Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Jamir Pitton Rissardo | -- | 2782 | 2023-09-02 04:50:29 | | | |
| 2 | Catherine Yang | Meta information modification | 2782 | 2023-09-04 02:49:15 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Pitton Rissardo, J.; Fornari Caprara, A.L. Alopecia Associated with Antiseizure Medication. Encyclopedia. Available online: https://encyclopedia.pub/entry/48756 (accessed on 08 February 2026).
Pitton Rissardo J, Fornari Caprara AL. Alopecia Associated with Antiseizure Medication. Encyclopedia. Available at: https://encyclopedia.pub/entry/48756. Accessed February 08, 2026.
Pitton Rissardo, Jamir, Ana Leticia Fornari Caprara. "Alopecia Associated with Antiseizure Medication" Encyclopedia, https://encyclopedia.pub/entry/48756 (accessed February 08, 2026).
Pitton Rissardo, J., & Fornari Caprara, A.L. (2023, September 02). Alopecia Associated with Antiseizure Medication. In Encyclopedia. https://encyclopedia.pub/entry/48756
Pitton Rissardo, Jamir and Ana Leticia Fornari Caprara. "Alopecia Associated with Antiseizure Medication." Encyclopedia. Web. 02 September, 2023.
Copy Citation
The mainstay of treatment for epilepsy is antiseizure medications (ASMs). Approximately 70% of individuals with epilepsy obtain seizure freedom with adequate ASMs therapy. Adverse effects of antiseizure medications (ASMs) remain one of the major causes of non-adherence. Cosmetic side effects (CSEs) are among the most commonly reported side effects of ASMs. Alopecia is one of the CSEs that has a high intolerance rate leading to poor therapeutical compliance.
alopecia
hair loss
cosmetic side effects
antiseizure medication
1. Valproate (VPA)
VPA is one of the most frequently used ASMs for treating generalized and focal seizures. It is also indicated for managing bipolar disorders, neuropathic pain, and migraine prophylaxis [1]. VPA is associated with neurological and cosmetic side effects [2]. Alopecia is among the 10 most commonly reported adverse effects of VPA use [3]. The incidence of alopecia secondary to VPA greatly varies from 0.5 to 24% [4]. The diagnosis is based on the history of hair loss or abnormalities following VPA administration. Additionally, it can be confirmed by performing pull tests and modified wash tests.
Hair loss most commonly occurs after three to six months of VPA introduction [5]. Apparently, there is a direct relationship between the occurrence of hair loss and blood levels of VPA. High VPA blood levels (80–150 mcg/L) are associated with alopecia occurrence in 28% of the individuals taking VPA. On the other hand, adequate blood level concentrations (25–50 mcg/L) of VPA are related to a 4 percent occurrence of alopecia [6]. However, a meta-analysis found no significant correlation between the dose or duration of VPA therapy and alopecia [7].
There are five main hypotheses to explain hair loss associated with VPA use [8][9]. The pathophysiology of hair loss includes biotin deficiency, hyperandrogenism, mineral deficiency, telogen effluvium, and vitamin D deficiency (Figure 1) [10][11][12][13][14]. The hair loss associated with VPA is typically incomplete and usually reversible after discontinuing the causative drug [15]. Treatment will include general measures, such as reassurance and hair care techniques. Additionally, some authors reported specific treatment options that should be carefully evaluated case-by-case (Table 1) [8][9][16][17][18][19][20][21][22][23].
