Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Felice Sorrentino | -- | 3029 | 2023-08-30 14:47:35 | | | |
| 2 | Jason Zhu | Meta information modification | 3029 | 2023-08-31 03:23:53 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Pados, G.; Gordts, S.; Sorrentino, F.; Nisolle, M.; Nappi, L.; Daniilidis, A. Adenomyosis and Infertility. Encyclopedia. Available online: https://encyclopedia.pub/entry/48648 (accessed on 08 February 2026).
Pados G, Gordts S, Sorrentino F, Nisolle M, Nappi L, Daniilidis A. Adenomyosis and Infertility. Encyclopedia. Available at: https://encyclopedia.pub/entry/48648. Accessed February 08, 2026.
Pados, George, Stephan Gordts, Felice Sorrentino, Michelle Nisolle, Luigi Nappi, Angelos Daniilidis. "Adenomyosis and Infertility" Encyclopedia, https://encyclopedia.pub/entry/48648 (accessed February 08, 2026).
Pados, G., Gordts, S., Sorrentino, F., Nisolle, M., Nappi, L., & Daniilidis, A. (2023, August 30). Adenomyosis and Infertility. In Encyclopedia. https://encyclopedia.pub/entry/48648
Pados, George, et al. "Adenomyosis and Infertility." Encyclopedia. Web. 30 August, 2023.
Copy Citation
Adenomyosis (the presence of ectopic endometrial glands and stroma below the endometrial–myometrial junction) is a benign condition which is increasingly diagnosed in younger women suffering from infertility. Adenomyosis is a common gynecological disorder, affecting women of reproductive age. It negatively affects in vitro fertilization, pregnancy and the live birth rate, as well as increases the risk of miscarriage. With the advent of non-invasive diagnoses with MRI and TVUS, the role of adenomyosis in infertility has been better recognized.
adenomyosis
diagnosis
classification
pathogenesis
1. Introduction
Adenomyosis is a benign condition of the uterus. It is characterized by the foci of the endometrial tissue invading the myometrium at a depth of at least 2.5 mm below the basal layer of the endometrium, which is typically surrounded by hyperplastic tissue. This can lead to the enlargement of the uterus. In addition, lymphatic and vascular channels penetrate the normal myometrium [1]. The symptomatology of adenomyosis typically includes chronic pelvic pain, dysmenorrhea, heavy menstrual periods, and infertility. Additionally, adenomyosis is associated with a greater incidence of anxiety, depression, and psychosocial stress [2]. However, approximately one-third of women with adenomyosis are asymptomatic. The etiology of adenomyosis is still uncertain, with many theories proposed [1][3][4][5][6][7]. A definite diagnosis of adenomyosis is made from histological examinations after hysterectomy as standard diagnostic criteria are still lacking when using imaging techniques such as a transvaginal ultrasound scan (TVUS) or magnetic resonance imaging (MRI). Thus, the prevalence of this disease has been reported with different ranges in many studies [2]. A majority of adenomyosis cases are reported in women in the fourth and fifth decades of life, while 5–25% of cases are patients younger than 39 years [6]. Recent studies have described the co-existence of adenomyosis with other pathologies such as leiomyomata (35–55% of cases) and endometriosis (65–70% of cases) [1][8]. The most common risk factors for adenomyosis are multiparity, an age of more than 40 years, and previous cesarean section or uterine surgery. However, recently, the disease has been increasingly diagnosed in younger women suffering from infertility [9]. In addition, a significant impact of adenomyosis on assisted reproductive technology outcomes has been reported [10].
