Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Zhang, M.; Ahmed, A.; Xu, L. Electrospun Nanofibers for Food Packaging. Encyclopedia. Available online: https://encyclopedia.pub/entry/48831 (accessed on 08 February 2026).
Zhang M, Ahmed A, Xu L. Electrospun Nanofibers for Food Packaging. Encyclopedia. Available at: https://encyclopedia.pub/entry/48831. Accessed February 08, 2026.
Zhang, Meng, Adnan Ahmed, Lan Xu. "Electrospun Nanofibers for Food Packaging" Encyclopedia, https://encyclopedia.pub/entry/48831 (accessed February 08, 2026).
Zhang, M., Ahmed, A., & Xu, L. (2023, September 05). Electrospun Nanofibers for Food Packaging. In Encyclopedia. https://encyclopedia.pub/entry/48831
Zhang, Meng, et al. "Electrospun Nanofibers for Food Packaging." Encyclopedia. Web. 05 September, 2023.
Copy Citation
With the strengthening of the public awareness of food safety and environmental protection, functional food packaging materials have received widespread attention. Nanofibers are considered as promising packaging materials due to their unique one-dimensional structure (high aspect ratio, large specific surface area) and functional advantages. Electrospinning, as a commonly used simple and efficient method for preparing nanofibers, can obtain nanofibers with different structures such as aligned, core-shell, and porous structures by modifying the devices and adjusting the process parameters.
electrospun nanofibers
food packaging
active packaging
1. Introduction
Food is prone to decay during storage, and the environmental humidity, storage temperature, and external light conditions will affect the quality of food. Meanwhile, the growth of microorganisms, enzyme-catalyzed food decomposition, and oxidation of food in contact with air will also lead to the growth of mold, loss of moisture, poor food flavour, and so on [1]. Food packaging can create a barrier between the environment and food, thereby preventing food corruption caused by foreign substance contamination and protecting food from chemical, physical, and biological hazards [2]. Food packaging can be generally characterized as edible or non-edible films and coatings. Films can be obtained by solvent casting, extrusion, and electrospinning. Coatings are directly applied to food surfaces by dipping, spraying, painting, or panning. Edible packaging, such as sugar coatings on tablets, gelatin films used on drug capsules [3], edible coatings on cheeses and fruits [4], and bio-based foams and hydrogels [5], can avoid packaging waste and reduce environmental damage. Plastic bags, plastic film, plastic wraps, and other types of plastic products are non-edible packaging. Further processing of plastic sheets using thermomechanical methods, such as thermoforming, extrusion blow molding, and blow molding, can form more complex packaging.
Currently, with the increasing demand for food safety and environmental protection, the use of natural biological and biodegradable packaging materials has increased. Moreover, a series of functional food packaging is being developed to retard food deterioration, improve food sensory properties, and ensure food safety. The ideal functional food packaging has enhanced antibacterial, antioxidant, water resistance, and other properties by adding some active substances or using physical and chemical modification (such as heat treatment, plasma treatment, and radiation treatment) [6], thus maintaining the quality and sensory properties of the food, improving food safety, and effectively prolonging the shelf life of food. In addition, food packaging can also act as an intelligent monitoring system to monitor the quality changes in food during storage in real time, thereby reminding consumers of the real-time quality of food and bringing about more healthy consumption for people [7].
In order to meet the changing needs of food packing, it is important to adopt nanotechnology and find new food packaging technologies [8][9]. Compared with bulk materials, nanomaterials have better physical, chemical, optical, mechanical, and catalytic properties, which makes the application of nanotechnology in food packaging very promising [10]. A recent development is the integration of active agents into packaging materials to improve food safety and quality. In particular, the combination of antioxidants and packaging materials will resist the deterioration of physical properties such as the flavor and color of food [11][12]. It is well known that polymers are the preferred materials for active food packaging because of their functional properties as well as their ability to carry active agents and their controlled release [13]. However, most active agents evaporate easily due to their high volatility [14], making it impossible to directly inject them through typical processing methods of polymers [15]. To overcome this problem, electrospinning (ES) technology can be applied to food packaging [16].
ES technology has been widely used in the manufacture of nanofibers due to its simple operation, low cost, wide application scope, and high production efficiency [17][18]. This technology can not only control the orientation, shape, and structure of nanofibers by modifying devices and adjusting process parameters, but also load various functional substances into nanofibers [19]. Furthermore, the ES process does not involve high temperature conditions, which can ensure the stability of active substances. Moreover, the solvent in the spinning solution will evaporate quickly during the ES process, greatly reducing the safety problems caused by toxic solvents in electrospun nanofibers [20]. Meanwhile, electrospun raw materials for food packaging are widely available [21]. These advantages of ES technology provide a good basis for the application of electrospun nanofibers in food packaging, and have attracted a great deal of attention [22]. Electrospun nanofibers have a high aspect ratio, large specific surface area, good physical and chemical properties, and will not be deformed under high temperature conditions. Electrospun nanofiber membranes (NFMs) used in food packaging are generally composed of polymers with good flexibility, fine feel, good air permeability, easy degradation, and low wear. NFMs have a large specific surface area and high porosity, which are suitable for the release of active substances [23]. Moreover, electrospun NFMs have good barrier properties, which can improve the closure of food packaging, reduce its permeability, and effectively inhibit the growth of microorganisms. They can also independently choose to filter oxygen and carbon dioxide, forming a natural air-conditioned packaging for fruits and vegetables to prolong the freshness of food. Therefore, electrospun NFMs have shown great potential for application in the field of food packaging.
2. Electrospun Raw Materials for Food Packaging
The raw materials of electrospun NFMs commonly used for food packaging are polymers, mainly including synthetic polymers and natural polymers [23][24][25][26]. Synthetic polymers are the main base materials for food packaging. However, some synthetic polymers are limited in resources and do not easily degrade [27]. For environmental protection, many reliable, safe, and biodegradable raw materials in the natural environment can be used for food packaging production through ES technology [28][29][30], and various fillers can be loaded into the base materials to enhance the mechanical, antimicrobial, and antioxidant properties of food packaging [31], as shown in Figure 1.

Figure 1. Electrospun raw materials used for food packaging.
2.1. Natural Polymers
Natural polymers, such as protein and sugar, are generally used as base materials in food packaging [28][29][30]. However, their low mechanical strength and poor spinnability limit their application in food packaging [32]. Food packaging materials generally require good hydrophobicity, with a WCA value greater than 90° [33], while most natural polymers have poor hydrophobicity. Therefore, their application in food packaging can be expanded by adding various fillers or combining them with other spinnable polymers.
2.1.1. Protein
Most proteins have poor spinnability. Poor spinnability of solutions means that spinning solutions cannot easily spin nanoscale fibers with good morphology, which may be due to the low conductivity or high viscosity of the spinning solutions. Moreover, most proteins have poor mechanical properties and hydrophobicity, which means that they do not perform well when used alone for packaging. Zein is a water-insoluble alcohol soluble protein from maize, which has good barrier properties for transport of gases, water vapor, or solutes, as well as good biocompatibility and biodegradability. However, when the zein concentration in the spinning solution is less than or equal to 10%, it is impossible to electrospin nanofibers. In recent years, zein films have already been applied as edible coatings on nuts to delay rancidity, and on tomatoes to delay color changes and weight loss and to maintain firmness during storage [34]. Zein-based NFMs prepared by ES have been widely used in food package. Roberta et al. [35] electrospun vanillin/zein NFMs, where the vanilla powder encapsulation rate reached 74%, as an alternative to synthetic polymers used in commercial applications of food packaging.