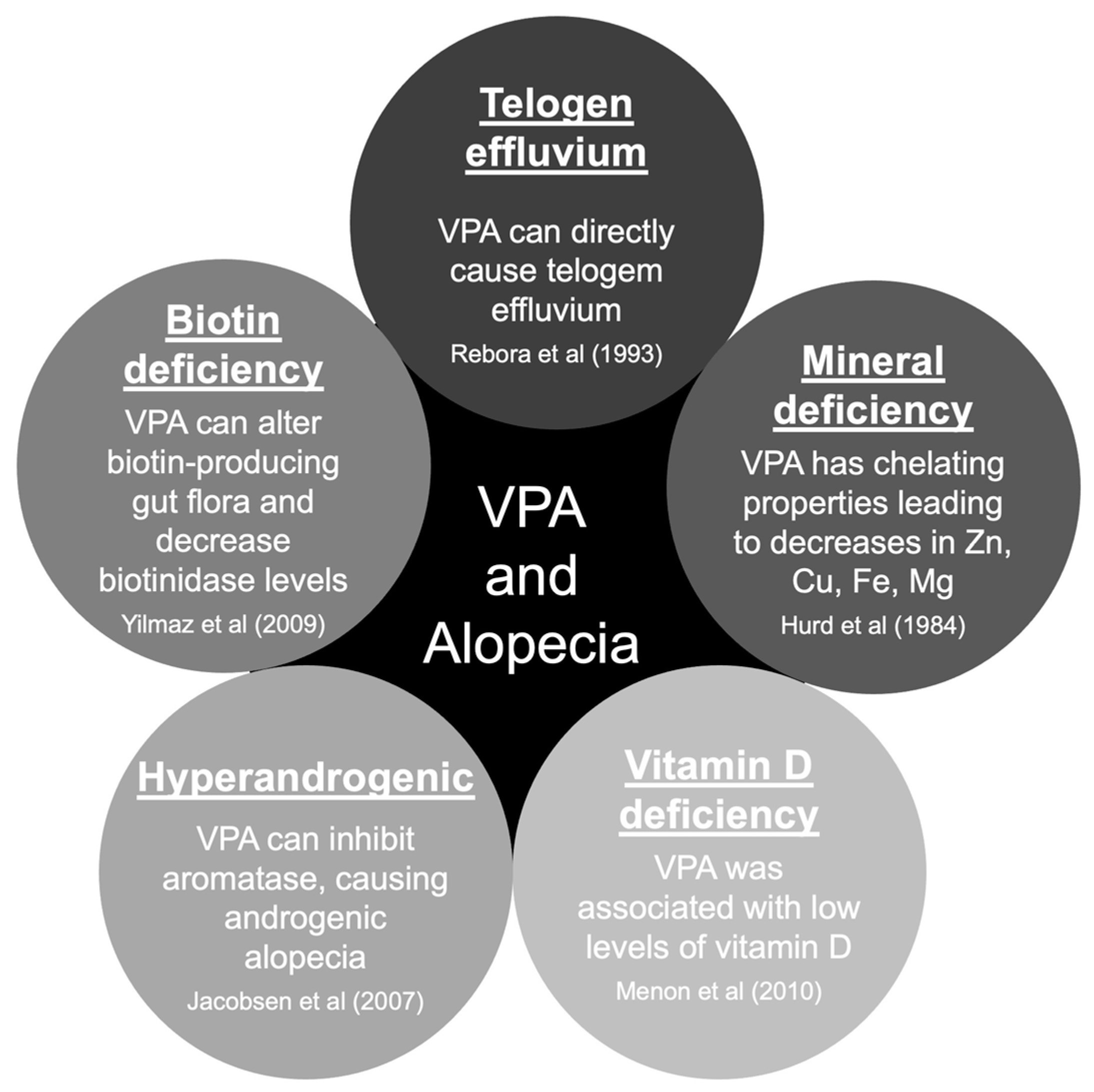
Table 1. Management of VPA-associated alopecia by Praharaj et al. [9] adapted by Rissardo et al.
| Management | Comment | Reference |
|---|---|---|
| General measures | Reassurance. Alopecia is a benign side effect and is usually reversible. Additionally, provide hair care techniques. Advise to use soft brushes and mild shampoos and avoid dyes, heated curlers, and hair dryers. | Praharaj et al. (2022) [9] |
| Adjustment of VPA dosage | If feasible, VPA should be discontinued. Dose reduction of VPA was associated with hair regrowth. Additionally, a gradual increase in VPA dose was effective in the management of neurological conditions without affecting hair growth in some cases. | Uehlinger et al. (1992) [16] Henriksen et al. (1982) [17] Wang et al. (2019) [8] |
| Mineral supplementation | Iron, copper, magnesium, selenium, and zinc could be useful in treating hair loss associated with VPA. Zinc and selenium supplementation can help prevent further hair loss and promote regrowth. | Fatemi et al. (1995) [18] Trost et al. (2006) [19] |
| Vitamin supplementation | Oral administration of biotin (10 mg/day) shorted hair regrowth time. Other vitamins can be prescribed, but there is no evidence of their efficacy. | Castro-Gago et al. (2010) [20] Yilmaz et al. (2009) [11] |
| Agomelatine administration | Agomelatine was associated with a reduction of hair loss related to VPA. Agomelatine use should be attempted when the offending drug cannot be discontinued. | Sahin et al. (2017) [21] |
| Minoxidil administration | Minoxidil was associated with a reduction of hair loss related to VPA. Minoxidil use should be attempted when the offending drug cannot be discontinued. | Thomson et al. (2017) [22] |
| Topical VPA therapy | Topical application of VPA was found to promote hair growth. | Kakunje et al. (2018) [23] |
| Others | Advise the patient not to take VPA during meals to reduce its chelating effect on metals. VPA can affect zinc and selenium absorption, two metals associated with hair growth. | Praharaj et al. (2022) [9] |
Abbreviations: VPA, valproate/valproic acid.
2. Carbamazepine (CBZ)
CBZ has a broad use for the treatment of focal seizures. It is also used for treating bipolar disorder and trigeminal neuralgia. Although CBZ is not as notorious for causing alopecia as VPA, the incidence of alopecia with it ranges from 0.3% to 6% [24][25]. In 1985, Shuper et al. reported the first case of CBZ-induced alopecia. An 8.5-year-old girl with headaches and multifocal epilepsy was managed with CBZ. Hair loss stopped after CBZ discontinuation, and hair regrowth was observed [26].
Pillans and Woods reported 177 cases of CBZ-induced alopecia in 1995 [6]. In 1992, Mattson et al. conducted a double-blind trial in 480 individuals with focal seizures and generalized tonic-clonic seizures using VPA or CBZ. Hair loss or abnormal hair texture was observed in 12 percent of subjects taking VPA and 6 percent taking CBZ [27].