2. Pathophysiology and Prevalence
The etiology of adenomyosis is still not clear as the exact underlying pathogenetic mechanisms are not completely known. Endometrial glands and stroma tissue are present in the myometrium. However, at least four theories have been proposed in the recent literature that try to explain a possible pathogenesis of the disease [1][3][4][5][6][7]. The most popular theory is based on invagination of the endometrial basalis into the myometrium [1]. This can be related to the weakening of the myometrium due to previous trauma, allowing endometrial growth into the injured mucosa and stromal invasion into the inner layer of the myometrium with glandular invasion, or it can be related to an abnormal immune phenomenon involving the local production of estrogen by adenomyotic tissue, activation of macrophages and B and T cells, and production of antibodies and stimulation of cytokines. Aromatase and estrogen enzymes are present in adenomyotic tissue leading to the local production of estrogens, which might enhance the growth and expansion of the endometriotic glands and stroma into the affected myometrium. A second theory describes a de novo origin of adenomyosis from misplaced pluripotent Müllerian remnants and it is supported by studies of the proliferative and biological properties of ectopic and eutopic endometrium that demonstrate distinct characteristics. Ectopic endometrium does not have the same response in hormonal changes. Secretory changes are limited, and cyclic properties are not similar with eutopic endometrium. Biological characteristics and changes within the myometrium and expression of growth factors and cytokines seem to be completely different in adenomyosis. All these support the theory that adenomyosis has a different origin from eutopic endometrium other than basal endometrium [1][3][4][5][6][7]. A third theory suggests that invagination of the myometrial basalis proceeds along the myometrial lymphatic system, leading to adenomyosis [1][3][4][5][6][7]. Finally, a recently proposed theory purported that adenomyosis originates from bone marrow stem cells, and it provided data supporting that bone-marrow-derived stem cells contribute to the regeneration of the endometrium. This theory suggests that bone-marrow-derived stem cells might also have a contribution to the formation of the new endometrium and also repopulation of areas of myometrium leading to local proliferation of endometrial glands and stromatic tissue [1][3][4][5][6][7].
The prevalence of the disease varies from 5% to 70%. This discrepancy is strongly related to the presence of different diagnostic classification systems that lack uniformity, as well as possible pathologist bias, but it is also related to differences in the patient populations of studies. Many papers have reported direct associations between adenomyosis and multiparity, perhaps due to the invasive nature of trophoblasts and the following invagination of the basalis layer, while others have provided data supporting higher rates of adenomyosis in women who previously underwent dilation and curettage [11][12]. On the other hand, the association between adenomyosis and previous uterine surgery is still unclear, while a higher incidence of intrinsic adenomyosis has been observed in patients with a history of previously induced abortions [13]. The diagnosis of adenomyosis appears to be more common in women between 40 and 50 years old (70–80%). Additionally, data from the recent literature have shown that the prevalence of the disease in women under 39 years old varies from 5% to 25%, while in postmenopausal woman, percentages of the disease drop down to 5–10%. [6]. Data from a recent study have shown that the diffuse type of adenomyosis is more common than the focal type, and the disease develops more often in the posterior than in the anterior wall of the uterus [14]. Recently, a new theory about the evolution and, thus, the pathogenesis of uterine adenomyosis as well as peritoneal and peripheral endometriosis has been published. Levendecker and his associates have proposed that tissue injury induced by uterine hyperperistalsis/dysperistalsis and repair (TIAR) causes adenomyosis. The TIAR hypothesis has recently been expanded to lump endometriosis and adenomyosis together as one disease, called archimetriosis [15][16][17][18]. It is evident that more than one mechanism is responsible for a cascade of changes that combine some of the above theories to explain the pathogenesis of adenomyosis.
3. Genetic and Epigenetic Alteration in Adenomyosis
Recently Konincks et al. [19] have proposed the genetic–epigenetic theory for endometriosis that can be equally applied to adenomyosis, as these two conditions share common patterns of aberrant gene expression [20][21]. These include pathways that favor increased estrogen production, decreased estrogen metabolism, estrogen receptor Beta (ER-β)-driven inflammatory process, and progesterone resistance due to decreased progesterone-receptor (PR) expression. An epithelial deficiency of the enzyme HSD17β2 can lead to aromatase overexpression in endometriotic stromal cells. The same mechanism may also be involved in adenomyotic epithelial cells, as endometriosis and adenomyosis share many molecular features. Excessive levels of local estradiol in adenomyosis may be given to estrogen excess and HSD17β2 deficiency [22]. Adenomyotic tissue appears to exhibit progesterone resistance and aberrant estrogen action regulated by ER-β with excessive production of prostaglandins that cause inflammation [23].
Cytochrome P450 (CYP) genes and catechol-O-methyltransferase (COMT) gene variants could increase the risk of an estrogen-dependent disease like adenomyosis [24].
There is an increased frequency of the C allele in the T/C and C/C genotypes of the CYP1A1 gene, A allele in the C/A and A/A genotypes of the CYP1A2 gene, and the T allele in the C/T and C/C genotypes of the CYP19 gene in patients with adenomyosis [24][25].
Epigenetic alterations have been detected in adenomyosis. Increased expression of deoxyribonucleic acid methytransferases (DNMTs) (enzymes that catalyze the transfer of a methyl group to DNA) was found in ectopic endometrium from patients with adenomyosis compared with controls [27]. Promoter hypermethylation of PR-B was detected in women with adenomyosis, leading to progesterone resistance [28].