Gelatin is a safe and harmless protein extracted from collagen, which has excellent barrier, mechanical, biodegradable, and biocompatible properties. It is widely used in the food, medicine, and cosmetics industries. However, gelatin has strong hydrophilicity, with a WAC of about 45°, and cannot protect food from water vapor for a long time [36]. Accordingly, other hydrophobic polymers or active substances are commonly used to mix with it for spinning so that the NFMs prepared can further extend the shelf life of food [37]. Tang et al. [38] used ES technology to prepare gelatin NFMs containing peppermint essential oil or chamomile essential oil. It was found that the addition of essential oil increased the hydrophobicity of the NFMs, improved the antibacterial activity of the NFMs, and compensated for the limitations of gelatin’s properties. Gelatin nanofibers containing 9% v/v chamomile essential oil turned out to be hydrophobic with a WAC of 101.3 ± 4.3°.
Soybean protein (SPI) has good biocompatibility, low cost, good and smooth film-forming properties, transparency and flexibility, but it is a globular protein with low solubility in organic solvents and poor mechanical properties, which complicates fiber formation through electrospinning. Daehwan et al. [39] electrospun SPI/PVA NFMs, and it was found that the mechanical properties of pure PVA NFMs were the best, and the mechanical properties of electrospun nanofibers decreased with the increase in SPI content. SPI is proposed as an excellent carrier material. Bruni et al. [40] formulated a hybrid emulsion of SPI and PVA, added the antioxidant β-carotene to the emulsion, and then prepared a packaging coating using ES. The experiments proved that an SPI emulsion could effectively encapsulate antioxidants, and could persistently release bioactive compounds when packaging food with it.
SF is a natural polymer fibrin extracted from silk, which has good physical and chemical properties as well as biocompatibility. Especially, SF has the functions of oxidation resistance and acting as a water vapor barrier. When it is used in packaging materials, it can effectively improve the shelf life of food [41]. However, due to its poor spinnability and strong hydrophilicity, it is often mixed with other materials to improve the NFM’s performance. Lin et al. [42] prepared SF nanofibers containing thyme essential oil. When PEO was added to the SF solution at a ratio of more than 20%, stable nanofibers could be prepared by ES. After plasma treatment, the NFMs could effectively release thyme essential oil to kill Salmonella typhi, which was an effective antibacterial packaging to extend the shelf life of food.
2.1.2. Sugar
Starch is a kind of natural polysaccharide, which has the characteristics of low cost, easy processing, being renewable, etc. Starch-derived edible food films have great potential as biodegradable food packaging materials because they reduce the overuse of traditional petroleum-based plastic [43]. However, there are a large number of hydrophilic hydroxyl groups in the starch molecule; the performance of starch NFMs for food packaging application can be improved by using self-assembly, grafting, and cross-linking. In order to overcome the problem of the ultra-low hydrophobicity of electrospun starch NFMs, Cai et al. [43] prepared a stearic acid (STA) coating through solution immersion, and changed the hydrophobicity of the NFMs by controlling the assembly of this coating on the surface of starch NFMs. The WAC of the NFMs increased from approximately 0° to 134.7° before and after coating the starch NFMs with STA. Zhang et al. [44] prepared starch/tea polyphenol NFMs and experimentally demonstrated that the addition of tea polyphenols endowed the NFMs with antioxidant properties, and with the increase in the cross-linking time from 0.5 to 2.5 h between tea polyphenols and starch, the WAC of the NFMs was significantly improved from 17.5° to 87.2°, and there was no negative impact on the antioxidant properties of the NFMs.
Cellulose is the most widely distributed and abundant polysaccharide in nature, being the main component of plant cell walls [45]. There are many kinds of cellulose, including carboxymethyl cellulose (CMC), hydroxypropyl cellulose (HPMC), ethyl cellulose, and cellulose acetate (CA). CMC has good biocompatibility and biodegradability, but it is easily soluble in water and has poor spinnability. Hashmi et al. [46] prepared PVA/polyvinylpyrrolidone (PVP)/CMC nanofibers by ES, and found that these nanofibers had a homogeneous morphology and exhibited better mechanical properties (tensile strength over 10 MPa) and hydrophobicity due to the cross-linking between PVA, PVP, and CMC. The WACs of PVA and PVA/PVP were 87.7° and 80.4°, respectively, and the WAC of PVA/PVP/CMC NFM increased to about 100° with the addition of CMC. Therefore, these nanofibers were used in food packaging to keep food fresh. HPMC has good film-forming ability, biocompatibility, and degradation ability. Aydogdu et al. [27] directly deposited HPMC nanofibers on polylactic acid (PLA) NFMs to generate double-layer NFM-based packaging, which was a preparation method to reduce the transparency of NFM-based packaging without increasing its permeability. Ethyl cellulose is a kind of artificially modified cellulose with a low manufacturing cost, excellent mechanical properties, and strong water resistance. It can be blended with other hydrophilic polymers to improve the mechanical properties and water resistance of NFMs. Niu et al. [47] prepared zein/ethyl cellulose/cinnamon essential oil nanofibers. The WAC of zein NFM was 54° at 1 s and 16° at 60 s. The WAC of ethyl cellulose NFM was 131°, which barely changed with time. After adding ethyl cellulose to zein NFMs, hydrogen bonds were formed between hydroxyl groups of ethyl cellulose and amino groups of zein, reducing the number of free hydrophilic groups and improving the water resistance of the NFMs. CA is formed by acetylation of cellulose, which is a low-cost cellulose derivative with good mechanical properties, biodegradability, and biocompatibility. Tarus et al. [48] prepared poly (vinyl chloride) (PVC)/CA/Ag NFMs, which had good tensile properties and an excellent antibacterial effect.
Chitosan (CS) is a safe, cheap, non-toxic natural polymer with excellent antibacterial effect, biocompatibility, and biodegradability. However, it is a cationic polymer with high density charges, which leads to high repulsion between its ionic groups, low spinnability, and poor mechanical properties produced when ES CS solutions [49]. In order to improve the spinnability of CS and the mechanical properties of NFMs, CS can be blended with other natural or synthetic polymers to prepare NFMs. Deng et al. [50] prepared CS/PEO/laurate arginine NFMs using ES, which exhibited an ultra-fine 3D porous structure and had excellent antibacterial activity. Duraiarasan et al. [51] prepared CS/PEO NFMs to encapsulate pomegranate peel extract. With the increase in CS content, the viscosity of the solution gradually increased and the fiber presented a bead shape. It was found that the composite fiber showed excellent mechanical properties and thermal stability. The tensile strength of pure PEO NFM was 2.4 ± 0.56 MPa. With the increase in the CS proportion, the tensile strength of CS/PEO NFM also increased, reaching a maximum of 10.8 ± 2.2 MPa. Furthermore, the NFM had an antibacterial effect on Escherichia coli (E. coli). The bacterial growth on beef was slower at 4 °C than at 25 °C. During psychrophilic storage, the total bacterial count reached 6.60 log cfu/g on the control, whereas for CS/PEO NFMs it reached only 2.96 log cfu/g after 10 days of storage, which showed it had great application potential in food packaging.