In 1997, Ikeda et al. published two individuals that developed hair loss secondary to CBZ therapy. In the first case, the patient was treated for focal epilepsy. Hair loss developed after three months of starting the drug. The blood concentration of CBZ was maintained at 5.0 mg/L. Hair regrowth after replacing the offending drug was observed. The second case reported by Ikeda et al. was an individual diagnosed with tuberous sclerosis with focal seizures. CBZ was started and maintained at a steady level with a blood concentration of 6.5 mg/L. Hair loss was observed after two months of starting CBZ therapy. After reducing the CBZ dose from 600 mg/day to 200 mg/day, hair shedding was reduced, and new hair began to grow [28]. Another case report from Oh et al. demonstrated hair loss four months after starting CBZ at 600 mg/day. The hair loss was reversed after decreasing the dose of CBZ to 200 mg/day [29].
3. Lamotrigine (LTG)
LTG is used to treat epilepsy as a monotherapy or as an adjunct to other antiseizure polytherapy. It is a first-line treatment for primary generalized tonic–clonic seizures, focal seizures, atypical absence seizures, myoclonic seizures, and atonic seizures [30]. Apart from using it as an ASM, it can also be used in mood disorders and depression management [31]. The incidence of hair loss associated with LTG is 0.8%. Interestingly, three in every four individuals who developed LTG-induced alopecia reported this side effect as a significant factor for LTG withdrawal [24].
LTG-induced epidermal necrolysis with some hair loss is well known. However, there are few reports of isolated hair loss without epidermal necrolysis associated with LTG in the literature [32]. In 2004, Patrizi et al. reported the first case of LTG-induced alopecia. A 24-year-old female with focal epilepsy was started on LTG and magnesium VPA. The magnesium VPA dose was decreased to 600 mg/day, and the LTG dosage was increased to 100 mg/day. After a few months, hair loss was observed. Trichogram ruled out androgenetic alopecia. LTG probably was the main cause for the development of alopecia, but the effect of VPA on hair structure cannot be excluded [33].
In 2006, Hillemacher et al. reported a case of a 63-year-old diagnosed with bipolar disorder. LTG was started and increased to 150 mg/day. Hair loss was noted in the third week of treatment. A trichogram showed hair with a prolonged telogen phase and dystrophic characteristics [34]. This strongly suggested telogen effluvium as the cause of LTG-induced alopecia. In 2017, Solmi et al. reported a patient affected by treatment-resistant major depressive disorder who was managed with LTG, and telogen effluvium was observed [35].
Tengstrand et al.’s study is one of the most significant articles in the literature regarding LTG-induced alopecia. The authors assessed 337 individuals from 19 countries who developed hair loss associated with LTG. In 291 reports, alopecia developed with LTG monotherapy. They found that alopecia should be considered a significant factor affecting adherence to LTG therapy. The time to onset of alopecia after LTG intake was variable, in which 17 individuals presented alopecia within one month, 48 presented between one and six months, and 31 presented after six months of LTG onset [36]. It is worth mentioning that Tengstrand et al.’s study assessed VigiBase reports, a database with limited information on patient demographic characteristics and clinical descriptions [36].
4. Levetiracetam (LEV)
LEV is considered a broad-spectrum ASM. This drug was approved by the FDA in 1999 for the management of epilepsy [37]. Interestingly, LEV differs in structure and mechanism of action from other marketed ASMs [38]. LEV has favorable pharmacokinetics and a low potential for drug interactions [39]. In a study with 1903 individuals, a 0.4% prevalence of hair loss due to LEV therapy was observed [24].
Hair loss is a rare adverse effect of LEV therapy. In 2014, Zou et al. reported five cases of LEV-induced alopecia. The doses of LEV ranged within 500–1000 mg/day. Hair loss secondary to LEV was observed to occur between three and eight weeks of LEV therapy. The individuals presented with diffuse non-scarring hair loss. Complete recovery from alopecia was seen in two out of five subjects. The other two patients noticed an improvement in hair loss after decreasing the dose from 1000 mg/day to 750 mg/day. One individual decided to continue with medication despite hair loss [40]. The authors concluded that the alopecia associated with LEV was due to telogen effluvium. Aghamollaii et al. published a case series of three patients that experienced hair loss with LEV [41].
5. Gabapentin (GBP)
GBP is associated with mild adverse events, such as somnolence, fatigue, ataxia, and dizziness, which are reported in about three in every four patients [42]. It is a first-line treatment for the management of neuropathic pain [43]. Eker et al. reported a case of alopecia with GBP therapy for neuropathic pain. The patient was started on GBP 1800 mg/day. Hair loss was noticed one week after the GBP therapy onset. Patchy areas of alopecia were noticed. When GBP was discontinued, hair regrowth was observed [44].