DNA hypomethylation and increased expression of a transcription factor, CCAAT/enhancer-binding protein β were associated with the development of adenomyosis [29].
In addition to DNA methylation, the aberrant expression and localization of class I histone deacetylases (HDACs) was also detected in women with adenomyosis. Indeed, the expression of HDAC1 and HDAC3 was increased in the eutopic and ectopic endometrium of adenomyosis patients compared to controls [28]. Furthermore, the use of an HDAC inhibitor (valproic acid) is effective in treating dysmenorrhea, hyperalgesia and myometrial infiltration in patients with adenomyosis [30]. These results suggest the involvement of histone modification in the pathogenesis of adenomyosis and confirm the opinion that adenomyosis may be an epigenetic disease like endometriosis.
4. Diagnosis and Classification
Traditionally, the standard method for accurate diagnosis of adenomyosis has been hysterectomy followed by histological examination of the endometrial invasion of the underlying myometrium [9]. The presence of adenomyosis is more common in the posterior wall, less common in the anterior wall and quite rare in the cornua or in areas close to cervical os [6]. Based on histopathological examinations, adenomyosis is classified as focal if circumscribed nodules of endometrial glands and stroma surrounded by normal myometrium are found in the specimens. Diffuse adenomyosis is characterized by endometrial glands and stroma distributed throughout the myometrium. Finally, adenomyomas are considered a subgroup of focal adenomyosis surrounded by hypertrophic myometrium [9]. Many classification systems have been proposed in recent decades [12][31][32][33][34].
Unfortunately, the histological criteria used for the diagnosis and staging of adenomyosis were not uniform. In addition, in many cases, there was no correlation between the extension of the disease and the severity of the clinical symptoms, and some of the studies were biased; thus, none of the proposed classification systems has been generally accepted [6][9]. Recent technological advances in imaging techniques, such as TVUS and MRI, have provided clinicians with non-invasive methods for the diagnosis of adenomyosis. Recently, MUSA (morphological uterus sonographic assessment) has been proposed as a standardized method for recognizing the typical features of adenomyosis on an ultrasound assessment. These features include asymmetrical thickening of the uterine walls, intra-myometrial cysts or/and hyperechoic islands, fan-shaped shadowing on the myometrium, myometrial echogenic sub-endometrial lines and buds, trans-lesional vascularity, and an irregular or interrupted junctional zone (JZ). These features have been recently modified by the same group, considering the presence of features like cysts, hyperechogenic islands and/or echogenic sub-endometrial line bubs as diagnostic and all other features as suspicious for adenomyosis [35].
Three-dimensional (3D) TVUS can be used for better visualization of the junctional zone with a specificity of 81% and sensitivity of 85% [36]. Features of adenomyosis on 3D TVUS include an irregular, interrupted junctional zone, a junctional zone thickness > 8 mm, and a significant difference between maximum and minimum thickness measurements of the junctional zone > 4 mm [37]. In a recent meta-analysis, two-dimensional TVUS had a sensitivity and specificity of 83.8% and 63.9%, respectively, and three-dimensional TVUS had a pooled sensitivity and specificity for all combined imaging characteristics of 88.9% and 56.0%, respectively [9]. The accuracy and sensitivity of TVUS decreases to as low as 33% when a coexisting pathology such as fibroids is present, especially when the volume of the fibroid is significantly increased. MRI has also been proven to be very accurate in diagnosing adenomyosis, although it is a more expensive method compared to TVUS. MRI findings considered diagnostic for adenomyosis include a large asymmetric uterus, an abnormal junctional zone to myometrial thickness ratio of more than 40%, and junctional zone thickening of 8 to 12 mm. Recent prospective studies have shown a sensitivity of 77% and a specificity of 89% for MRI, while it seems more reasonable to opt for MRI when other uterine abnormalities such as fibroids are also present, with a sensitivity of 67% and a specificity of 82% [6]. The combination of both techniques offers the highest sensitivity for preoperative diagnosis [37]. Over the years, different classification systems have been proposed based on MRI or TVUS findings of adenomyosis in relation to histological and clinical findings of the disease [38][39][40][41][42][43][44].
