
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Seeta Devi | -- | 3744 | 2023-08-31 15:30:08 | | | |
| 2 | Jason Zhu | -1 word(s) | 3743 | 2023-09-01 07:31:02 | | |
Video Upload Options
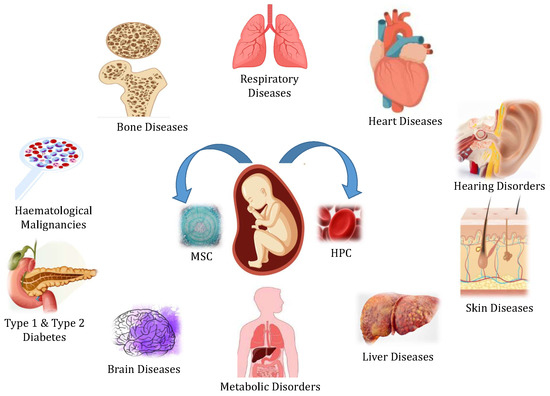
Umbilical cord blood (UCB) is a rich source of hematopoietic cells that can be used to replace bone marrow components. Many blood disorders and systemic illnesses are increasingly being treated with stem cells as regenerative medical therapy. Collected blood has been stored in either public or private banks for allogenic or autologous transplantation.
1. Introduction
2. UCB as a Regenerative Medicine
2.1. Stem Cell Transplants
2.2. Ex Vivo Modulation Strategies to Enhance the Therapeutic Potential of UCB
| Donor Type |
Cell Source |
2016, No. |
2016, Col % |
2017, No. |
2017, Col % |
2018, No. |
2018, Col % |
2019, No. |
2019, Col % |
2020, No. |
2020, Col % |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Allogenic | Bone Marrow | 2011 | 23 | 2071 | 23 | 2179 | 23 | 2014 | 21 | 1507 | 17 |
| Allogenic | Cord Blood | 682 | 8 | 621 | 7 | 557 | 6 | 512 | 5 | 422 | 5 |
| Allogenic | Peripheral Blood | 6065 | 69 | 6343 | 70 | 6580 | 71 | 6865 | 73 | 7097 | 79 |
| Autologous | Bone Marrow | 22 | <1 | 35 | <1 | 27 | <1 | 23 | <1 | 22 | <1 |
| Autologous | Cord Blood | 0 | 0 | 0 | 0 | 4 | <1 | 2 | <1 | 1 | <1 |
| Autologous | Peripheral Blood | 12,847 | 100 | 13,337 | 100 | 13,477 | 100 | 13,710 | 100 | 12,951 | 100 |
2.3. Evolutions in the Use of Umbilical Cord Mesenchymal Stem Cells (MSCs)
3. Collection and Cryopreservation of UCB
3.1. Umbilical Cord Blood Collection
3.2. Cryopreservation of Umbilical Cord Blood
C) [53]. Cryopreservative agents must be used for the cell’s survival and to maintain the structural integrity of the cells. An alternative procedure to cryopreservation, is vitrification, in which a solidification of the aqueous system occurs, without the crystallization and growth of ice. Umbilical cord blood is cryopreserved using an automated microprocessor-controlled cell freezer. Over 20 min, an equivalent amount of the cryoprotectant dimethyl sulfoxide (DMSO) is gently and slowly added to the autologous plasma. According to the cryopreservation protocol, the temperature is gradually reduced to −80 °
4. Public and Private Umbilical Cord Blood Banking
-
Jeevan Stem Cell Bank was founded in 1995 and is supported by the Tamil Nadu government. These banks primarily treat leukemia, thalassemia, and other hematological disorders.
-
The Reliance Dhirubhai Ambani Life Sciences Center in Thane, Maharashtra, is supported by Reliance Life Sciences Pvt. Ltd. Free UCB; collection and storage services are provided.
-
The School of Tropical Medicine (STM) established Kolkata’s first public cord blood bank.
-
StemCyte Inc., Apollo Hospital Enterprises Ltd., and Cadila Pharmaceuticals Ltd. founded StemCyte India. The bank provides collection, processing, testing, and storage services for private and public umbilical cord blood units, and therapeutic applications.
-
LifeCell was established in 2004 in technological collaboration with cryo-cell international. The primary goal of this bank was to assist patients in receiving lifesaving stem cell transplants to increase their chances of receiving the same stem cells.