The first case of GBP-induced alopecia was reported in 1997 by Picard et al. A 15-year-old girl with seizures was taking CBZ and phenytoin. GBP 1800 mg/day was prescribed as add-on therapy. The patient experienced alopecia during the second month of treatment with GBP. Hair loss was diffuse without bald patches. GBP was discontinued, but CBZ and phenytoin were continued. Hair regrowth was observed three weeks after GBP discontinuation, and alopecia was completely reversed within one month [45].
6. Topiramate (TPM)
TPM may cause alopecia in approximately one percent of its users [46]. According to Chen et al., alopecia prevalence was 1.7% among 230 TPM users. TPM was discontinued in all the individuals who developed alopecia because the patients decided to stop the treatment [24]. Chuang et al. reported a 15-year-old girl with frontal lobe epilepsy who developed hair loss after two months of TPM adjunctive therapy. The hair loss was reversible upon discontinuation of the drug. There was a recurrence of alopecia after the TPM rechallenge [47]. Another case report demonstrated hair loss that started after three months of starting TPM at a dosage of 50 mg/day for migraine headaches. Hair started regrowing with a dose reduction to 25 mg/day, and alopecia recurred after the reintroduction of a 50 mg/day dosage of TPM [48].
Lagrand et al. described a case of an individual who developed alopecia with different medications for managing her tremor-dominant Parkinson’s disease. She presented hair loss after the introduction, in different moments, of levodopa/benserazide, propranolol, and TPM [49]. Their case is interesting because it suggests a possible genetic predisposition for the development of alopecia after the administration of determined groups of medications.
7. Phenytoin (PHT)
As part of the Columbia and Yale ASM Database Project, Chen et al. reviewed patient records, including demographics, medical history, ASM use, and side effects for 1903 adult patients (≥16 years of age) newly started on an ASM. Cosmetic side effects were determined by patient or physician reports in the medical record, including acne, gingival hyperplasia, hair loss, hirsutism, and weight gain. PHT was taken by 404 out of 1903 patients. In total, 0.3% attributed hair loss due to PHT and 0.3% intolerability due to hair loss [24]. Herranz et al. assessed the clinical side effects of long-term monotherapy of phenobarbital, primidone, PHT, CBZ, and VPA in 392 pediatric individuals. PHT was more commonly associated with cosmetic side effects than the other investigated drugs. However, there was no report of PHT-induced alopecia. Instead, hirsutism was observed in 9% of children taking PHT [50]. Interestingly, VPA was the drug most commonly associated with alopecia, which occurred in 0.8% of children [51].
Kuhne et al. described a case of Munchausen by proxy syndrome mimicking childhood-onset systemic lupus erythematosus. A patient presenting with malar rash, photosensitivity, alopecia, arthralgia, arterial hypertension, macroscopic hematuria, seizure, and positive antinuclear antibodies was reported. The pediatric patient received high doses of PHT, which led to drug-induced lupus erythematosus [52]. Thus, subjects presenting with alopecia during PHT therapy should be assessed for other clinical manifestations suggesting autoimmune diseases. There are two other cases in the literature with alopecia secondary to PHT-induced lupus erythematosus [53][54].
Hirsutism is more frequent than alopecia as an adverse effect of PHT [51]. In this context, a study assessed the effectiveness of PHT in suppressing chemotherapy-induced hair loss. The authors revealed that oral PHT could significantly suppress hair loss due to cyclophosphamide therapy in rats. PHT co-administration was related to improved hair growth, increased hair-shaft thickness, and reduced skin lipid peroxidation [55].
8. Pregabalin (PGB)
Isolated alopecia secondary to PGB was uncommonly described in the literature. Chen et al. found only one patient who developed alopecia among 143 PGB users [24]. In another study with the Netherlands Pharmacovigilance Centre Lareb data, the incidence of PGB-induced alopecia was 0.07% [56]. Noteworthily, PGB dose may be directly associated with hair loss. In Wistar rats, alopecia was more frequently observed with higher doses of PGB [57].
Turgut et al. reported an adult female diagnosed with fibromyalgia. PGB 75 mg/day was started, and the dose was increased to 150 mg/day after one week. Within three weeks of PGB therapy, significant hair loss was observed. PGB was discontinued. Complete hair regrowth was observed after two weeks of PGB withdrawal [58].
In clinical practice, PGB-induced alopecia is commonly seen as part of drug reactions with eosinophilia and systemic symptoms (DRESS). However, it was scarcely reported in the literature. Suh et al. described an individual presenting with alopecia and pruritic erythema after PGB use. DRESS diagnosis was made. After PGB discontinuation, hair regrowth was observed [59].
9. Perampanel (PMP)
PMP has been approved as an add-on treatment for refractory focal seizures and primary generalized tonic–clonic seizures in idiopathic generalized epilepsy [60]. The most frequently reported side effects are dizziness and fatigue. In this context, the only described CSE were weight gain (7.4–19.2%) and skin rash (10.6%) [61][62][63]. It is worth mentioning that these CSEs were only observed in specific populations, such as Asian individuals [63].
