamin D supplementation is crucial. Recent research shows promising evidence of its positive impact on urogenital health and sexual functioning in postmenopausal women. However, robust randomized controlled trials are lacking, with variations in participant characteristics, dosage, intervention length, and assessment methods. Objective indicators like the vaginal maturation index should be combined with subjective measures to strengthen evidence of vitamin D's effects, in measuring sexual functioning. Comprehensive investigations will inform evidence-based guidelines and personalized interventions for postmenopausal women. Filling knowledge gaps will provide clearer insights for clinicians and facilitate tailored strategies to enhance urogenital health and well-being. Figure 3 summarizes all potential therapeutic effects of vitamin D on urogenital health and sexual functions.
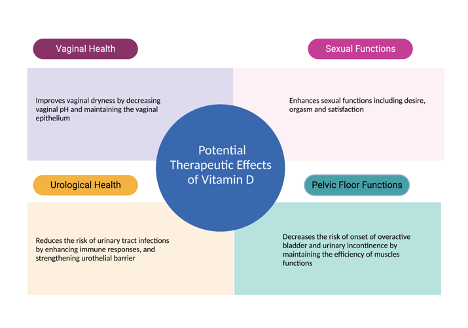
Figure 3. Therapeutic Effects of Vitamin D on Urogenital Functions.
References
- Gandhi, J.; Chen, A.; Dagur, G.; Suh, Y.; Smith, N.; Cali, B.; Khan, S.A. Genitourinary syndrome of menopause: An overview of clinical manifestations, pathophysiology, etiology, evaluation, and management. Am. J. Obstet. Gynecol. 2016, 215, 704–711.
- North American Menopause Society. Management of symptomatic vulvovaginal atrophy: 2013 position statement of The North American Menopause Society. Menopause 2013, 20, 888–902.
- Angelou, K.; Grigoriadis, T.; Diakosavvas, M.; Zacharakis, D.; Athanasiou, S. The Genitourinary Syndrome of Menopause: An Overview of the Recent Data. Cureus 2020, 12, e7586.
- Faubion, S.S.; Sood, R.; Kapoor, E. Genitourinary Syndrome of Menopause: Management Strategies for the Clinician. Mayo Clin. Proc. 2017, 92, 1842–1849.
- Hugenholtz, F.; van der Veer, C.; Terpstra, M.L.; Borgdorff, H.; van Houdt, R.; Bruisten, S.; Geerlings, S.E.; van de Wijgert, J. Urine and vaginal microbiota compositions of postmenopausal and premenopausal women differ regardless of recurrent urinary tract infection and renal transplant status. Sci. Rep. 2022, 12, 2698.
- Nappi, R.E.; Martini, E.; Cucinella, L.; Martella, S.; Tiranini, L.; Inzoli, A.; Brambilla, E.; Bosoni, D.; Cassani, C.; Gardella, B. Addressing Vulvovaginal Atrophy (VVA)/Genitourinary Syndrome of Menopause (GSM) for Healthy Aging in Women. Front. Endocrinol. 2019, 10, 561.
- Portman, D.J.; Gass, M.L.; Vulvovaginal Atrophy Terminology Consensus Conference Panel. Genitourinary syndrome of menopause: New terminology for vulvovaginal atrophy from the International Society for the Study of Women’s Sexual Health and the North American Menopause Society. Menopause 2014, 21, 1063–1068.
- Domoney, C.; Short, H.; Particco, M.; Panay, N. Symptoms, attitudes and treatment perceptions of vulvo-vaginal atrophy in UK postmenopausal women: Results from the REVIVE-EU study. Post Reprod. Health 2020, 26, 101–109.
- Krychman, M.; Graham, S.; Bernick, B.; Mirkin, S.; Kingsberg, S.A. The Women’s EMPOWER Survey: Women’s Knowledge and Awareness of Treatment Options for Vulvar and Vaginal Atrophy Remains Inadequate. J. Sex. Med. 2017, 14, 425–433.
- Moral, E.; Delgado, J.L.; Carmona, F.; Caballero, B.; Guillan, C.; Gonzalez, P.M.; Suarez-Almarza, J.; Velasco-Ortega, S.; Nieto, C.; as the writing group of the GENISSE study. Genitourinary syndrome of menopause. Prevalence and quality of life in Spanish postmenopausal women. The GENISSE study. Climacteric 2018, 21, 167–173.
- Nappi, R.E.; de Melo, N.R.; Martino, M.; Celis-Gonzalez, C.; Villaseca, P.; Rohrich, S.; Palacios, S. Vaginal Health: Insights, Views & Attitudes (VIVA-LATAM): Results from a survey in Latin America. Climacteric 2018, 21, 397–403.