5. Ethical Concerns in UCB Banking
Recommendations of Professional Organizations Regarding UCB
References
- Zakrzewski, W.; Dobrzyński, M.; Szymonowicz, M.; Rybak, Z. Stem cells: Past, present, and future. Stem Cell Res. Ther. 2019, 10, 1–22.
- Burns, C.E.; Zon, L.I. Portrait of a stem cell. Dev. Cell 2002, 3, 612–613.
- Preston, S.; Alison, M.; Forbes, S.; Direkze, N.; Poulsom, R.; Wright, N. The new stem cell biology: Something for everyone. Mol. Pathol. 2003, 56, 86.
- Cai, J.; Weiss, M.L.; Rao, M.S. In search of “stemness”. Exp. Hematol. 2004, 32, 585–598.
- McGuckin, C.P.; Forraz, N. Umbilical cord blood stem cells-an ethical source for regenerative medicine. Med. Law 2008, 27, 147.
- Vazin, T.; Freed, W.J. Human embryonic stem cells: Derivation, culture, and differentiation: A review. Restor. Neurol. Neurosci. 2010, 28, 589–603.
- Meshorer, E. What are embryonic stem cells and how can they help us? Front. Young Minds 2020, 8, 32.
- Murata, M.; Kawabe, K.; Hatta, T.; Maeda, S.; Fujita, M. Current status of umbilical cord blood storage and provision to private biobanks by institutions handling childbirth in Japan. BMC Med. Ethics 2022, 23, 92.
- Gupta, A.O.; Wagner, J.E. Umbilical cord blood transplants: Current status and evolving therapies. Front. Pediatr. 2020, 8, 570282.
- Khaddour, K.; Hana, C.K.; Mewawalla, P. Hematopoietic stem cell transplantation. In StatPearls ; StatPearls Publishing: Tampa, FL, USA, 2021.
- Pascutti, M.; Erkelens, M.; Nolte, M. Impact of viral infections on hematopoiesis: From beneficial to detrimental effects on bone marrow output. Front Immunol. 2016, 7, 364.
- Theunissen, K.; Verfaillie, C.M. A multifactorial analysis of umbilical cord blood, adult bone marrow and mobilized peripheral blood progenitors using the improved ML-IC assay. Exp. Hematol. 2005, 33, 165–172.
- Hildbrand, P.; Cirulli, V.; Prinsen, R.C.; Smith, K.A.; Torbett, B.E.; Salomon, D.R.; Crisa, L. The role of angiopoietins in the development of endothelial cells from cord blood CD34+ progenitors. Blood 2004, 104, 2010–2019.
- Gluckman, E. Milestones in umbilical cord blood transplantation. Blood Rev. 2011, 25, 255–259.
- Liao, Y.; Geyer, M.B.; Yang, A.J.; Cairo, M.S. Cord blood transplantation and stem cell regenerative potential. Exp. Hematol. 2011, 39, 393–412.
- Götherström, C.; Ringdén, O.; Tammik, C.; Zetterberg, E.; Westgren, M.; Le Blanc, K. Immunologic properties of human fetal mesenchymal stem cells. Am. J. Obstet. Gynecol. 2004, 190, 239–245.
- North, T.E.; Goessling, W.; Walkley, C.R.; Lengerke, C.; Kopani, K.R.; Lord, A.M.; Weber, G.J.; Bowman, T.V.; Jang, I.H.; Grosser, T.; et al. Prostaglandin E2 regulates vertebrate haematopoietic stem cell homeostasis. Nature 2007, 447, 1007–1011.
- Flynn, P.; Yingling, J.; Shoemaker, D. Converging technologies to enable induced pluripotent stem cells in drug discovery. Regen. Med. 2010, 5, 489–491.
- Malgieri, A.; Kantzari, E.; Patrizi, M.P.; Gambardella, S. Bone marrow and umbilical cord blood human mesenchymal stem cells: State of the art. Int. J. Clin. Exp. Med. 2010, 3, 248.
- Garbuzova-Davis, S.; Rodrigues, M.C.; Mirtyl, S.; Turner, S.; Mitha, S.; Sodhi, J.; Suthakaran, S.; Eve, D.J.; Sanberg, C.D.; Kuzmin-Nichols, N.; et al. Multiple intravenous administrations of human umbilical cord blood cells benefit in a mouse model of ALS. PLoS ONE 2012, 7, e31254.
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317.