Rohracher et al. pooled observational data of PMP comprising a full analysis set of 2396 individuals. Only one individual reported alopecia during the first year of PMP therapy [64]. Villanueva et al. assessed the safety and efficacy of long-term PMP in 464 subjects. The incidence of PMP-induced alopecia was 0.2% [65].
The mechanism of action of PMP involves a non-competitive antagonism of the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor. The AMPA receptors are considered the major subtype of ionotropic glutamate receptors [66]. Noteworthy glutamate can promote hair growth in mice models. Additionally, it is believed that glutamic acid can control hair follicle proliferation, decreasing gene expression related to apoptosis in the skin and increasing cell viability and proliferation in human keratinocytes [67]. Therefore, the low levels of glutamate in PMP therapy may be associated with hair loss.
10. Phenobarbital, Vigabatrin, Tiagabine, and Trimethadione
Phenobarbital-induced isolated alopecia was rarely described. Alopecia was usually described as part of an anticonvulsant hypersensitivity syndrome induced by phenobarbital. Huang et al. reported an individual presenting with alopecia in the convalescent status of phenobarbital-induced anticonvulsant hypersensitivity syndrome. Skin histology revealed peri-follicular, peri-bulbar, and supra-bulbar lymphocyte infiltration [68]. In the literature, two other cases of alopecia were present in the convalescent period of phenobarbital hypersensitivity syndrome [69][70]. All individuals reported they had a favorable prognosis with complete hair regrowth within three months [68][69][70]. Huang et al. proposed that phenobarbital could promote lymphocyte infiltration into the peri-follicular, peri-bulbar, and supra-bulbar anatomical regions. Additionally, phenobarbital dose may be associated with the incidence of alopecia. Ghorani-Azam et al. systematically reviewed the literature concerning phenobarbital in neonates and pediatrics that were treated for seizures. Interestingly, alopecia was a reported sign of phenobarbital poisoning [71].
Vigabatrin has also been reported to cause hair loss. In Lampl et al., 5 out of 52 patients who received vigabatrin for focal seizures experienced moderate hair loss or changes in hair structure. The complaints began after three to seven weeks of initiating vigabatrin treatment. Hair loss recovery was seen in all patients after cessation of treatment [72]. Vigabatrin showed different results in rodent studies. It was observed that mice tolerated high doses of vigabatrin, but Sprague Dawley rats developed acute alopecia and body weight gain. These CSEs were improved within six months of vigabatrin discontinuation [73].
Tiagabine may increase the level of γ-aminobutyric acid. Vossler et al. assessed the adverse effects of long-term tiagabine use in 292 subjects. Only one patient developed alopecia secondary to tiagabine [74]. Another study by Mercke et al. found an incidence of 1% of tiagabine-induced alopecia [46].
Trimethadione is historically important for managing absence seizures and refractory temporal lobe epilepsy. There are some case reports in the literature about alopecia associated with this medication [75].
References
- McKinney, P.A.; Finkenbine, R.D.; DeVane, C.L. Alopecia and mood stabilizer therapy. Ann. Clin. Psychiatry 1996, 8, 183–185.
- Johannessen, C.U. Mechanisms of action of valproate: A commentatory. Neurochem. Int. 2000, 37, 103–110.
- Rissardo, J.P.; Caprara, A.L.; Durante, Í. Valproate-associated Movement Disorder: A Literature Review. Prague Med. Rep. 2021, 122, 140–180.
- Schmidt, D. Adverse effects of valproate. Epilepsia 1984, 25, 44–49.
- Völzke, E.; Doose, H. Dipropylacetate (Dépakine, Ergenyl) in the treatment of epilepsy. Epilepsia 1973, 14, 185–193.
- Pillans, P.I.; Woods, D.J. Drug-associated alopecia. Int. J. Dermatol. 1995, 34, 149–158.
- Beydoun, A.; Sackellares, J.C.; Shu, V. Safety and efficacy of divalproex sodium monotherapy in partial epilepsy: A double-blind, concentration-response design clinical trial. Depakote Monotherapy for Partial Seizures Study Group. Neurology 1997, 48, 182–188.
- Wang, X.; Wang, H.; Xu, D.; Zhu, L.; Liu, L. Risk of valproic acid-related alopecia: A systematic review and meta-analysis. Seizure 2019, 69, 61–69.
- Praharaj, S.K.; Munoli, R.N.; Udupa, S.T.; Vaidyanathan, S. Valproate-associated hair abnormalities: Pathophysiology and management strategies. Hum. Psychopharmacol. 2022, 37, e2814.
- Rebora, A. Telogen effluvium: An etiopathogenetic theory. Int. J. Dermatol. 1993, 32, 339–340.
- Yilmaz, Y.; Tasdemir, H.A.; Paksu, M.S. The influence of valproic acid treatment on hair and serum zinc levels and serum biotinidase activity. Eur. J. Paediatr. Neurol. 2009, 13, 439–443.
- Hurd, R.W.; Rinsvelt, H.A.; Wilder, B.J.; Karas, B.; Maenhaut, W.; Reu, L. Selenium, zinc, and copper changes with valproic acid: Possible relation to drug side effects. Neurology 1984, 34, 1393–1395.