Cyclodextrin is a cyclic oligosaccharide which is often used to encapsulate various drugs in food packaging research. Wen et al. [52] encapsulated cinnamon essential oil (CEO) into a β-cyclodextrin (β-CD) inclusion complex to prepare biodegradable antibacterial materials and improve the antibacterial activity of nanofibers. Sharif et al. [53] prepared β-CD NFMs containing cuminaldehyde. The experiment proved that acrolein was encapsulated in β-CD at a very high encapsulation rate, and the encapsulated nanofibers had a uniform morphology (Figure 2). After high temperature treatment, the weight loss rate of cuminaldehyde/β-CD NFMs was smaller than that of cuminaldehyde and β-CD, indicating an improvement in the thermal stability of the NFMs. Culturing E. coli and Staphylococcus aureus (S. aureus) on cuminaldehyde/β-CD NFMs, the results showed that the surviving populations of both microorganisms were obviously reduced for cuminaldehyde/β-CD fibers, indicating the inhibition of bacterial growth.
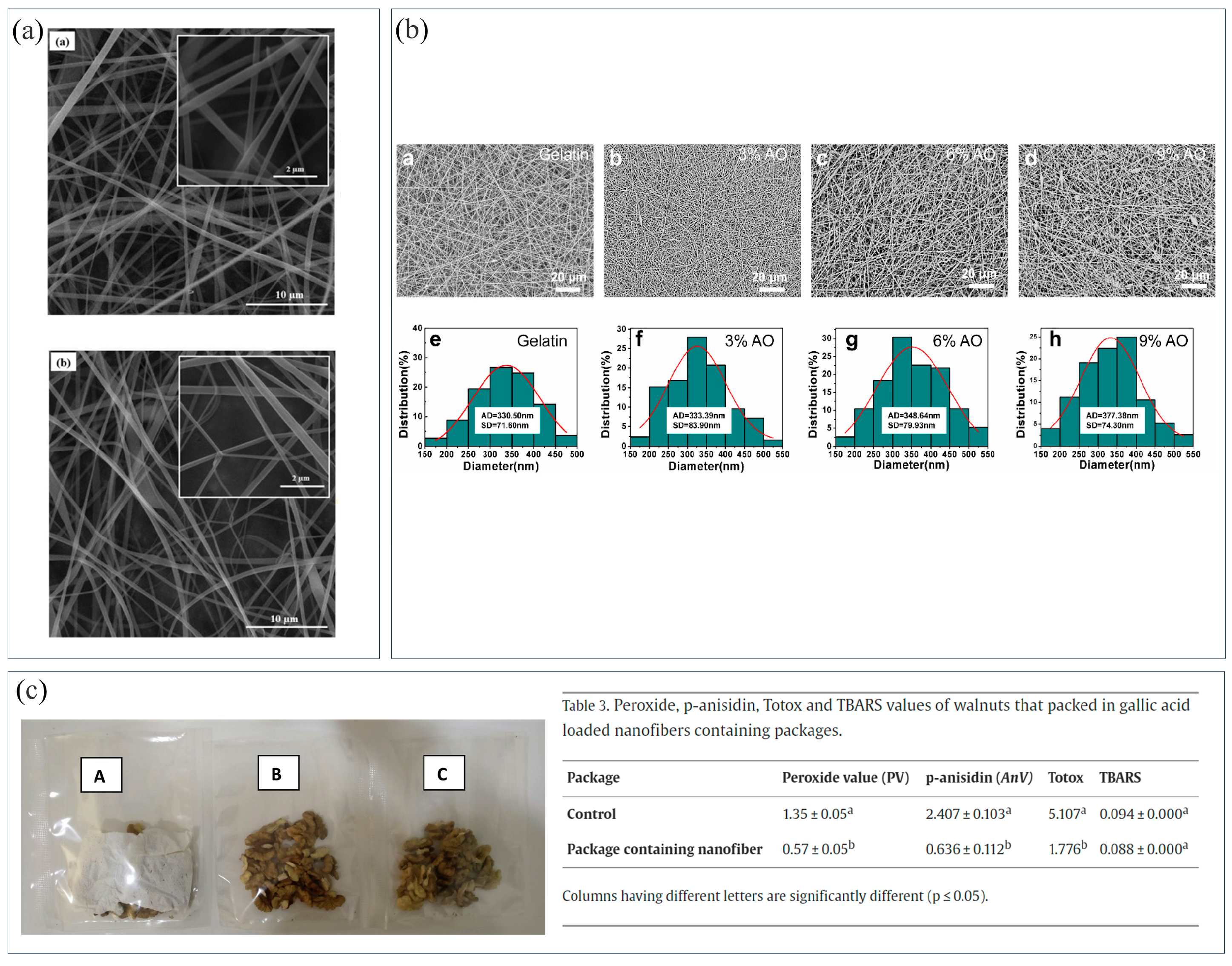
Figure 2. (a) SEM images and average fiber diameter of fibers: (a) hydroxypropyl-β-cyclodextrin (β-CD) fibers; and (b) cuminaldehyde/β-CD inclusion complex (CUM/β-CD) fibers. (b) The addition of angelica essential oil (AO) increased the diameter of gelatin nanofibers; (c) NFMs loaded with gallic acid for packaging walnuts: (left figure; A) 10% galllic acid loaded nanofiber, (B,C) control.
2.2. Synthetic Polymers
Many bio-based polymers have poor spinnability, and the morphology and properties of electrospun nanofibers produced using them are poor, limiting their application in food packaging. Synthetic polymers have the advantages of a low cost, easy production, being lightweight, and good flexibility, and their moderate addition can improve the mechanical properties of packaging materials, which are widely used in the production of disposable packaging.
2.2.1. Non-Degradable Polymers
Conventional packaging materials, including petroleum-based plastics, paper, metal, and glass, are used in food packaging. Most petroleum-based plastics belong to non-degradable polymers and have the characteristics of rigidity, flexibility, barrier properties, low cost, and ease in processing, making them one of the most extensively used packaging materials. Common petroleum-based plastics include polyethylene, polypropylene, PVC, etc. [54]. PVC is generally made using the thermoplastic method with the advantages of easy processing, being non-flammable, non-deformable, and low cost, and is often applied as cling films in the agricultural and processed food markets. Tarus et al. [48] used ES technology to prepare PVC/CA/Ag NFMs with enhanced tensile properties and antibacterial effects. The tensile characteristics of flexible packaging materials can be described as inferior (<1 MPa), marginal (1–10 MPa), good (10–100 MPa), or superior (100 MPa). Accordingly, the tensile characteristics of CA electrospun NFMs with an average strength of 8.6 MPa could be described as marginal, while PVC NFMs had an average strength of 13 MPa, exhibiting good mechanical properties as a packaging material. PVC/CA NFMs loaded with Ag nanoparticles (NPs) displayed inhibited growth of yeast and mold after the incubation period. After growing bacteria on the NFMs, the number of fungi on PVC/CA NFM was about 0.4 cfu/cm2, while that on Ag NPs loaded NFM was only about 0.1 cfu/cm2.
2.2.2. Degradable Polymers
Although adding non-degradable synthetic polymers can improve the spinnability of spinning solutions, they are terrible for environmental protection. Therefore, degradable synthetic polymers are more widely used in electrospun nanofiber-based food packaging, commonly including polycaprolactone (PCL), PLA, and PVA, etc.