- Williams, K.J.; Picou, A.A.; Kish, S.L.; Giraldo, A.M.; Godke, R.A.; Bondioli, K.R. Isolation and characterization of porcine adipose tissue-derived adult stem cells. Cells Tissues Organs 2008, 188, 251–258.
- Liao, H.T.; Chen, C.T. Osteogenic potential: Comparison between bone marrow and adipose-derived mesenchymal stem cells. World J. Stem Cells 2014, 6, 288.
- Wang, J.; Cai, X.; Wang, Z.; Xu, Q.; Li, K.; Hua, C. Isolation and identification of exosomes from human adipose-derived mesenchymal stem cells. Chin. J. Tissue Eng. Res. 2019, 23, 2651.
- Cotten, C.M.; Murtha, A.P.; Goldberg, R.N.; Grotegut, C.A.; Smith, P.B.; Goldstein, R.F.; Fisher, K.A.; Gustafson, K.E.; Waters-Pick, B.; Swamy, G.K.; et al. Feasibility of autologous cord blood cells for infants with hypoxic-ischemic encephalopathy. J. Pediatr. 2014, 164, 973–979.
- Vendrame, M.; Gemma, C.; Mesquita, D.D.; Collier, L.; Bickford, P.C.; Sanberg, C.D.; Sanberg, P.R.; Pennypacker, K.R.; Willing, A.E. Anti-inflammatory effects of human cord blood cells in a rat model of stroke. Stem Cells Dev. 2005, 14, 595–604.
- Harris, D.T.; Badowski, M.; Ahmad, N.; Gaballa, M.A. The potential of cord blood stem cells for use in regenerative medicine. Expert Opin. Biol. Ther. 2007, 7, 1311–1322.
- Taguchi, A.; Soma, T.; Tanaka, H.; Kanda, T.; Nishimura, H.; Yoshikawa, H.; Tsukamoto, Y.; Iso, H.; Fujimori, Y.; Stern, D.M.; et al. Administration of CD34+ cells after stroke enhances neurogenesis via angiogenesisin a mouse model. J. Clin. Investig. 2004, 114, 330–338.
- Nikfarjam, S.; Rezaie, J.; Zolbanin, N.M.; Jafari, R. Mesenchymal stem cell derived-exosomes: A modern approach in translational medicine. J. Transl. Med. 2020, 18, 449.
- Kim, Y.J.; mi Yoo, S.; Park, H.H.; Lim, H.J.; Kim, Y.L.; Lee, S.; Seo, K.W.; Kang, K.S. Exosomes derived from human umbilical cord blood mesenchymal stem cells stimulates rejuvenation of human skin. Biochem. Biophys. Res. Commun. 2017, 493, 1102–1108.
- Harris, D.; He, X.; Badowski, M.; Nicols, J. Regenerative medicine of the eye: A short review. In Stem Cell Repair and Regeneration; Imperial College Press: London, UK, 2008; Volume 3, pp. 211–225.
- Laughlin, M.J.; Barker, J.; Bambach, B.; Koc, O.N.; Rizzieri, D.A.; Wagner, J.E.; Gerson, S.L.; Lazarus, H.M.; Cairo, M.; Stevens, C.E.; et al. Hematopoietic engraftment and survival in adult recipients of umbilical-cord blood from unrelated donors. N. Engl. J. Med. 2001, 344, 1815–1822.
- Moodley, Y.; Atienza, D.; Manuelpillai, U.; Samuel, C.S.; Tchongue, J.; Ilancheran, S.; Boyd, R.; Trounson, A. Human umbilical cord mesenchymal stem cells reduce fibrosis of bleomycin-induced lung injury. Am. J. Pathol. 2009, 175, 303–313.
- Undale, A.H.; Westendorf, J.J.; Yaszemski, M.J.; Khosla, S. Mesenchymal stem cells for bone repair and metabolic bone diseases. In Mayo Clinic Proceedings; Elsevier: Amsterdam, The Netherlands, 2009; Volume 84, pp. 893–902.
- Chorath, K.; Willis, M.; Morton-Gonzaba, N.; Moreira, A. Mesenchymal stem cells for sensorineural hearing loss: A systematic review of preclinical studies. Mol. Biol. Rep. 2020, 47, 4723–4736.