- Jacobsen, N.W.; Halling-Sorensen, B.; Birkved, F.K. Inhibition of human aromatase complex (CYP19) by antiepileptic drugs. Toxicol. In Vitro 2008, 22, 146–153.
- Menon, B.; Harinarayan, C.V. The effect of anti epileptic drug therapy on serum 25-hydroxyvitamin D and parameters of calcium and bone metabolism--a longitudinal study. Seizure 2010, 19, 153–158.
- Moattari, C.R.; Jafferany, M. Psychological Aspects of Hair Disorders: Consideration for Dermatologists, Cosmetologists, Aesthetic, and Plastic Surgeons. Ski. Appendage Disord. 2022, 8, 186–194.
- Uehlinger, C.; Barrelet, L.; Touabi, M.; Baumann, P. Alopecia and mood stabilizers: Two case reports. Eur. Arch. Psychiatry Clin. Neurosci. 1992, 242, 85–88.
- Henriksen, O.; Johannessen, S.I. Clinical and pharmacokinetic observations on sodium valproate—A 5-year follow-up study in 100 children with epilepsy. Acta Neurol. Scand. 1982, 65, 504–523.
- Fatemi, S.H.; Calabrese, J.R. Treatment of valproate-induced alopecia. Ann. Pharmacother. 1995, 29, 1302.
- Trost, L.B.; Bergfeld, W.F.; Calogeras, E. The diagnosis and treatment of iron deficiency and its potential relationship to hair loss. J. Am. Acad. Dermatol. 2006, 54, 824–844.
- Castro-Gago, M.; Gómez-Lado, C.; Eirís-Puñal, J.; Díaz-Mayo, I.; Castiñeiras-Ramos, D.E. Serum biotinidase activity in children treated with valproic acid and carbamazepine. J. Child. Neurol. 2010, 25, 32–35.
- Sahin, E.K.; Can, S.S.; Caykoylu, A.; Atagun, M.I. Agomelatine may alleviate valproate induced hair loss. J. Psych. Neurol. Sci. 2017, 30, 269–270.
- Thomson, S.R.; Mamulpet, V.; Adiga, S. Sodium Valproate Induced Alopecia: A Case Series. J. Clin. Diagn. Res. 2017, 11, 1–2.
- Kakunje, A.; Prabhu, A.; Priya, E.S.; Karkal, R.; Kumar, P.; Gupta, N.; Rahyanath, P.K. Valproate: It’s Effects on Hair. Int. J. Trichol. 2018, 10, 150–153.
- Chen, B.; Choi, H.; Hirsch, L.J.; Moeller, J.; Javed, A.; Kato, K.; Legge, A.; Buchsbaum, R.; Detyniecki, K. Cosmetic side effects of antiepileptic drugs in adults with epilepsy. Epilepsy Behav. 2015, 42, 129–137.
- Pellock, J.M. Carbamazepine side effects in children and adults. Epilepsia 1987, 28, 64–70.
- Shuper, A.; Stahl, B.; Weitz, R. Carbamazepine-induced hair loss. Drug Intell. Clin. Pharm. 1985, 19, 924–925.
- Mattson, R.H.; Cramer, J.A.; Collins, J.F. A comparison of valproate with carbamazepine for the treatment of complex partial seizures and secondarily generalized tonic-clonic seizures in adults. N. Engl. J. Med. 1992, 327, 765–771.
- Ikeda, A.; Shibasaki, H.; Shiozaki, A.; Kimura, J. Alopecia with carbamazepine in two patients with focal seizures. J. Neurol. Neurosurg. Psychiatry 1997, 63, 549–550.
- Oh, S.H.; Kim, D.S.; Kwon, Y.S.; Lee, J.H.; Lee, K.H. Concurrence of palmoplantar psoriasiform eruptions and hair loss during carbamazepine treatment. Acta Derm. Venereol. 2008, 88, 532–533.
- Goldenberg, M.M. Overview of drugs used for epilepsy and seizures: Etiology, diagnosis, and treatment. Pharm. Ther. 2010, 35, 392–415.
- Rybakowski, J.K. Mood Stabilizers of First and Second Generation. Brain Sci. 2023, 13, 741.
- Shirazi, Z.; Inaloo, S. Intravenous immunoglobulin in the treatment of lamotrigine- induced toxic epidermal necrolysis. Iran. J. Allergy Asthma Immunol. 2008, 7, 239–241.
- Patrizi, A.; Savoia, F.; Negosanti, F.; Posar, A.; Santucci, M.; Neri, I. Telogen effluvium caused by magnesium valproate and lamotrigine. Acta Derm. Venereol. 2005, 85, 77–78.
- Hillemacher, T.; Bleich, S.; Kornhuber, J.; Frieling, H. Hair loss as a side effect of lamotrigine treatment. Am. J. Psychiatry 2006, 163, 1451.
- Solmi, M.; Tamiello, G.I.; Manuli, G. Lamotrigine Induces Hair Loss in a Patient with Treatment-Resistant Major Depressive Disorder. Am. J. Ther. 2017, 24, 611–612.