PCL is a fossil derived polymer with excellent biocompatibility, biodegradability, and non-toxicity, which can be combined with other types of polymers to enhance its application performance. Beikzadeh et al. [55] electrospun PCL/ethyl cellulose/gelatin/zataria multiflora essential oil (ZEO)/ZnO NP NFMs, which had appropriate biocompatibility on account of a cell viability obtained above 80% at designated times. The NFMs had high cell survival, and can be used in food packaging. Liu et al. [56] used hydrophobic PCL to encapsulate hydrophilic anthocyanin, preparing hydrophobic food packaging for monitoring the freshness of food.
PLA is one of the most studied degradable polymers used to develop antimicrobial materials in recent years; it can extend the shelf life of food. In food packaging, various active drugs are often encapsulated into PLA fibers to obtain antimicrobial packaging materials. Vidal et al. [57] electrospun core-shell NFMs based on lauryl arginine ethyl ester (LAE), cellulose nanocrystals (CNCs), and PLA. Here, PLA effectively coated antibacterial drugs, slowed down the rate of drug release, and enhanced the antibacterial properties of packaging materials.
PVA has excellent mechanical properties, good biodegradability, and biocompatibility, and can effectively block gas. Narayanan et al. [58] prepared PVA NFMs coated with γ-cyclodextrin and ferulic acid (FA) which achieved effective release of FA while enhancing the thermal stability. PVA also has good hydrophilicity and can be highly soluble in water. It is often combined with other additives in food packaging and undergoes simple graft modification to improve the water resistance of NFMs. Yu et al. [59] electrospun PVA/clove oil (CO) NFMs and, through heat treatment, the hydroxyl group of PVA interacted with the carboxyl group of CA, thus effectively improving the thermal stability, mechanical properties, and water resistance of the NFMs.
2.3. Various Fillers
A variety of fillers have been added to NFMs for functional food packaging, improving qualities such as antibacterial, antioxidant, and vinyl degradation.
2.3.1. Metals and Their Oxides
Metals or metal oxide NPs have good mechanical, thermal, antibacterial, barrier, and optical properties and have been used in food packaging in recent years. The interaction of electrons generated by these NPs with water or atmospheric oxygen produces reactive oxygen species (ROS) (hydroxyl radicals, superoxide anions, hydrogen peroxide) which can interact with bacteria to enhance the antibacterial activity of NFMs. Valerini et al. [60] sputtered and deposited Ag NPs on PCL NFMs, coating PCL with Ag NPs. This coating not only gave the fibers the antibacterial properties of Ag NPs, but also maintained the hydrophobicity of PCL. Kowsalya et al. [61] obtained stable Ag NPs using green synthesis from grape peel and incorporated them into PVA to prepare green organic nanofibers with good antibacterial properties. Zhang et al. [62] prepared NFMs with different ratios of TiO2 NPs using ES. NFMs with 5% TiO2 were measured to have high photocatalytic activity for the degradation of ethylene. Mayorga et al. [63] used compression molded poly (3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV) NFMs as a bottom layer, and coated them with PHBV-based NFMs containing CuO NPs to prepare bilayer structured NFMs, thus increasing their antibacterial activity. Rapa et al. [64] prepared NFMs containing PHBV and 1 wt% Fe-doped ZnO NPs, which had shown significant antibacterial effects against pseudomonas aeruginosa.
However, the increased amount of ROS produced by metal oxide NPs can induce cell damage or oxidative stress, which is contrary to their positive use as antimicrobials and antioxidants. For example, kidney diseases and hepatic injury may occur as a consequence of a single oral dose of ZnO NPs. The gastrointestinal tract offers a chance for ZnO NP ingestion, which may readily pass through biological barriers and enter the circulatory system [65]. The use and discharge of titanium oxide can have an impact on people and nature, raising the risk of harm to the environment and human health [66].
2.3.2. Carbon Materials
Carbon nanotube (CNT) is a good material for enzyme immobilization. Liu et al. [67] electrospun regenerated cellulose/carboxylated CNTs/graphene oxide (GO) NFMs to immobilize lysozyme. Carboxyl groups on CNTs (or GO) were activated to react with the nucleophilic amino functionality at the side chains of amino acids in lysozyme, realizing the immobilization of lysozyme and providing antibacterial properties for the composite NFMs. However, the prolonged exposure to carbon NPs outside NFMs may bind to proteins or other biomolecules in the body, enter the skin and bloodstream, and be toxic to the skin and lungs [68]. Graphene has outstanding mechanical flexibility, excellent electrical and thermal conductivity, as well as optical transparency [69][70]. It has multiple conservation functional groups, which can interact with polar solutions or polymers to promote the dispersion of graphene in the polymer matrix [71]. Sergio et al. [72] embedded graphene into poly (ethylene-co-vinyl alcohol) and found that graphene could be effectively dispersed in the polymer. The composite NFM had high conductivity and could be used as a smart label in food packaging.
2.3.3. Phase Change Materials (PCMs)
PCMs are substances that undergo phase change at a specific temperature. When the external temperature changes, they can absorb and release heat energy, thus maintaining a constant temperature of food during food transport and storage [73]. However, due to their poor thermal stability and low thermal conductivity, PCMs cannot be directly used in packaging [47]. Encapsulating PCMs in a polymer through ES technology can effectively protect them from external influences [74]. Wilson et al. [75] used polystyrene (PS) as an encapsulation base for the commercial PCM RT15. RT15 could be effectively encapsulated in the PS matrix with an encapsulation rate of 78%, thereby improving its heat storage performance and maintaining the quality of packaged refrigerated food. Rocio et al. [76] encapsulated dodecane in PCL/PLA with a submicron droplet size using the ES technique, thus improving the thermal storage capacity of dodecane and maintaining food quality when packaging temperature-sensitive products with the electrospun NFMs.
2.3.4. Bioactive Compounds
Aromatic herbs are effective antimicrobial agents and antioxidants, containing beneficial phytochemicals such as terpenoids, phenols and derivatives, flavonoids, coumarins, quinine, saponins, tannins, and alkaloids, etc. [77]. These compounds are derived from plants and are generally less toxic to mammals and humans [78][79]. Accordingly, plenty of studies have focused on the extraction of bioactive compounds from plants to be applied in food packaging [80].
Among natural bioactive drugs, essential oils have the advantages of good biodegradability, antibacterial and antioxidant properties, and few side effects, and so are widely used in food packaging. The hydrophobic characteristics of essential oils enable them to partition into the cell membranes and mitochondria of the lipid layer, altering cell permeability and disrupting the cell wall structures, thus causing crucial molecules and ions to leak from the bacterial cells, thus leading to bacterial death [81]. However, the volatility, strong smell, and other problems of essential oils also limit their application in food packaging. Their long-term release can be effectively controlled by immobilizing them on a polymer matrix through ES [79]. Liu et al. [80] used the emulsion ES method to encapsulate cinnamon essential oil (CEO) into CS nanofibers. With time, CEO diffused to the fiber surface, and its release gradually increased. CS and CEO had a synergistic antibacterial effect, maintaining antibacterial activity as CEO slowly released. Fonseca et al. [82] encapsulated thyme essential oil in starch nanofibers, with an encapsulation rate of about 99%, and it effectively expressed antioxidant activity. Zhou et al. [37] prepared gelatin nanofibers containing angelica essential oil (AO). The addition of AO increased the diameter of gelatin nanofibers (Figure 2b), improved their hydrophobicity, and enabled the nanofibers to have good antioxidant activity and inhibit Gram-negative and -positive bacteria.