5. Effect of Adenomyosis on Fertility
The exact mechanism that causes infertility in women diagnosed with adenomyosis remains elusive. One of the reasons for the difficulty in accurately predicting the negative effects of adenomyosis on fertility is perhaps its high correlation with endometriosis. Adenomyosis appears to destruct the normal architecture of the myometrium, leading to the impairment of the uterine mechanisms that are important for implantation and consequent conception. The disruption of the normal junctional zone may lead to abnormal contractility, thus negatively affecting implantation. Additionally, it is not clear enough if concurrent gynecological diseases like myomas could contribute negatively to fertility. Possible mechanisms through which adenomyosis causes impairment of implantation have been described in the recent literature, including anatomical distortion of the uterine cavity, disturbed uterine peristalsis and sperm transport, dysfunctional hyperperistalsis of the inner myometrium, increased intrauterine pressure, a disturbance in normal myocyte contractility with a subsequent loss of normal rhythmic contraction, altered sex steroid hormone pathways, increased inflammatory markers and oxidative stress, the reduced expression of implantation markers, a lack of expression of adhesion molecules, and altered function of the gene for embryonic development (the HOXA 10 gene) [14]. Different locations in the female genital tract and the negative impact of adenomyosis on the individual steps of reproduction are shown in Figure 1 [45].
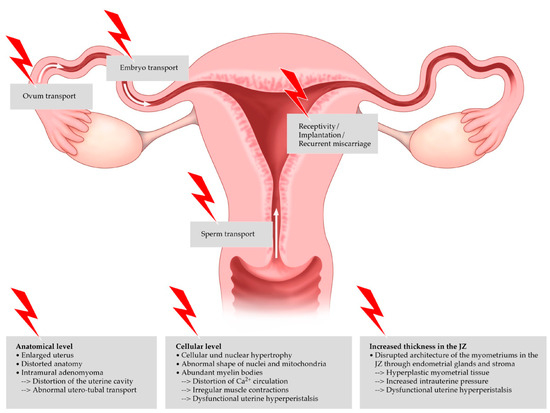
Figure 1. Negative impact of adenomyosis on the individual steps of reproduction.
Other suggested mechanisms are focused on P450 (P450arom) and mRNA expression, which seem to be present in women with adenomyosis, leading to lower clinical pregnancy rates [46]. Leukemia inhibitory factor (LIF) has been demonstrated to be dysregulated in women with adenomyosis, thus impairing implantation [47]. All these factors are hypothesized to contribute to the reduction in pregnancy rates. It has become more than obvious from the literature that adenomyosis indeed has a negative impact on fertility. Recent meta-analyses have provided data associating adenomyosis and increased risk for miscarriages, 31% in women with adenomyosis and 14.1% in non-affected women [48]. The extension and type of adenomyosis appear to be important factors that affect fertility. According to a multicenter prospective study, the presence of numerous morphological features on ultrasound worsens the reproductive outcome. Clinical pregnancy decreased from 42.7% in women with no adenomyosis to 22.9% and 13.0% in those with four and seven ultrasound diagnostic features of adenomyosis, respectively [49]. A recent cross-sectional study supported that the prevalence of adenomyosis detected de novo by a 2D-TVUS in a population of young, infertile women was 7.5%.