- Tengstrand, M.; Star, K.; Puijenbroek, E.P.; Hill, R. Alopecia in association with lamotrigine use: An analysis of individual case safety reports in a global database. Drug Saf. 2010, 33, 653–658.
- Stephen, L.J.; Kelly, K.; Parker, P.; Brodie, M.J. Levetiracetam monotherapy--outcomes from an epilepsy clinic. Seizure 2011, 20, 554–557.
- Hovinga, C.A. Levetiracetam: A novel antiepileptic drug. Pharmacotherapy 2001, 21, 1375–1388.
- Neyens, L.G.; Alpherts, W.C.; Aldenkamp, A.P. Cognitive effects of a new pyrrolidine derivative (levetiracetam) in patients with epilepsy. Prog. Neuropsychopharmacol. Biol. Psychiatry 1995, 19, 411–419.
- Zou, X.; Hong, Z.; Zhou, D. Hair loss with levetiracetam in five patients with epilepsy. Seizure 2014, 23, 158–160.
- Aghamollaii, V.; Khan, Z.G.; Maneshi, A.; Ghaeli, P. Role of Zinc Supplementation in the Treatment of Levetiracetam-Induced Hair Loss: A Case Series. J. Pharm. Care. 2017, 4, 44–45.
- Goa, K.L.; Sorkin, E.M. Gabapentin. A review of its pharmacological properties and clinical potential in epilepsy. Drugs 1993, 46, 409–427.
- Rose, M.A.; Kam, P.C. Gabapentin: Pharmacology and its use in pain management. Anaesthesia 2002, 57, 451–462.
- Eker, H.E.; Cok, O.Y.; Aribogan, A. Alopecia associated with gabapentin in the treatment of neuropathic pain. J. Pain Symptom Manag. 2009, 37, 5–6.
- Picard, C.; Jonville-Bera, A.P.; Billard, C.; Autret, E. Alopecia associated with gabapentin: First case. Ann. Pharmacother. 1997, 31, 1260.
- Mercke, Y.; Sheng, H.; Khan, T.; Lippmann, S. Hair loss in psychopharmacology. Ann. Clin. Psychiatry 2000, 12, 35–42.
- Chuang, Y.C.; Chang, W.N.; Chen, I.L.; Yang, J.Y.; Ho, J.C.; Kuo, H.W. Topiramate-induced hair loss: Case report. Dermatol. Psychosom. 2002, 3, 183–184.
- Ghafoor, I.; Hosseini, H. Hair Loss Following The Topiramate Treatment. J. Babol. Univ. Med. Sci. 2017, 19, 71–74.
- Lagrand, T.J.; Lehn, A.C. Tremor Drugs in the Crosshairs. Tremor Other Hyperkinetic Mov. 2021, 11, 52.
- Herranz, J.L.; Armijo, J.A.; Arteaga, R. Clinical side effects of phenobarbital, primidone, phenytoin, carbamazepine, and valproate during monotherapy in children. Epilepsia 1988, 29, 794–804.
- Wallace, S.J. A comparative review of the adverse effects of anticonvulsants in children with epilepsy. Drug Saf. 1996, 15, 378–393.
- Kuhne, A.C.; Pitta, A.C.; Galassi, S.C.; Gonçalves, A.M.; Cardoso, A.C.; Paz, J.A.; Campos, L.M.; Silva, C.A. Munchausen by proxy syndrome mimicking childhood-onset systemic lupus erythematosus. Lupus 2019, 28, 249–252.
- Mangalvedhekar, S.S.; Gogtay, N.J.; Manjula, S.; Kadam, V.S.; Dalvi, S.S.; Shah, P.U.; Badakere, S.S.; Pradhan, V.D.; Kshirsagar, N.A. Phenytoin associated alopecia: Drug induced lupus. J. Assoc. Physicians India 2001, 49, 929–930.
- Neki, N.S.; Shah, D.M. Phenytoin induced alopecia & Lupus: A case report. RGUHS J. Med. Sci. 2015, 5, 188–189.
- Onaolapo, A.Y.; Adebayo, A.A.; Onaolapo, O.J. Oral phenytoin protects against experimental cyclophosphamide-chemotherapy induced hair loss. Pathophysiology 2018, 25, 31–39.
- Harmark, L.; Puijenbroek, E.; Straus, S.; Grootheest, K. Intensive monitoring of pregabalin: Results from an observational, Web-based, prospective cohort study in the Netherlands using patients as a source of information. Drug Saf. 2011, 34, 221–231.
- Morse, D.C.; Henck, J.W.; Bailey, S.A. Developmental Toxicity Studies with Pregabalin in Rats: Significance of Alterations in Skull Bone Morphology. Birth Defects Res. B Dev. Reprod. Toxicol. 2016, 107, 94–107.
- Turgut, C.; İzki, A.A. Hair loss due to pregabaline: A case report. Med. Res. Rep. 2020, 3, 41–45.
- Suh, J.H.; Oh, W.J.; Park, K.Y.; Seo, S.J.; Hong, C.K. DRESS syndrome induced by pregabalin in postherpetic neuralgia. Korean J. Derm. 2016, 68, 475–476.