- Nappi, R.E.; Kingsberg, S.; Maamari, R.; Simon, J. The CLOSER (CLarifying Vaginal Atrophy’s Impact On SEx and Relationships) survey: Implications of vaginal discomfort in postmenopausal women and in male partners. J. Sex. Med. 2013, 10, 2232–2241.
- Palma, F.; Volpe, A.; Villa, P.; Cagnacci, A.; as the writing group of the AGATA study. Vaginal atrophy of women in postmenopause. Results from a multicentric observational study: The AGATA study. Maturitas 2016, 83, 40–44.
- Mac Bride, M.B.; Rhodes, D.J.; Shuster, L.T. Vulvovaginal atrophy. Mayo Clin. Proc. 2010, 85, 87–94.
- Lee, A.; Lee, M.R.; Lee, H.H.; Kim, Y.S.; Kim, J.M.; Enkhbold, T.; Kim, T.H. Vitamin D Proliferates Vaginal Epithelium through RhoA Expression in Postmenopausal Atrophic Vagina tissue. Mol. Cells 2017, 40, 677–684.
- Jalali-Chimeh, F.; Gholamrezaei, A.; Vafa, M.; Nasiri, M.; Abiri, B.; Darooneh, T.; Ozgoli, G. Effect of Vitamin D Therapy on Sexual Function in Women with Sexual Dysfunction and Vitamin D Deficiency: A Randomized, Double-Blind, Placebo Controlled Clinical Trial. J. Urol. 2019, 201, 987–993.
- Askin, M.; Koc, E.M.; Soyoz, M.; Aksun, S.; Aydogmus, S.; Sozmen, K. Relationship between Postmenopausal Vitamin D Level, Menopausal Symptoms and Sexual Functions. J. Coll. Physicians Surg. Pak. 2019, 29, 823–827.
- Hertting, O.; Holm, A.; Luthje, P.; Brauner, H.; Dyrdak, R.; Jonasson, A.F.; Wiklund, P.; Chromek, M.; Brauner, A. Vitamin D induction of the human antimicrobial Peptide cathelicidin in the urinary bladder. PLoS ONE 2010, 5, e15580.
- Lorenzen, M.; Boisen, I.M.; Mortensen, L.J.; Lanske, B.; Juul, A.; Blomberg Jensen, M. Reproductive endocrinology of vitamin D. Mol. Cell. Endocrinol. 2017, 453, 103–112.
- Skowronska, P.; Pastuszek, E.; Kuczynski, W.; Jaszczol, M.; Kuc, P.; Jakiel, G.; Woclawek-Potocka, I.; Lukaszuk, K. The role of vitamin D in reproductive dysfunction in women—A systematic review. Ann. Agric. Environ. Med. 2016, 23, 671–676.
- Kamronrithisorn, T.; Manonai, J.; Vallibhakara, S.A.; Sophonsritsuk, A.; Vallibhakara, O. Effect of Vitamin D Supplement on Vulvovaginal Atrophy of the Menopause. Nutrients 2020, 12, 2876.
- Jefferson, K.K.; Parikh, H.I.; Garcia, E.M.; Edwards, D.J.; Serrano, M.G.; Hewison, M.; Shary, J.R.; Powell, A.M.; Hollis, B.W.; Fettweis, J.M.; et al. Relationship between vitamin D status and the vaginal microbiome during pregnancy. J. Perinatol. 2019, 39, 824–836.
- Duque, G.; El Abdaimi, K.; Macoritto, M.; Miller, M.M.; Kremer, R. Estrogens (E2) regulate expression and response of 1,25-dihydroxyvitamin D3 receptors in bone cells: Changes with aging and hormone deprivation. Biochem. Biophys. Res. Commun. 2002, 299, 446–454.
- Abban, G.; Yildirim, N.B.; Jetten, A.M. Regulation of the vitamin D receptor and cornifin beta expression in vaginal epithelium of the rats through vitamin D3. Eur. J. Histochem. 2008, 52, 107–114.
- Donders, G.G.G.; Ruban, K.; Bellen, G.; Grinceviciene, S. Pharmacotherapy for the treatment of vaginal atrophy. Expert Opin Pharmacother. 2019, 20, 821–835.
- Bikle, D.; Teichert, A.; Hawker, N.; Xie, Z.; Oda, Y. Sequential regulation of keratinocyte differentiation by 1,25(OH)2D3, VDR, and its coregulators. J. Steroid Biochem. Mol. Biol. 2007, 103, 396–404.
- Fadiel, A.; Lee, H.H.; Demir, N.; Richman, S.; Iwasaki, A.; Connell, K.; Naftolin, F. Ezrin is a key element in the human vagina. Maturitas 2008, 60, 31–41.