6. Treatment and Reproductive Outcomes
The primary indication for the treatment of adenomyosis is the presence of symptomatology negatively affecting a patient’s daily life [1]. Although the standard method of treatment for adenomyosis is hysterectomy, the use of conservative medical or surgical options offers relief of symptoms and maintenance of fertility of patients. Nonsteroidal anti-inflammatory drugs (NSAIDs) and the hormonal control of excessive cyclic bleeding are considered the first lines of conservative medical management. Unfortunately, none of the available medical therapies can treat symptoms of adenomyosis while still allowing patients to conceive [6]. Suppressive hormonal treatments such as the continuous use of oral contraceptive pills, high-dose progestins, the levonorgestrel-releasing intrauterine device (LNG-IUD), danazol, aromatase inhibitors, selective progesterone receptor modulators, and gonadotropin-releasing hormone agonist (GnRH-a) can temporarily improve symptoms and induce the regression of adenomyosis [6][50]. Recent data have shown that only GnRH-a treatment with add-back estrogen therapy can be beneficial for infertile women with adenomyosis because of its positive effect on endometrial implantation markers, leading to improved implantation rates. In addition, a reduction of lesion size and patient quality of life has been demonstrated to be another factor which might also improve chances of conception. The long-term preparation of the endometrium with GnRH-a therapy for 2 to 4 months, before frozen embryo transfer, in women with adenomyosis undergoing IVF is associated with significantly higher clinical pregnancy, implantation, and ongoing pregnancy rates [9][50][51][52][53]. Also, pre-treatment with the LNG-IUD for 3 months before embryo transfer has been proposed to improve the reproductive outcomes of patients undergoing in vitro fertilization with a significantly increased ongoing pregnancy rate (41.8% versus 29.5%). Unfortunately, there are no published RCTs available having evaluated the efficacy of GnRH agonist pre-treatment in patients with adenomyosis. The surgical treatment of adenomyosis-related infertility remains a highly controversial issue regarding the impact of surgery on reproductive outcomes. There is still a lack of consensus on the rationale for removing the pathology in order to improve fertility. Many methods and techniques such as electrocoagulation and adenomyomectomy, with or without myomectomy, have been described, either by laparoscopy, hysteroscopy, or laparotomy. Each method has its own advantages and risks. Crucial factors to be taken into account are the proper removal of the pathology, the degree of residual disease, and the methods for setting and reconstructing the uterine wall. Proper conservative surgery could be an alternative treatment for infertile women with adenomyosis as successful pregnancies have been reported in many cases. Conservative surgical treatment aims to balance the advantages of removing the affected area against the disadvantages of leaving a possibly defective uterine wall. Factors like the extent of excision of the myometrial defect, the reconstruction technique, postoperative infection and the surgeon’s experience are quite important. Even the use of electrodiathermy instead of a cold knife during the operation might affect the wound healing and integrity of the myometrium [54]. Pertinent risks after an operation include the development of abdominal and intrauterine adhesions, placenta accreta and uterine rupture, especially during the second and third trimester of pregnancy [54]. Therefore, establishing an optimum conservative surgical technique for adenomyosis is difficult, and several operative options (open or laparoscopic), surgical techniques (complete or partial adenomyomectomy), and modified surgical techniques (U-shaped suturing, overlapping flaps, the triple-flap method, and transverse H-incisions) have been proposed. Regarding safety and the future risk of uterine rupture, for 113 women treated by the triple-flap technique, 81.4% had normal blood flow, as demonstrated by Doppler, with a 31.4% pregnancy rate and no cases of uterine rupture [54]. In women who underwent conservative surgeries for infertility treatment, pregnancy rates ranged from 25.0% to 61.5% and the miscarriage rates ranged from 11.1% to 25.0% [14]. Another recent study analyzed data from 18 facilities worldwide. Conservative surgical treatment was performed on 2365 infertile women with adenomyosis, and the postoperative pregnancy rate varied between 17.5% and 72.7%. In total, 449 pregnancies were confirmed, and 363 (80.8%) resulted in deliveries. However, artificial reproductive technology (ART) largely contributed to this relatively high pregnancy rate [54]. A review from 2014 concluded that the complete excision of localized adenomyosis in younger women is associated with a 50% delivery rate, while in women older than 40 years old, pregnancy rates were very low after cytoreductive surgery [40]. Conservative surgical treatment for uterine adenomyosis is associated with a higher risk of spontaneous rupture in a future pregnancy. A literature review suggested that the risk of uterine rupture due to pregnancy, after the removal of a uterine adenomyosis, is >1.0% compared to 0.26% in pregnancies following a myomectomy [54].
References
- Aleksandrovych, V.; Basta, P.; Gil, K. Current facts constituting an understanding of the nature of adenomyosis. Adv. Clin. Exp. Med. 2019, 28, 839–846.
- Martone, S.; Centini, G.; Exacoustos, C.; Zupi, E.; Afors, K.; Zullo, F.; Maneschi, F.; Habib, N.; Lazzeri, L. Pathophysiologic mechanisms by which adenomyosis predisposes to postpartum haemorage and other obstetric complications. Med. Hypotheses 2020, 143, 109833.
- Ferenczy, A. Pathophysiology of adenomyosis. Hum. Reprod. Updat. 1998, 4, 312–322.
- Zou, Y.; Liu, F.-Y.; Wang, L.-Q.; Guo, J.-B.; Yang, B.-C.; Wan, X.-D.; Wang, F.; He, M.; Huang, O.-P. Downregulation of DNA methyltransferase 3 alpha promotes cell proliferation and invasion of ectopic endometrial stromal cells in adenomyosis. Gene 2017, 604, 41–47.