Phenolic compounds are widely found in fruits, vegetables, cereals, and tea, and have a variety of biological activities, such as anticancer, antitumor, antioxidation, etc. Their common feature is that all molecules contain phenol groups, thus possessing antioxidation properties [83]. Dumitriu et al. [84] prepared PCL/vitamin E (α-tocopherol) NFMs using ES, which had good antioxidant properties. Phenolic compounds not only have antioxidant properties, but also have antibacterial and antifungal properties. Phenolic compounds in microalgae can effectively replace synthetic compounds. Kuntzler et al. [85] prepared CS/PEO NFMs coated with microalgae phenols, which had a good inhibitory effect on S. aureus. Gallic acid has good bioactivity, antibacterial, and antioxidant properties, however, it is sensitive to temperature, pH, oxygen, and light, and tastes bitter, which limits its use in food packaging. Aydogdu et al. [86] encapsulated gallic acid in HPMC/PEO NFMs using ES. The bioactivity of gallic acid was effectively retained in the NFMs, which could extend the shelf life of food. The NFM loaded with 10% gallic acid were chosen to package walnuts due to its higher loading efficiency and antioxidant activity (Figure 2c). Curcumin is a natural yellow-orange polyphenol compound with low molecular weight, which has good anti-inflammatory and antiviral effects. In addition, it is also an effective antibacterial agent and antioxidant. Wang et al. [87] prepared curcumin/zein NFMs, which exhibited excellent antioxidant and antibacterial activities against E. coli and S. aureus. Curcumin is also a natural food colorant which can be used to detect food deterioration. Luo et al. [88] electrospun curcumin/zein NFMs to effectively monitor the freshness of food. Tea polyphenol is a kind of polyphenol compound extracted from tea which is a non-toxic antioxidant and can effectively delay the deterioration of food. Zhang et al. [44] prepared starch/tea polyphenol NFMs using ES. Cross-linking between tea polyphenol and starch improved the hydrophobicity of NFMs, while the addition of tea polyphenol gave the NFMs antioxidant activity. Jaboticaba is a natural fruit rich in anthocyanins which has a high antioxidant and antibacterial effect. Avila et al. [89] extracted bioactive compounds from Jaboticaba peels using an immersion method. The extracts contained high amounts of phenolic substances and anthocyanins, and the prepared NFMs containing the extracts had excellent antibacterial and antioxidant properties. Aloe vera is an ancient medicinal plant which is widely used in the medical and cosmetic industries. Solaberrieta et al. [90] obtained NFMs with antioxidant activity by adding aloe vera extract to PEO. Bitter orange is a citrus plant containing phenols, flavonoids, and vitamins, which has excellent antioxidant properties. Rashidi et al. [91] prepared ethyl cellulose/SPI/bitter-orange-peel-extract NFMs. The NFMs with 20% concentration of the extract had high antioxidant properties and could effectively inhibit the growth of pathogenic bacteria.
References
- Mohammadi, Z.B.; Zhang, F.; Kharazmi, M.S.; Jafari, S.M. Nano-Biocatalysts for Food Applications; Immobilized Enzymes within Different Nanostructures. Crit. Rev. Food Sci. 2022, 19, 1–19.
- Sameen, D.E.; Ahmed, S.; Lu, R.; Li, R.; Dai, J.; Qin, W.; Zhang, Q.; Li, S.; Liu, Y. Electrospun Nanofibers Food Packaging: Trends and Applications in Food Systems. Crit. Rev. Food Sci. 2022, 62, 6238–6251.
- Shlush, E.; Davidovich-Pinhas, M. Bioplastics for Food Packaging. Trends Food Sci. Technol. 2022, 125, 66–80.
- Kumar, L.; Ramakanth, D.; Akhila, K.; Gaikwad, K.K. Edible Films and Coatings for Food Packaging Applications: A Review. Environ. Chem. Lett. 2022, 20, 875–900.
- Petersen, K.; Nielsen, P.V.; Bertelsen, G.; Lawther, M.; Olsen, M.B.; Nilsson, N.H.; Mortensen, G. Potential of Biobased Materials for Food Packaging. Trends Food Sci. Technol. 1999, 10, 52–68.
- Sharma, R.; Jafari, S.M.; Sharma, S. Antimicrobial Bio-Nanocomposites and Their Potential Applications in Food Packaging. Food Control 2020, 112, 107086.
- Ahari, H.; Soufiani, S.P. Smart and Active Food Packaging: Insights in Novel Food Packaging. Front. Microbiol. 2021, 12, 657233.
- Beachley, V.; Katsanevakis, E.; Zhang, N.; Wen, X. A Novel Method to Precisely Assemble Loose Nanofiber Structures for Regenerative Medicine Applications. Adv. Healthc. Mater. 2013, 2, 343–351.
- Xie, J.; Li, X.; Lipner, J.; Manning, C.N.; Schwartz, A.G.; Thomopoulos, S.; Xia, Y. “Aligned-to-Random” Nanofiber Scaffolds for Mimicking the Structure of the Tendon-to-Bone Insertion Site. Nanoscale 2010, 2, 923–926.
- Biswas, R.; Alam, M.; Sarkar, A.; Haque, M.I.; Hasan, M.M.; Hoque, M. Application of Nanotechnology in Food: Processing, Preservation, Packaging and Safety Assessment. Heliyon 2022, 8, e11795.
- Han, D.; Steckl, A.J. Superhydrophobic and Oleophobic Fibers by Coaxial Electrospinning. Langmuir 2009, 25, 9454–9462.
- Sun, Z.C.; Zussman, E.; Yarin, A.L.; Wendorff, J.H.; Greiner, A. Compound Core-Shell Polymer Nanofibers by Co-Electrospinning. Adv. Mater. 2003, 15, 1929–1932.
- Luo, G.; Teh, K.S.; Liu, Y.; Zang, X.; Wen, Z.; Lin, L. Direct-Write, Self-Aligned Electrospinning on Paper for Controllable Fabrication of Three-Dimensional Structures. ACS Appl. Mater. Inter. 2015, 7, 27765–27770.
- Zhao, Y.; Cao, X.; Jiang, L. Bio-Mimic Multichannel Microtubes by a Facile Method. J. Am. Chem. Soc. 2007, 129, 764–765.
- Fuh, Y.K.; Wang, B.S. Near Field Sequentially Electrospun Three-Dimensional Piezoelectric Fibers Arrays for Self-Powered Sensors of Human Gesture Recognition. Nano Energy 2016, 30, 677–683.
- Terada, D.; Kobayashi, H.; Zhang, K.; Tiwari, A.; Yoshikawa, C.; Hanagata, N. Transient Charge-Masking Effect of Applied Voltage on Electrospinning of Pure Chitosan Nanofibers from Aqueous Solutions. Sci. Technol. Adv. Mat. 2012, 13, 9.
- Ghaderpour, A.; Hoseinkhani, Z.; Yarani, R.; Mohammadiani, S.; Amiri, F.; Mansouri, K. Altering the Characterization of Nanofibers by Changing the Electrospinning Parameters and Their Application in Tissue Engineering, Drug Delivery, and Gene Delivery Systems. Polym. Advan Technol. 2021, 32, 1924–1950.
- Xu, H.; Fan, P.; Xu, L. Cuo/Zno/Cqds@Pan Nanocomposites with Ternary Heterostructures for Enhancing Photocatalytic Performance. Catalysts 2023, 13, 110.
- Chinnappan, B.A.; Krishnaswamy, M.; Xu, H.; Hoque, M.E. Electrospinning of Biomedical Nanofibers/Nanomembranes: Effects of Process Parameters. Polymers 2022, 14, 3719.
