Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Giulia Magnani | -- | 2923 | 2023-08-30 11:51:41 | | | |
| 2 | Catherine Yang | Meta information modification | 2923 | 2023-08-31 03:09:27 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Magnani, G. Antithrombotic Therapy in Polyvascular Disease. Encyclopedia. Available online: https://encyclopedia.pub/entry/48640 (accessed on 08 February 2026).
Magnani G. Antithrombotic Therapy in Polyvascular Disease. Encyclopedia. Available at: https://encyclopedia.pub/entry/48640. Accessed February 08, 2026.
Magnani, Giulia. "Antithrombotic Therapy in Polyvascular Disease" Encyclopedia, https://encyclopedia.pub/entry/48640 (accessed February 08, 2026).
Magnani, G. (2023, August 30). Antithrombotic Therapy in Polyvascular Disease. In Encyclopedia. https://encyclopedia.pub/entry/48640
Magnani, Giulia. "Antithrombotic Therapy in Polyvascular Disease." Encyclopedia. Web. 30 August, 2023.
Copy Citation
Patients presenting with atherosclerosis involving more than one vascular bed, such as those with peripheral artery disease (PAD) and concomitant coronary artery disease (CAD), constitute a particularly vulnerable group characterized by enhanced residual long-term risk for major adverse cardiac events (MACE), as well as major adverse limb events (MALE). Antithrombotic therapy is the mainstay of secondary prevention in both patients with PAD or CAD; however, the optimal intensity of such therapy is still a topic of debate, particularly in the post-acute and long-term setting.
coronary artery disease
peripheral artery disease
polyvascular disease
anti-thrombotic therapy
1. Single Antiplatelet Strategy
Single antiplatelet therapy with aspirin is still the most frequently used antiplatelet drug in both chronic CAD and PAD, with a class I recommendation, level of evidence A, in secondary prevention of CCS and in both symptomatic carotid and lower-extremity PAD (LE-PAD) patients [1][2][3][4]. Clopidogrel monotherapy has been studied in the Clopidogrel versus Aspirin in Patients at Risk of Ischemic Events (CAPRIE) enrolling a broad population with stable atherosclerotic cardiovascular disease (ASCVD). In this trial, clopidogrel monotherapy reduced ischemic events compared to aspirin monotherapy [5]. In a post hoc analysis of patients with symptomatic PAD, clopidogrel was associated with an additional one fourth risk reduction in MACE compared to aspirin (HR 0.76, 95% CI: 0.64–0.91, p = 0.003) [6] and the European PAD guidelines suggest clopidogrel as the preferred antiplatelet drug (Class IIb, Level of evidence C), whereas the ACC/AHA guidelines did not make a preference statement between agents [2][7]. Although the efficacy of clopidogrel has been confirmed in an extensive meta-analysis including PAD patients who underwent revascularization [8], more recent evidence did not corroborate the superiority of clopidogrel over aspirin as SAPT in PAD patients [9]. This may be partially traced back to the so called “antiplatelet non-responsiveness”, a condition of high on-treatment platelet reactivity present in up to 50% of patients with critical limb ischemia and associated to a significantly increased CV events risk [10]. More recently, in the Examining Use of Ticagrelor in Peripheral Artery Disease (EUCLID) trial, enrolling 13,185 patients with symptomatic PAD, more potent P2Y12 inhibition with ticagrelor monotherapy, did not reduce MACE (HR 1.02, 95% CI: 0.92–1.13, p = 0.65) or lower-limb revascularization (HR 0.95, 95% CI: 0.87–1.05, p = 0.30) compared to clopidogrel, with a similar rate of major bleeding (HR 1.10, 95% CI: 0.84–1.43, p = 0.49) [11]. The use of SAPT with ticagrelor may, therefore, be reserved to PAD patients who are non-responders to clopidogrel. Although neutral, the EUCLID trial is of clinical utility, raising awareness of a higher CV risk in the subgroup of PAD patients with concomitant CAD (n = 4032, 29%), that had higher composite rates of CV death, MI, and ischemic stroke (15.3% vs. 8.9%, HR 1.50, 95% CI: 1.13–1.99; p = 0.005) compared to those without CAD (Figure 1, Panel B).