- Enatsu, A.; Harada, T.; Yoshida, S.; Iwabe, T.; Terakawa, N. Adenomyosis in a patient with the Rokitansky-Kuster-Hauser syndrome. Fertil. Steril. 2000, 73, 862–863.
- Garcia, L.; Isaacson, K. Adenomyosis: Review of the Literature. J. Minim. Invasive Gynecol. 2011, 18, 428–437.
- Zhai, J.; Vannuccini, S.; Petraglia, F.; Giudice, L.C. Adenomyosis: Mechanisms and Pathogenesis. Semin. Reprod. Med. 2020, 38, 129–143.
- Tan, J.; Yong, P.; Bedaiwy, M.A. A critical review of recent advances in the diagnosis, classification, and management of uterine adenomyosis. Curr. Opin. Obstet. Gynecol. 2019, 31, 212–221.
- Vannuccini, S.; Petraglia, F. Recent advances in understanding and managing adenomyosis. F1000Research 2019, 8, 283.
- Abu Hashim, A.; Elaraby, S.; Fouda, A.; El Rakhawy, M.E. The prevalence of adenomyosis in an infertile population: A cross-sectional study. Reprod. Biomed. Online 2020, 40960, 842–850.
- Vercellini, P.; Parazzini, F.; Oldani, S.; Panazza, S.; Bramante, T.; Crosignani, P.G. Surgery: Adenomyosis at hysterectomy: A study on frequency distribution and patient characteristics. Hum. Reprod. 1995, 10, 1160–1162.
- Levgur, M.; Abdai, M.A.; Tucker, A. Adenomyosis: Symptoms, histology, and pregnancy terminations. Obstet. Gynecol. 2000, 95, 688–691.
- Kobayashi, H.; Matsubara, S.; Imanaka, S. Clinicopathological features of different subtypes in adenomyosis: Focus on early lesions. PLoS ONE 2021, 16, e0254147.
- Tamura, H.; Kishi, H.; Kitade, M.; Asai-Sato, M.; Tanaka, A.; Murakami, T.; Minegishi, T.; Sugino, N. Clinical outcomes of infertility treatment for women with adenomyosis in Japan. Reprod. Med. Biol. 2017, 16, 276–282.
- Leyendecker, G.; Wildt, L.; Mall, G. The pathophysiology of endometriosis and adenomyosis: Tissue injury and repair. Arch. Gynecol. Obstet. 2009, 280, 529–538.
- Leyendecker, G.; Wildt, L. A new concept of endometriosis and adenomyosis: Tissue injury and repair (TIAR). Horm. Mol. Biol. Clin. Investig. 2011, 5, 125–142.
- Leyendecker, G.; Bilgicyildirim, A.; Inacker, M.; Stalf, T.; Huppert, P.; Mall, G.; Böttcher, B.; Wildt, L. Adenomyosis and endometriosis. Re-visiting their association and further insights into the mechanisms of auto-traumatisation. An MRI study. Arch. Gynecol. Obstet. 2015, 291, 917–932.
- Leyendecker, G.; Wildt, L.; Laschke, M.W.; Mall, G. Archimetrosis: The evolution of a disease and its extant presentation: Pathogenesis and pathophysiology of archimetrosis (uterine adenomyosis and endometriosis). Arch. Gynecol. Obstet. 2022, 21, 93–112.
- Koninckx, P.R.; Ussia, A.; Adamyan, L.; Wattiez, A.; Gomel, V.; Martin, D.C. Pathogenesis of endometriosis: The genet-ic/epigenetic theory. Fertil. Steril. 2019, 111, 327–340.
- Benagiano, G.; Brosens, I. Adenomyosis and endometriosis have a common origin. J. Obstet. Gynecol. India 2011, 61, 146–152.
- Vannuccini, S.; Tosti, C.; Carmona, F.; Huang, S.J.; Chapron, C.; Guo, S.W.; Petraglia, F. Pathogenesis of adenomyosis: An update on molecular mechanisms. Reprod Biomed Online 2017, 35, 592–601.
- Bulun, S.E.; Yilmaz, B.D.; Sison, C.; Miyazaki, K.; Bernardi, L.; Liu, S.; Kohlmeier, A.; Yin, P.; Milad, M.; Wei, J. Endometriosis. Endocr. Rev. 2019, 40, 1048–1079.
- Artymuk, N.; Zotova, O.; Gulyaeva, L. Adenomyosis: Genetics of estrogen metabolism. Horm. Mol. Biol. Clin. Investig. 2019, 37, 37.