- Altoparlak, U.; Kadanali, A.; Kadanali, S. Correlation of urinary tract infections with the vaginal colonization in postmenopausal women. Mikrobiyol. Bul. 2004, 38, 377–383.
- Yildirim, B.; Kaleli, B.; Duzcan, E.; Topuz, O. The effects of postmenopausal Vitamin D treatment on vaginal atrophy. Maturitas 2004, 49, 334–337.
- Rad, P.; Tadayon, M.; Abbaspour, M.; Latifi, S.M.; Rashidi, I.; Delaviz, H. The effect of vitamin D on vaginal atrophy in postmenopausal women. Iran J. Nurs. Midwifery Res. 2015, 20, 211–215.
- Saeideh, Z.; Raziyeh, M.; Soghrat, F. Comparing the effects of continuous hormone replacement therapy and tibolone on the genital tract of menopausal women; a randomized controlled trial. J. Reprod. Infertil. 2010, 11, 183–187.
- Mucci, M.; Carraro, C.; Mancino, P.; Monti, M.; Papadia, L.S.; Volpini, G.; Benvenuti, C. Soy isoflavones, lactobacilli, Magnolia bark extract, vitamin D3 and calcium. Controlled clinical study in menopause. Minerva Ginecol. 2006, 58, 323–334.
- Checa, M.A.; Garrido, A.; Prat, M.; Conangla, M.; Rueda, C.; Carreras, R. A comparison of raloxifene and calcium plus vitamin D on vaginal atrophy after discontinuation of long-standing postmenopausal hormone therapy in osteoporotic women. A randomized, masked-evaluator, one-year, prospective study. Maturitas 2005, 52, 70–77.
- Bala, R.; Kaur, H.; Nagpal, M. Authenticity of vitamin D in modified vaginal health index in geriatric subjects. Int. J. Reprod. Contracept. Obstet. Gynecol. 2016, 5, 4119–4122.
- Carranza-Lira, S.; Amador-Perez, C.; Macgregor-Gooch, A.L.; Estrada-Moscoso, I. Changes in maturation index and vaginal dryness in postmenopausal women who use or not calcitriol. Rev. Med. Inst. Mex. Seguro Soc. 2012, 50, 537–540.
- Radnia, N.; Hosseini, S.T.; Vafaei, S.Y.; Pirdehghan, A.; Mehrabadi, N.L. The effect of conjugated estrogens vaginal cream and a combined vaginal cream of vitamins D and E in the treatment of genitourinary syndrome. J. Fam. Med. Prim. Care 2023, 12, 507–516.
- Porterfield, L.; Wur, N.; Delgado, Z.S.; Syed, F.; Song, A.; Weller, S.C. Vaginal Vitamin E for Treatment of Genitourinary Syndrome of Menopause: A Systematic Review of Randomized Controlled Trials. J. Menopausal. Med. 2022, 28, 9–16.
- Hillier, S.L.; Lau, R.J. Vaginal microflora in postmenopausal women who have not received estrogen replacement therapy. Clin. Infect. Dis. 1997, 25 (Suppl. S2), S123–S126.
- Cauci, S.; Driussi, S.; De Santo, D.; Penacchioni, P.; Iannicelli, T.; Lanzafame, P.; De Seta, F.; Quadrifoglio, F.; de Aloysio, D.; Guaschino, S. Prevalence of bacterial vaginosis and vaginal flora changes in peri- and postmenopausal women. J. Clin. Microbiol. 2002, 40, 2147–2152.
- Dennerstein, G.J.; Ellis, D.H. Oestrogen, glycogen and vaginal candidiasis. Aust. N. Z. J. Obstet. Gynaecol. 2001, 41, 326–328.
- Raz, R.; Stamm, W.E. A controlled trial of intravaginal estriol in postmenopausal women with recurrent urinary tract infections. N. Engl. J. Med. 1993, 329, 753–756.
- Gupta, S.; Kumar, N.; Singhal, N.; Kaur, R.; Manektala, U. Vaginal microflora in postmenopausal women on hormone replacement therapy. Indian J. Pathol. Microbiol. 2006, 49, 457–461.
- Fischer, G.; Bradford, J. Vulvovaginal candidiasis in postmenopausal women: The role of hormone replacement therapy. J. Low. Genit. Tract. Dis. 2011, 15, 263–267.
- Tarry, W.; Fisher, M.; Shen, S.; Mawhinney, M. Candida albicans: The estrogen target for vaginal colonization. J. Surg. Res. 2005, 129, 278–282.
- Bodnar, L.M.; Krohn, M.A.; Simhan, H.N. Maternal vitamin D deficiency is associated with bacterial vaginosis in the first trimester of pregnancy. J. Nutr. 2009, 139, 1157–1161.