- Zhao, L.; Liu, P.; He, J. Sudden Solvent Evaporation in Bubble Electrospinning for Fabrication of Unsmooth Nanofibers. Therm. Sci. 2017, 21, 1827–1832.
- Zhao, L.; Duan, G.; Zhang, G.; Yang, H.; He, S.; Jiang, S. Electrospun Functional Materials Toward Food Packaging Applications: A Review. Nanomaterials 2020, 10, 150.
- Zhang, C.; Li, Y.; Wang, P.; Zhang, H. Electrospinning of Nanofibers: Potentials and Perspectives for Active Food Packaging. Compr. Rev. Food Sci. Food Saf. 2020, 19, 479–502.
- Alp-Erbay, E.; Yesilsu, A.F.; Ture, M. Fish Gelatin Antimicrobial Electrospun Nanofibers for Active Food-Packaging Applications. J. Nano Res. 2019, 56, 80–97.
- Kara, H.H.; Xiao, F.; Sarker, M.; Jin, T.Z.; Sousa, A.M.M.; Liu, C.; Tomasula, P.M.; Liu, L. Antibacterial Poly (Lactic Acid) (Pla) Films Grafted with Electrospun Pla/Allyl Isothiocyanate Fibers for Food Packaging. J. Appl. Polym. Sci. 2016, 133, 8.
- Lan, W.; Liang, X.; Lan, W.; Ahmed, S.; Liu, Y.; Qin, W. Electrospun Polyvinyl Alcohol/D-Limonene Fibers Prepared by Ultrasonic Processing for Antibacterial Active Packaging Material. Molecules 2019, 24, 767.
- Moreno, M.A.; Orqueda, M.E.; Gomez-Mascaraque, L.G.; Isla, M.I.; Lopez-Rubio, A. Crosslinked Electrospun Zein-Based Food Packaging Coatings Containing Bioactive Chilto Fruit Extracts. Food Hydrocolloid 2019, 95, 496–505.
- Aydogdu, A.; Yildiz, E.; Ayhan, Z.; Aydogdu, Y.; Sumnu, G.; Sahin, S. Nanostructured Poly (Lactic Acid)/Soy Protein/Hpmc Films by Electrospinning for Potential Applications in Food Industry. Eur. Polym. J. 2019, 112, 477–486.
- Antunes, M.D.; Dannenberg, G.D.S.; Fiorentini, A.M.; Pinto, V.Z.; Lim, L.; Zavareze, E.D.R.; Dias, A.R.G. Antimicrobial Electrospun Ultrafine Fibers from Zein Containing Eucalyptus Essential Oil/Cyclodextrin Inclusion Complex. Int. J. Biol. Macromol. 2017, 104, 874–882.
- Azeredo, H.M.C.; Barud, H.; Farinas, C.S.; Vasconcellos, V.M.; Claro, A.M. Bacterial Cellulose as a Raw Material for Food and Food Packaging Applications. Front. Sustain. Food Syst. 2019, 3, 7.
- Diez-Pascual, A.M.; Diez-Vicente, A.L. Antimicrobial and Sustainable Food Packaging Based on Poly (Butylene Adipate-Co-Terephthalate) and Electrospun Chitosan Nanofibers. RSC Adv. 2015, 5, 93095–93107.
- Yildirim, S.; Rocker, B.; Pettersen, M.K.; Nilsen-Nygaard, J.; Ayhan, Z.; Rutkaite, R.; Radusin, T.; Suminska, P.; Marcos, B.; Coma, V. Active Packaging Applications for Food. Compr. Rev. Food Sci. Food Saf. 2018, 17, 165–199.
- Espindola-Gonzalez, A.; Martinez-Hernandez, A.L.; Fernandez-Escobar, F.; Castano, V.M.; Brostow, W.; Datashvili, T.; Velasco-Santos, C. Natural-Synthetic Hybrid Polymers Developed via Electrospinning: The Effect of Pet in Chitosan/Starch System. Int. J. Mol. Sci. 2011, 12, 1908–1920.
- Cui, C.; Gao, L.; Dai, L.; Ji, N.; Qin, Y.; Shi, R.; Qiao, Y.; Xiong, L.; Sun, Q. Hydrophobic Biopolymer-Based Films: Strategies, Properties, and Food Applications. Food Eng. Rev. 2023, 15, 360–379.
- Dhall, R.K. Advances in Edible Coatings for Fresh Fruits and Vegetables: A Review. Crit. Rev. Food Sci. 2013, 53, 435–450.
- Campardelli, R.; Pettinato, M.; Drago, E.; Perego, P. Production of Vanillin-Loaded Zein Sub-Micron Electrospun Fibers for Food Packaging Applications. Chem. Eng. Technol. 2021, 44, 1390–1396.
- Figueroa-Lopez, K.J.; Castro-Mayorga, J.L.; Andrade-Mahecha, M.M.; Cabedo, L.; Lagaron, J.M. Antibacterial and Barrier Properties of Gelatin Coated by Electrospun Polycaprolactone Ultrathin Fibers Containing Black Pepper Oleoresin of Interest in Active Food Biopackaging Applications. Nanomaterials 2018, 8, 199.
- Zhou, Y.; Miao, X.; Lan, X.; Luo, J.; Luo, T.; Zhong, Z.; Gao, X.; Mafang, Z.; Ji, J.; Wang, H.; et al. Angelica Essential Oil Loaded Electrospun Gelatin Nanofibers for Active Food Packaging Application. Polymers 2020, 12, 299.
- Tang, Y.; Zhou, Y.; Lan, X.; Huang, D.; Luo, T.; Ji, J.; Mafang, Z.; Miao, X.; Wang, H.; Wang, W. Electrospun Gelatin Nanofibers Encapsulated with Peppermint and Chamomile Essential Oils as Potential Edible Packaging. J. Agr. Food Chem. 2019, 67, 2227–2234.
- Cho, D.; Netravali, A.N.; Joo, Y.L. Mechanical Properties and Biodegradability of Electrospun Soy Protein Isolate/Pva Hybrid Nanofibers. Polym. Degrad. Stab. 2012, 97, 747–754.
- Bruni, G.P.; de Oliveira, J.P.; Gomez-Mascaraque, L.G.; Fabra, M.J.; Martins, V.G.; Zavareze, E.D.R.; Lopez-Rubio, A. Electrospun Beta-Carotene-Loaded Spi: Pva Fiber Mats Produced by Emulsion-Electrospinning as Bioactive Coatings for Food Packaging. Food Packag. Shelf Life 2020, 23, 100426.
- Low, J.T.; Yusoff, N.I.S.M.; Othman, N.; Wong, T.; Wahit, M.U. Silk Fibroin-Based Films in Food Packaging Applications: A Review. Compr. Rev. Food Sci. Food Saf. 2022, 21, 2253–2273.
- Lin, L.; Liao, X.; Cui, H. Cold Plasma Treated Thyme Essential Oil/Silk Fibroin Nanofibers Against Salmonella Typhimurium in Poultry Meat. Food Packag. Shelf Life 2019, 21, 100337.
- Cai, J.; Zhang, D.; Zhou, R.; Zhu, R.; Fei, P.; Zhu, Z.; Cheng, S.; Ding, W. Hydrophobic Interface Starch Nanofibrous Film for Food Packaging: From Bioinspired Design to Self-Cleaning Action. J. Agr. Food Chem. 2021, 69, 5067–5075.