- Tong, X.; Li, Z.; Wu, Y.; Fu, X.; Zhang, Y.; Fan, H. COMT 158G/A and CYP1B1 432C/G polymorphisms increase the risk of endometriosis and adenomyosis: A meta-analysis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2014, 179, 17–21.
- Guo, S.-W. Epigenetics of endometriosis. Mol. Hum. Reprod. 2009, 15, 587–607.
- Gordts, S.; Koninckx, P.; Brosens, I. Pathogenesis of deep endometriosis. Fertil. Steril. 2017, 108, 872–885.e1.
- Liu, X.; Guo, S.-W. Aberrant immunoreactivity of deoxyribonucleic acid methyltransferases in adenomyosis. Gynecol. Obstet. Investig. 2012, 74, 100–108.
- Nie, J.; Liu, X.; Guo, S.-W. Promoter Hypermethylation of Progesterone Receptor Isoform B (PR-B) in Adenomyosis and Its Rectification by a Histone Deacetylase Inhibitor and a Demethylation Agent. Reprod. Sci. 2010, 17, 995–1005.
- Xiang, Y.; Sun, Y.; Yang, B.; Yang, Y.; Zhang, Y.; Yu, T.; Huang, H.; Zhang, J.; Xu, H. Transcriptome sequencing of adenomyosis eutopic endometrium: A new insight into its pathophysiology. J. Cell. Mol. Med. 2019, 23, 8381–8391.
- Liu, X.; Guo, S.-W. Valproic acid alleviates generalized hyperalgesia in mice with induced adenomyosis. J. Obstet. Gynaecol. Res. 2011, 37, 696–708.
- Bird, C.C.; McElin, T.W.; Manalo-Estrella, P. The elusive adenomyosis of the uterus—Revisited. Am. J. Obstet. Gynecol. 1972, 112, 583–593.
- Hulka, C.A.; Hall, D.A.; McCarthy, K.; Simeone, J. Sonographic Findings in Patients with Adenomyosis: Can Sonography Assist in Predicting Extent of Disease? Am. J. Roentgenol. 2002, 179, 379–383.
- Sammour, A.; Pirwany, I.; Usubutun, A.; Arseneau, J.; Tulandi, T. Correlations between Extent and Spread of Adenomyosis and Clinical Symptoms. Gynecol. Obstet. Investig. 2002, 54, 213–216.
- Vercellini, P.; Viganò, P.; Somigliana, E.; Daguati, R.; Abbiati, A.; Fedele, L. Adenomyosis: Epidemiological factors. Best Pract. Res. Clin. Obstet. Gynaecol. 2006, 20, 465–477.
- Harmen, M.J.; Van den Bosch, T.; De Leeuw, R.A.; Dueholm, M.; Exacoustos, C.; Valentin, L.; Hehenkamp, W.J.K.; Groenman, F.; Uyn, C.D.E.; Rasmussen, C.; et al. Consensus on revised definitions of Morphological Uterus Sonographic Assessment (MUSA) features of adenomyosis: Results of modified Delphi procedure. Ultrasound Obstet. Gynecol. 2022, 60, 118–131.
- Marques, A.L.S.; Andres, M.P.; Mattos, L.A.; Gonçalves, M.O.; Baracat, E.C.; Abrão, M.S. Association of 2D and 3D transvaginal ultrasound findings with adenomyosis in symptomatic women of reproductive age: A prospective study. Clinics 2021, 76, e2981.
- Tellum, T.; Nygaard, S.; Lieng, M. Noninvasive Diagnosis of Adenomyosis: A Structured Review and Meta-analysis of Diagnostic Accuracy in Imaging. J. Minim. Invasive Gynecol. 2020, 27, 408–418.e3.
- Gordts, S.; Brosens, J.J.; Fusi, L.; Benagiano, G.; Brosens, I. Uterine adenomyosis: A need for uniform terminology and consensus classification. Reprod. Biomed. Online 2008, 17, 244–248.
- Kishi, Y.; Suginami, H.; Kuramori, R.; Yabuta, M.; Suginami, R.; Taniguchi, F. Four subtypes of adenomyosis assessed by magnetic resonance imaging and their specification. Am. J. Obstet. Gynecol. 2012, 207, 114.e1–114.e7.
- Grimbizis, G.F.; Mikos, T.; Tarlatzis, B. Uterus-sparing operative treatment for adenomyosis. Fertil. Steril. 2014, 101, 472–487.e8.