- Kaur, H.; Bala, R.; Nagpal, M. Role of Vitamin D in urogenital health of geriatric participants. J. Midlife Health 2017, 8, 28–35.
- Ginkel, P.D.; Soper, D.E.; Bump, R.C.; Dalton, H.P. Vaginal flora in postmenopausal women: The effect of estrogen replacement. Infect. Dis. Obstet. Gynecol. 1993, 1, 94–97.
- Krysiak, R.; Gilowska, M.; Okopien, B. Sexual function and depressive symptoms in young women with low vitamin D status: A pilot study. Eur. J. Obstet. Gynecol. Reprod. Biol. 2016, 204, 108–112.
- Inal, Z.O.; Inal, H.A.; Gorkem, U. Sexual function and depressive symptoms in primary infertile women with vitamin D deficiency undergoing IVF treatment. Taiwan J. Obstet. Gynecol. 2020, 59, 91–98.
- Sarebani, Z.; Chegini, V.; Chen, H.; Aali, E.; Mirzadeh, M.; Abbaspour, M.; Griffiths, M.D.; Alimoradi, Z. Effect of vitamin D vaginal suppository on sexual functioning among postmenopausal women: A three-arm randomized controlled clinical trial. Obstet. Gynecol. Sci. 2023, 66, 208–220.
- Vitale, S.G.; Caruso, S.; Rapisarda, A.M.C.; Cianci, S.; Cianci, A. Isoflavones, calcium, vitamin D and inulin improve quality of life, sexual function, body composition and metabolic parameters in menopausal women: Result from a prospective, randomized, placebo-controlled, parallel-group study. Prz. Menopauzalny 2018, 17, 32–38.
- Provvedini, D.M.; Tsoukas, C.D.; Deftos, L.J.; Manolagas, S.C. 1,25-dihydroxyvitamin D3 receptors in human leukocytes. Science 1983, 221, 1181–1183.
- Adorini, L.; Giarratana, N.; Penna, G. Pharmacological induction of tolerogenic dendritic cells and regulatory T cells. Semin. Immunol. 2004, 16, 127–134.
- Wang, T.T.; Nestel, F.P.; Bourdeau, V.; Nagai, Y.; Wang, Q.; Liao, J.; Tavera-Mendoza, L.; Lin, R.; Hanrahan, J.W.; Mader, S.; et al. Cutting edge: 1,25-dihydroxyvitamin D3 is a direct inducer of antimicrobial peptide gene expression. J. Immunol. 2004, 173, 2909–2912.
- Tian, H.; Miao, J.; Zhang, F.; Xiong, F.; Zhu, F.; Li, J.; Wang, X.; Chen, S.; Chen, J.; Huang, N.; et al. Non-histone nuclear protein HMGN2 differently regulates the urothelium barrier function by altering expression of antimicrobial peptides and tight junction protein genes in UPEC J96-infected bladder epithelial cell monolayer. Acta Biochim. Pol. 2018, 65, 93–100.
- Sayeed, I.; Turan, N.; Stein, D.G.; Wali, B. Vitamin D deficiency increases blood-brain barrier dysfunction after ischemic stroke in male rats. Exp. Neurol. 2019, 312, 63–71.
- Won, S.; Sayeed, I.; Peterson, B.L.; Wali, B.; Kahn, J.S.; Stein, D.G. Vitamin D prevents hypoxia/reoxygenation-induced blood-brain barrier disruption via vitamin D receptor-mediated NF-kB signaling pathways. PLoS ONE 2015, 10, e0122821.
- Alperin, M.; Burnett, L.; Lukacz, E.; Brubaker, L. The mysteries of menopause and urogynecologic health: Clinical and scientific gaps. Menopause 2019, 26, 103–111.
- Zhang, Q.; Zhang, Z.; He, X.; Liu, Z.; Shen, L.; Long, C.; Wei, G.; Liu, X.; Guo, C. Vitamin D levels and the risk of overactive bladder: A systematic review and meta-analysis. Nutr. Rev. 2023, nuad049.
- Schulte-Uebbing, C.; Schlett, S.; Craiut, D.; Bumbu, G. Stage I and II Stress Incontinence (SIC): High dosed vitamin D may improve effects of local estriol. Dermato-Endocrinology 2016, 8, e1079359.
- Dallosso, H.M.; McGrother, C.W.; Matthews, R.J.; Donaldson, M.M.; Leicestershire, M.R.C.I.S.G. Nutrient composition of the diet and the development of overactive bladder: A longitudinal study in women. Neurourol. Urodyn. 2004, 23, 204–210.
- Arjmand, M.; Abbasi, H.; Behforouz, A. The effect of vitamin D on urgent urinary incontinence in postmenopausal women. Int. Urogynecol. J. 2023, 34, 1955–1960.