- Zhang, D.; Chen, L.; Cai, J.; Dong, Q.; Din, Z.; Hu, Z.; Wang, G.; Ding, W.; He, J.; Cheng, S. Starch/Tea Polyphenols Nanofibrous Films for Food Packaging Application: From Facile Construction to Enhance Mechanical, Antioxidant and Hydrophobic Properties. Food Chem. 2021, 360, 129922.
- Wang, M.; Fang, Y.; Li, Q.; Bai, L.; Yu, H.; Liu, S.; Li, J.; Chen, W. Nanostructures of Plant Cell Walls and Individualization Methodology of Nanofibrillated Cellulose. Acta Polym. Sin. 2020, 51, 586–597.
- Hashmi, M.; Ullah, S.; Ullah, A.; Saito, Y.; Haider, M.K.; Bie, X.; Wada, K.; Kim, I.S. Carboxymethyl Cellulose (Cmc) Based Electrospun Composite Nanofiber Mats for Food Packaging. Polymers 2021, 13, 302.
- Niu, B.; Zhan, L.; Shao, P.; Xiang, N.; Sun, P.; Chen, H.; Gao, H. Electrospinning of Zein-Ethyl Cellulose Hybrid Nanofibers with Improved Water Resistance for Food Preservation. Int. J. Biol. Macromol. 2020, 142, 592–599.
- Tarus, B.K.; Mwasiagi, J.I.; Fadel, N.; Al-Oufy, A.; Elmessiry, M. Electrospun Cellulose Acetate and Poly(Vinyl Chloride) Nanofiber Mats Containing Silver Nanoparticles for Antifungi Packaging. Sn Appl. Sci. 2019, 1, 245.
- Dash, M.; Chiellini, F.; Ottenbrite, R.M.; Chiellini, E. Chitosan-a Versatile Semi-Synthetic Polymer in Biomedical Applications. Prog. Polym. Sci. 2011, 36, 981–1014.
- Deng, L.; Taxipalati, M.; Zhang, A.; Que, F.; We, H.; Feng, F.; Zhang, H. Electrospun Chitosan/Poly(Ethylene Oxide)/Lauric Arginate Nanofibrous Film with Enhanced Antimicrobial Activity. J. Agr. Food Chem. 2018, 66, 6219–6226.
- Surendhiran, D.; Li, C.; Cui, H.; Lin, L. Fabrication of High Stability Active Nanofibers Encapsulated with Pomegranate Peel Extract Using Chitosan/Peo for Meat Preservation. Food Packag. Shelf Life 2020, 23, 100439.
- Wen, P.; Zhu, D.; Feng, K.; Liu, F.; Lou, W.; Li, N.; Zong, M.; Wu, H. Fabrication of Electrospun Polylactic Acid Nanofilm Incorporating Cinnamon Essential Oil/Beta-Cyclodextrin Inclusion Complex for Antimicrobial Packaging. Food Chem. 2016, 196, 996–1004.
- Sharif, N.; Golmakani, M.; Hajjari, M.M.; Aghaee, E.; Ghasemi, J.B. Antibacterial Cuminaldehyde/Hydroxypropyl-Beta-Cyclodextrin Inclusion Complex Electrospun Fibers Mat: Fabrication and Characterization. Food Packag. Shelf Life 2021, 29, 100738.
- Sid, S.; Mor, R.S.; Kishore, A.; Sharanagat, V.S. Bio-Sourced Polymers as Alternatives to Conventional Food Packaging Materials: A Review. Trends Food Sci. Technol. 2021, 115, 87–104.
- Beikzadeh, S.; Hosseini, S.M.; Mofid, V.; Ramezani, S.; Ghorbani, M.; Ehsani, A.; Mortazavian, A.M. Electrospun Ethyl Cellulose/Poly Caprolactone/Gelatin Nanofibers: The Investigation of Mechanical, Antioxidant, and Antifungal Properties for Food Packaging. Int. J. Biol. Macromol. 2021, 191, 457–464.
- Liu, L.; Zhang, J.; Zou, X.; Arslan, M.; Shi, J.; Zhai, X.; Xiao, J.; Wang, X.; Huang, X.; Li, Z.; et al. A High-Stable and Sensitive Colorimetric Nanofiber Sensor Based on Pcl Incorporating Anthocyanins for Shrimp Freshness. Food Chem. 2022, 377, 131909.
- Vidal, C.P.; Velasquez, E.; Galotto, M.J.; de Dicastillo, C.L. Development of an Antibacterial Coaxial Bionanocomposite Based on Electrospun Core/Shell Fibers Loaded with Ethyl Lauroyl Arginate and Cellulose Nanocrystals for Active Food Packaging. Food Packag. Shelf Life 2022, 31, 100802.
- Narayanan, V.; Mani, M.K.; Thambusamy, S. Electrospinning Preparation and Spectral Characterizations of the Inclusion Complex of Ferulic Acid and Gamma-Cyclodextrin with Encapsulation into Polyvinyl Alcohol Electrospun Nanofibers. J. Mol. Struct. 2020, 1221, 128767.
- Yu, D.; Feng, Y.; Xu, J.; Kong, B.; Liu, Q.; Wang, H. Fabrication, Characterization, and Antibacterial Properties of Citric Acid Crosslinked Pva Electrospun Microfibre Mats for Active Food Packaging. Packag. Technol. Sci. 2021, 34, 361–370.
- Valerini, D.; Tammaro, L.; Vitali, R.; Guillot, G.; Rinaldi, A. Sputter-Deposited Ag Nanoparticles on Electrospun Pcl Scaffolds: Morphology, Wettability and Antibacterial Activity. Coatings 2021, 11, 345.
- Kowsalya, E.; MosaChristas, K.; Balashanmugam, P.; Selvi, T.A.; Rani, J.C. Biocompatible Silver Nanoparticles/Poly(Vinyl Alcohol) Electrospun Nanofibers for Potential Antimicrobial Food Packaging Applications. Food Packag. Shelf Life 2019, 21, 100379.
- Zhu, Z.; Zhang, Y.; Zhang, Y.; Shang, Y.; Zhang, X.; Wen, Y. Preparation of Pan@TiO2 Nanofibers for Fruit Packaging Materials with Efficient Photocatalytic Degradation of Ethylene. Materials 2019, 12, 896.
- Mayorga, J.L.C.; Rovira, M.J.F.; Mas, L.C.; Moragas, G.S.; Cabello, J.M.L. Antimicrobial Nanocomposites and Electrospun Coatings Based on Poly(3-Hydroxybutyrate-Co-3-Hydroxyvalerate) and Copper Oxide Nanoparticles for Active Packaging and Coating Applications. J. Appl. Polym. Sci. 2018, 135, 45673.
- Rapa, M.; Stefan, M.; Popa, P.A.; Toloman, D.; Leostean, C.; Borodi, G.; Vodnar, D.C.; Wrona, M.; Salafranca, J.; Nerin, C.; et al. Electrospun Nanosystems Based on Phbv and Zno for Ecological Food Packaging. Polymers 2021, 13, 2123.
- Esmaeillou, M.; Moharamnejad, M.; Hsankhani, R.; Tehrani, A.A.; Maadi, H. Toxicity of Zno Nanoparticles in Healthy Adult Mice. Environ. Toxicol. Pharmacol. 2013, 35, 67–71.