- Sierra-Sánchez, Á.; Montero-Vilchez, T.; Quiñones-Vico, M.I.; Sanchez-Diaz, M.; Arias-Santiago, S. Current advanced therapies based on human mesenchymal stem cells for skin diseases. Front. Cell Dev. Biol. 2021, 9, 643125.
- Broxmeyer, H.E.; Douglas, G.W.; Hangoc, G.; Cooper, S.; Bard, J.; English, D.; Arny, M.; Thomas, L.; Boyse, E.A. Human umbilical cord blood as a potential source of transplantable hematopoietic stem/progenitor cells. Proc. Natl. Acad. Sci. USA 1989, 86, 3828–3832.
- Kielpinski, G.; Prinzi, S.; Duguid, J.; Du Moulin, G. Roadmap to approval: Use of an automated sterility test method as a lot release test for Carticel®, autologous cultured chondrocytes. Cytotherapy 2005, 7, 531–541.
- Harris, D.; Schumacher, M.; Rychlik, S.; Booth, A.; Acevedo, A.; Rubinstein, P.; Bard, J.; Boyse, E. Collection, separation and cryopreservation of umbilical cord blood for use in transplantation. Bone Marrow Transplant. 1994, 13, 135–143.
- M-Reboredo, N.; Díaz, A.; Castro, A.; Villaescusa, R. Collection, processing and cryopreservation of umbilical cord blood for unrelated transplantation. Bone Marrow Transplant. 2000, 26, 1263–1270.
- Mousavi, S.H.; Zarrabi, M.; Abroun, S.; Ahmadipanah, M.; Abbaspanah, B. Umbilical cord blood quality and quantity: Collection up to transplantation. Asian J. Transfus. Sci. 2019, 13, 79.
- Fung, M.K.; Eder, A.; Spitalnik, S.L.; Westhoff, C.M. Technical Manual; AABB: Bethesda, MD, USA, 2017.
- Solves, P.; Moraga, R.; Saucedo, E.; Perales, A.; Soler, M.; Larrea, L.; Mirabet, V.; Planelles, D.; Carbonell-Uberos, F.; Monleon, J.; et al. Comparison between two strategies for umbilical cord blood collection. Bone Marrow Transplant. 2003, 31, 269–273.
- Tamburini, A.; Malerba, C.; Picardi, A.; Amadori, S.; Calugi, A. Placental/umbilical cord blood: Experience of St. Eugenio Hospital collection center. Transplant. Proc. 2005, 37, 2670–2672.
- Nakagawa, R.; Watanabe, T.; Kawano, Y.; Kanai, S.; Suzuya, H.; Kaneko, M.; Watanabe, H.; Okamoto, Y.; Kuroda, Y.; Nakayama, T.; et al. Analysis of maternal and neonatal factors that influence the nucleated and CD34+ cell yield for cord blood banking. Transfusion 2004, 44, 262–267.
- Jan, R.H.; Wen, S.H.; Shyr, M.H.; Chiang, B.L. Impact of maternal and neonatal factors on CD34+ cell count, total nucleated cells, and volume of cord blood. Pediatr. Transplant. 2008, 12, 868–873.
- Bassiouny, M.; El-Chennawi, F.; Mansour, A.; Yahia, S.; Darwish, A. Optimal method for collection of umbilical cord blood: An E gyptian trial for a public cord blood bank. Transfusion 2015, 55, 1263–1268.
- Harris, D.T. Cord blood banking for transplantation. Can. J. Clin. Med. 1997, 4, 4–12.
- Solves, P.; Mirabet, V.; Blanquer, A.; Delgado-Rosas, F.; Planelles, D.; Andrade, M.; Carbonell-Uberos, F.; Soler, M.A.; Roig, R. A new automatic device for routine cord blood banking: Critical analysis of different volume reduction methodologies. Cytotherapy 2009, 11, 1101–1107.
- Solves, P.; Mirabet, V.; Roig, R. Volume reduction in routine cord blood banking. Curr. Stem Cell Res. Ther. 2010, 5, 362–366.
- Akel, S.; Regan, D.; Wall, D.; Petz, L.; McCullough, J. Current thawing and infusion practice of cryopreserved cord blood: The impact on graft quality, recipient safety, and transplantation outcomes. Transfusion 2014, 54, 2997–3009.
- Roura, S.; Pujal, J.M.; Gálvez-Montón, C.; Bayes-Genis, A. The role and potential of umbilical cord blood in an era of new therapies: A review. Stem Cell Res. Ther. 2015, 6, 123.