- Bazot, M.; Daraï, E. Role of transvaginal sonography and magnetic resonance imaging in the diagnosis of uterine adenomyosis. Fertil. Steril. 2018, 109, 389–397.
- Lazzeri, L.; Morosetti, G.; Centini, G.; Monti, G.; Zupi, E.; Piccione, E.; Exacoustos, C. A sonographic classification of adenomyosis: Interobserver reproducibility in the evaluation of type and degree of the myometrial involvement. Fertil. Steril. 2018, 110, 1154–1161.e3.
- Van den Bosch, T.; De Bruin, A.M.; De Leeuw, R.A.; Dueholm, M.; Exacoustos, C.; Valentin, L.; Bourne, T.; Timmerman, D.; Huirne, J.A.F. Sonographic classification and reporting system for diagnosing adenomyosis. Ultrasound Obstet Gynecol. 2019, 53, 576–582.
- Exacoustos, C.; Morosetti, G.; Conway, F.; Camilli, S.; Martire, F.G.; Lazzeri, L.; Piccione, E.; Zupi, E. New Sonographic Classification of Adenomyosis: Do Type and Degree of Adenomyosis Correlate to Severity of Symptoms? J. Minim. Invasive Gynecol. 2020, 27, 1308–1315.
- Günther, V.; Allahqoli, L.; Gitas, G.; Maass, N.; Tesch, K.; Ackermann, J.; Rosam, P.; Mettler, L.; von Otte, S.; Alkatout, I. Impact of Adenomyosis on Infertile Patients—Therapy Options and Reproductive Outcomes. Biomedicines 2022, 10, 3245.
- Brosens, J.; Verhoeven, H.; Campo, R.; Gianaroli, L.; Gordts, S.; Hazekamp, J.; Hägglund, L.; Mardesic, T.; Varila, E.; Zech, J.; et al. High endometrial aromatase P450 mRNA expression is associated with poor IVF outcome. Hum. Reprod. 2004, 19, 352–356.
- Xiao, Y.; Sun, X.; Yang, X.; Zhang, J.; Xue, Q.; Cai, B.; Zhou, Y. Leukemia inhibitory factor is dysregulated in the endometrium and uterine flushing fluid of patients with adenomyosis during implantation window. Fertil. Steril. 2010, 94, 85–89.
- Vercellini, P.; Consonni, D.; Dridi, D.; Bracco, B.; Frattaruolo, M.P.; Somigliana, E. Uterine adenomyosis and in vitro fertilization outcome: A systematic review and meta-analysis. Hum. Reprod. 2014, 29, 964–977.
- Mavrelos, D.; Holland, T.K.; O’Donovan, O.; Khalil, M.; Ploumpidis, G.; Jurkovic, D.; Khalaf, Y. The impact of adenomyosis on the outcome of IVF–embryo transfer. Reprod. Biomed. Online 2017, 35, 549–554.
- Dueholm, M.; Aagaard, J. Adenomyosis and IVF/ICSI treatment: Clinical considerations and recommendations. Expert Rev. Endocrinol. Metab. 2018, 13, 177–179.
- Younes, G.; Tulandi, T. Effects of adenomyosis on in vitro fertilization treatment outcomes: A meta-analysis. Fertil. Steril. 2017, 108, 483–490.e3.
- Nirgianakis, K.; Kalaitzopoulos, D.R.; Schwartz, A.S.K.; Spaanderman, M.; Kramer, B.W.; Mueller, M.D.; Mueller, M. Fertility, pregnancy and neonatal outcomes of patients with adenomyosis: A systematic review and meta-analysis. Reprod. Biomed. Online 2021, 42, 185–206.
- Zhang, X.-P.; Zhang, Y.-F.; Shi, R.; Zhang, Y.-J.; Zhang, X.-L.; Hu, X.-M.; Hu, X.-Y.; Hu, Y.-J. Pregnancy outcomes of infertile women with ultrasound-diagnosed adenomyosis for in vitro fertilization and frozen–thawed embryo transfer. Arch. Gynecol. Obstet. 2021, 304, 1089–1096.
- Osada, H. Uterine adenomyosis and adenomyoma: The surgical approach. Fertil. Steril. 2018, 109, 406–417.
More
Information
Subjects:
Obstetrics & Gynaecology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
549
Revisions:
2 times
(View History)
Update Date:
31 Aug 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