- Yang, Y.; Doudrick, K.; Bi, X.; Hristovski, K.; Herckes, P.; Westerhoff, P.; Kaegi, R. Characterization of Food-Grade Titanium Dioxide: The Presence of Nanosized Particles. Environ. Sci. Technol. 2014, 48, 6391–6400.
- Liu, Y.; Edwards, J.V.; Prevost, N.; Huang, Y.; Chen, J.Y. Physico- and Bio-Activities of Nanoscale Regenerated Cellulose Nonwoven Immobilized with Lysozyme. Mater. Sci. Eng. C 2018, 91, 389–394.
- Yayehrad, A.T.; Wondie, G.B.; Marew, T. Different Nanotechnology Approaches for Ciprofloxacin Delivery Against Multidrug-Resistant Microbes. Infect. Drug Resist. 2022, 15, 413–426.
- Balandin, A.A.; Ghosh, S.; Bao, W.; Calizo, I.; Teweldebrhan, D.; Miao, F.; Lau, C.N. Superior Thermal Conductivity of Single-Layer Graphene. Nano Lett. 2008, 8, 902–907.
- Zhu, N.; Liu, W.; Xue, M.; Xie, Z.; Zhao, D.; Zhang, M.; Chen, J.; Cao, T. Graphene as a Conductive Additive to Enhance the High-Rate Capabilities of Electrospun Li4Ti5O12 for Lithium-Ion Batteries. Electrochim. Acta 2010, 55, 5813–5818.
- Yang, S.; Lei, P.; Shan, Y.; Zhang, D. Preparation and Characterization of Antibacterial Electrospun Chitosan/Poly (Vinyl Alcohol)/Graphene Oxide Composite Nanofibrous Membrane. Appl. Surf. Sci. 2018, 435, 832–840.
- Torres-Giner, S.; Echegoyen, Y.; Teruel-Juanes, R.; Badia, J.D.; Ribes-Greus, A.; Lagaron, J.M. Electrospun Poly(Ethylene-Co-Vinyl Alcohol)/Graphene Nanoplatelets Composites of Interest in Intelligent Food Packaging Applications. Nanomaterials 2018, 8, 745.
- Perez-Masiá, R.; Fabra, M.J.; Chalco-Sandoval, W.; López-Rubio, A.; Lagaron, J.M. Development by Electrohydrodynamic Processing of Heat Storage Materials for Multisectorial Applications; Springer: Berlin/Heidelberg, Germany, 2015.
- Perez-Masia, R.; Lopez-Rubio, A.; Fabra, M.J.; Lagaron, J.M. Use of Electrohydrodynamic Processing to Develop Nanostructured Materials for the Preservation of the Cold Chain. Innov. Food Sci. Emerg. 2014, 26, 415–423.
- Chalco-Sandoval, W.; Fabra, M.J.; Lopez-Rubio, A.; Lagaron, J.M. Use of Phase Change Materials to Develop Electrospun Coatings of Interest in Food Packaging Applications. J. Food Eng. 2017, 192, 122–128.
- Perez-Masia, R.; Lopez-Rubio, A.; Fabra, M.J.; Lagaron, J.M. Biodegradable Polyester-Based Heat Management Materials of Interest in Refrigeration and Smart Packaging Coatings. J. Appl. Polym. Sci. 2013, 130, 3251–3262.
- Savoia, D. Plant-Derived Antimicrobial Compounds: Alternatives to Antibiotics. Future Microbiol. 2012, 7, 979–990.
- Agrimonti, C.; White, J.C.; Tonetti, S.; Marmiroli, N. Antimicrobial Activity of Cellulosic Pads Amended with Emulsions of Essential Oils of Oregano, Thyme and Cinnamon Against Microorganisms in Minced Beef Meat. Int. J. Food Microbiol. 2019, 305, 108246.
- Stoleru, E.; Brebu, M. Stabilization Techniques of Essential Oils by Incorporation into Biodegradable Polymeric Materials for Food Packaging. Molecules 2021, 26, 6307.
- Liu, Y.; Wang, S.; Zhang, R.; Lan, W.; Qin, W. Development of Poly(Lactic Acid)/Chitosan Fibers Loaded with Essential Oil for Antimicrobial Applications. Nanomaterials 2017, 7, 194.
- Basavegowda, N.; Baek, K. Synergistic Antioxidant and Antibacterial Advantages of Essential Oils for Food Packaging Applications. Biomolecules 2021, 11, 1267.
- Fonseca, L.M.; Radunz, M.; Hackbart, H.C.D.S.; Silva, F.T.D.; Camargo, T.M.; Bruni, G.P.; Monks, J.L.F.; Zavareze, E.D.R.; Dias, A.R.G. Electrospun Potato Starch Nanofibers for Thyme Essential Oil Encapsulation: Antioxidant Activity and Thermal Resistance. J. Sci. Food Agr. 2020, 100, 4263–4271.
- Lv, Q.; Long, J.; Gong, Z.; Nong, K.; Liang, X.; Qin, T.; Huang, W.; Yang, L. Current State of Knowledge on the Antioxidant Effects and Mechanisms of Action of Polyphenolic Compounds. Nat. Prod. Commun. 2021, 16, 1934578X211027745.
- Dumitriu, R.P.; Mitchell, G.R.; Davis, F.J.; Vasile, C. Functionalized Coatings by Electrospinning for Anti-Oxidant Food Packaging. Procedia Manuf. 2017, 12, 59–65.
- Kuntzler, S.G.; Costa, J.A.V.; de Morais, M.G. Development of Electrospun Nanofibers Containing Chitosan/Peo Blend and Phenolic Compounds with Antibacterial Activity. Int. J. Biol. Macromol. 2018, 117, 800–806.
- Aydogdu, A.; Sumnu, G.; Sahin, S. Fabrication of Gallic Acid Loaded Hydroxypropyl Methylcellulose Nanofibers by Electrospinning Technique as Active Packaging Material. Carbohyd Polym. 2019, 208, 241–250.
- Wang, H.; Hao, L.; Wang, P.; Chen, M.; Jiang, S.; Jiang, S. Release Kinetics and Antibacterial Activity of Curcumin Loaded Zein Fibers. Food Hydrocolloid 2017, 63, 437–446.
- Luo, X.; Lim, L. Curcumin-Loaded Electrospun Nonwoven as a Colorimetric Indicator for Volatile Amines. LWT Food Sci. Technol. 2020, 128, 109493.
- Avila, L.B.; Fontes, M.R.V.; Zavareze, E.D.R.; Moraes, C.C.; Morais, M.M.; Rosa, G.S.D. Recovery of Bioactive Compounds from Jaboticaba Peels and Application into Zein Ultrafine Fibers Produced by Electrospinning. Polymers 2020, 12, 2916.
- Solaberrieta, I.; Jimenez, A.; Cacciotti, I.; Garrigos, M.C. Encapsulation of Bioactive Compounds from Aloe Vera Agrowastes in Electrospun Poly (Ethylene Oxide) Nanofibers. Polymers 2020, 12, 1323.
- Rashidi, M.; Mansour, S.S.; Mostashari, P.; Ramezani, S.; Mohammadi, M.; Ghorbani, M. Electrospun Nanofiber Based on Ethyl Cellulose/Soy Protein Isolated Integrated with Bitter Orange Peel Extract for Antimicrobial and Antioxidant Active Food Packaging. Int. J. Biol. Macromol. 2021, 193, 1313–1323.
More
Information
Subjects:
Food Science & Technology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
677
Revisions:
3 times
(View History)
Update Date:
07 Sep 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