- Hunt, C.J. Cryopreservation: Vitrification and controlled rate cooling. Stem Cell Bank. 2017, 1590, 41–77.
- Lane, T.; Plunkett, M.; Buenviaje, J.; Law, P.; Wu, L.; Patterson, H. Recovery of leukocytes in cord blood units after cryopreservation by controlled rate freeze in DMSO and storage in vapor phase liquid nitrogen. In Proceedings of the Blood; The American Society of Hematology: Washington, DC, USA, 2001; Volume 98, p. 180A.
- Lysak, D.; Brychtová, M.; Leba, M.; Čedíková, M.; Georgiev, D.; Jindra, P.; Vlas, T.; Holubova, M. Long-Term Cryopreservation Does Not Affect Quality of Peripheral Blood Stem Cell Grafts: A Comparative Study of Native, Short-Term and Long-Term Cryopreserved Haematopoietic Stem Cells. Cell Transplant. 2021, 30, 09636897211036004.
- Rubinstein, P.; Dobrila, L.; Rosenfield, R.E.; Adamson, J.W.; Migliaccio, G.; Migliaccio, A.R.; Taylor, P.E.; Stevens, C.E. Processing and cryopreservation of placental/umbilical cord blood for unrelated bone marrow reconstitution. Proc. Natl. Acad. Sci. USA 1995, 92, 10119–10122.
- Broxmeyer, H.E.; Lee, M.R.; Hangoc, G.; Cooper, S.; Prasain, N.; Kim, Y.J.; Mallett, C.; Ye, Z.; Witting, S.; Cornetta, K.; et al. Hematopoietic stem/progenitor cells, generation of induced pluripotent stem cells, and isolation of endothelial progenitors from 21-to 23.5-year cryopreserved cord blood. Blood J. Am. Soc. Hematol. 2011, 117, 4773–4777.
- Miura, J.; Minegishi, M.; Itoh, T.; Kitaura, T.; Fukawa, N.; Takahashi, H.; Suzuki, A.; Kudo, Y.; Narita, A.; Sato, Y.; et al. Quality evaluation of umbilical cord blood progenitor cells cryopreserved with a small-scale automated liquid nitrogen system. Cryobiology 2008, 57, 178–181.
- Young, W. Plasma-depleted versus red cell-reduced umbilical cord blood. Cell Transplant. 2014, 23, 407–415.
- Chow, R.; Lin, A.; Tonai, R.; Bolanos, R.; Connor, C.; Mendoza, A.; Heminger, R.; Chow, M.; Ho, E.; Kang, J.; et al. Cell recovery comparison between plasma depletion/reduction-and red cell reduction-processing of umbilical cord blood. Cytotherapy 2011, 13, 1105–1119.
- Barker, J.N.; Weisdorf, D.J.; DeFor, T.E.; Blazar, B.R.; McGlave, P.B.; Miller, J.S.; Verfaillie, C.M.; Wagner, J.E. Transplantation of 2 partially HLA-matched umbilical cord blood units to enhance engraftment in adults with hematologic malignancy. Blood 2005, 105, 1343–1347.
- Scaradavou, A.; Brunstein, C.G.; Eapen, M.; Le-Rademacher, J.; Barker, J.N.; Chao, N.; Cutler, C.; Delaney, C.; Kan, F.; Isola, L.; et al. Double unit grafts successfully extend the application of umbilical cord blood transplantation in adults with acute leukemia. Blood J. Am. Soc. Hematol. 2013, 121, 752–758.
- Murdoch, B.; Marcon, A.R.; Caulfield, T. The law and problematic marketing by private umbilical cord blood banks. BMC Med. Ethics 2020, 21, 52.
- Shearer, W.T.; Lubin, B.H.; Cairo, M.S.; Notarangelo, L.D.; Hord, J.; Crouch, G.; Hale, G.; Harper, J.; Lipton, J.; Rogers, Z.; et al. Cord blood banking for potential future transplantation. Pediatrics 2017, 140, e20172695.
- Gerdfaramarzi, M.S.; Bazmi, S.; Kiani, M.; Afshar, L.; Fadavi, M.; Enjoo, S.A. Ethical challenges of cord blood banks: A scoping review. J. Med. Life 2022, 15, 735.
- Petrini, C. Umbilical cord blood collection, storage and use: Ethical issues. Blood Transfus. 2010, 8, 139.