- Franco, V.; Crema, F.; Iudice, A.; Zaccara, G.; Grillo, E. Novel treatment options for epilepsy: Focus on perampanel. Pharmacol. Res. 2013, 70, 35–40.
- French, J.A.; Krauss, G.L.; Biton, V.; Squillacote, D.; Yang, H.; Laurenza, A.; Kumar, D.; Rogawski, M.A. Adjunctive perampanel for refractory partial-onset seizures: Randomized phase III study 304. Neurology 2012, 79, 589–596.
- French, J.A.; Krauss, G.L.; Wechsler, R.T.; Wang, X.F.; DiVentura, B.; Brandt, C.; Trinka, E.; O’Brien, T.J.; Laurenza, A.; Patten, A.; et al. Perampanel for tonic-clonic seizures in idiopathic generalized epilepsy A randomized trial. Neurology 2015, 85, 950–957.
- Lin, K.L.; Lin, J.J.; Chou, M.L.; Hung, P.C.; Hsieh, M.Y.; Chou, I.J.; Lim, S.N.; Wu, T.; Wang, H.S. Efficacy and tolerability of perampanel in children and adolescents with pharmacoresistant epilepsy: The first real-world evaluation in Asian pediatric neurology clinics. Epilepsy Behav. 2018, 85, 188–194.
- Rohracher, A.; Zimmermann, G.; Villanueva, V.; Garamendi, I.; Sander, J.W.; Wehner, T.; Shankar, R.; Ben-Menachem, E.; Brodie, M.J.; Pensel, M.C.; et al. Perampanel in routine clinical use across Europe: Pooled, multicenter, observational data. Epilepsia 2018, 59, 1727–1739.
- Villanueva, V.; Garcés, M.; López-González, F.J.; Rodriguez-Osorio, X.; Toledo, M.; Salas-Puig, J.; González-Cuevas, M.; Campos, D.; Serratosa, J.M.; González-Giráldez, B.; et al. Safety, efficacy and outcome-related factors of perampanel over 12 months in a real-world setting: The FYDATA study. Epilepsy Res. 2016, 126, 201–210.
- Johansen, T.N.; Greenwood, J.R.; Frydenvang, K.; Madsen, U.; Krogsgaard-Larsen, P. Stereostructure-activity studies on agonists at the AMPA and kainate subtypes of ionotropic glutamate receptors. Chirality 2003, 15, 167–179.
- Jara, C.P.; Berti, B.A.; Mendes, N.F.; Engel, D.F.; Zanesco, A.M.; Souza, G.F.; Bezerra, R.M.; Bagatin, J.T.; Maria-Engler, S.S.; Morari, J.; et al. Glutamic acid promotes hair growth in mice. Sci. Rep. 2021, 11, 15453.
- Huang, Y.L.; Hsieh, M.Y.; Hsiao, P.F.; Sheen, J.M.; Yu, H.R.; Kuo, H.C.; Chen, S.T.; Huang, J.L.; Yang, K.D.; Lee, W.I. Alopecia areata universalis after phenobarbital-induced anti-convulsant hypersensitivity syndrome. Immunol. Investig. 2009, 38, 383–397.
- Knutsen, A.P.; Shah, M.; Schwarz, K.B.; Tsai, C.C. Graft versus host-like illness in a child with phenobarbital hypersensitivity. Pediatrics 1986, 78, 581–584.
- Bavdekar, S.B.; Muranjan, M.N.; Gogtay, N.J.; Kantharia, V.; Kshirsagar, N.A. Anticonvulsant hypersensitivity syndrome: Lymphocyte toxicity assay for the confirmation of diagnosis and risk assessment. Ann. Pharmacother. 2004, 38, 1648–1650.
- Ghorani-Azam, A.; Balali-Mood, M.; Riahi-Zanjani, B.; Darchini-Maragheh, E.; Sadeghi, M. Acute Phenobarbital Poisoning for the Management of Seizures in Newborns and Children; A Systematic Literature Review. CNS Neurol. Disord. Drug Targets 2021, 20, 174–180.
- Lampl, Y.; Gilad, R.; Sarova-Pinchas, I.; Barak, Y. Hair loss-an adverse reaction to treatment with vigabatrin. Acta Therap. 1996, 22, 51–55.
- Graham, D. Neuropathology of vigabatrin. Br. J. Clin. Pharmacol. 1989, 27, 43–45.
- Vossler, D.G.; Morris, G.L.; Harden, C.L.; Montouris, G.; Faught, E.; Kanner, A.M.; Fix, A.; French, J.A. Tiagabine in clinical practice: Effects on seizure control and behavior. Epilepsy Behav. 2013, 8, 211–216.
- Holowach, J.; Sanden, H.V. Alopecia as a side effect of treatment of epilepsy with trimethadione: Report of two cases. N. Engl. J. Med. 1960, 263, 1187.
More
Information
Subjects:
Neurosciences
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.7K
Revisions:
2 times
(View History)
Update Date:
04 Sep 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