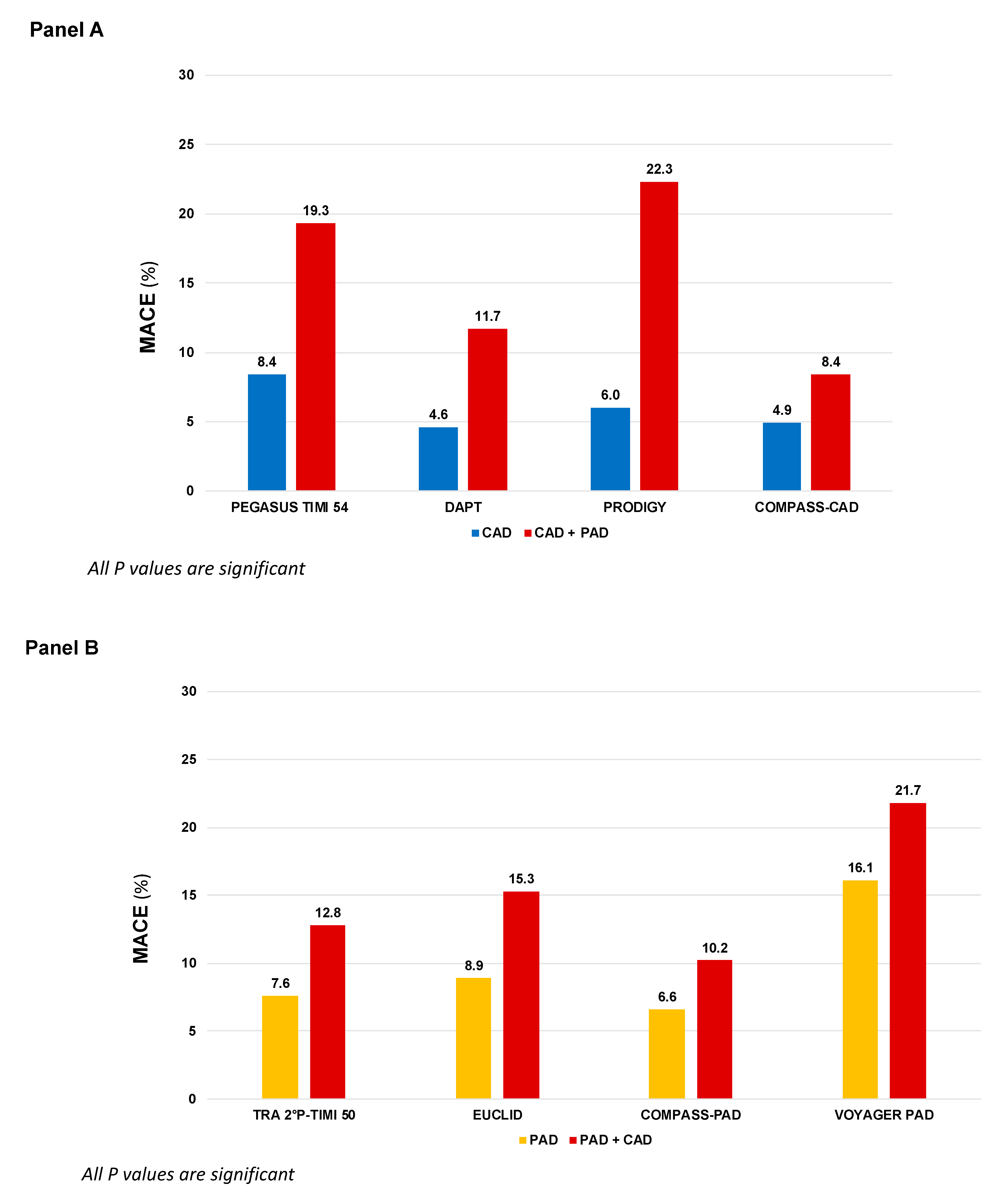
Figure 1. MACE incidence according to different antithrombotic strategies in patients randomized in large clinical trials primarily based on coronary artery disease (Panel A) or peripheral artery disease (Panel B). COMPASS: Cardiovascular Outcomes for People Using Anticoagulation Strategies; CAD: Coronary artery disease; PAD: Peripheral artery disease; DAPT: Dual antiplatelet therapy; EUCLID: Examining Use of Ticagrelor in Peripheral Artery Disease; PEGASUS-TIMI 54: Prevention of Cardiovascular Events in Patients with Prior Heart Attack Using Ticagrelor Compared to Placebo on a Background of Aspirin–Thrombolysis in Myocardial Infarction 54; TRA 2P–TIMI 50: Thrombin Receptor Antagonist in Secondary Prevention of Atherothrombotic Ischemic Events—Thrombolysis in Myocardial Infarction 50; PRODIGY: Prolonging Dual Antiplatelet Treatment After Grading Stent-Induced Intimal Hyperplasia Study; VOYAGER-PAD: Vascular Outcomes Study of ASA (acetylsalicylic acid) Along with Rivaroxaban in Endo-vascular or Surgical Limb Revascularization for PAD.
2. Dual Antiplatelet Therapy
Aspirin, through the inhibition of the cyclooxygenase 1, and prasugrel, ticagrelor or clopidogrel, blocking the platelet receptor for adenosine diphosphate P2Y12, reduce the production of thromboxane A2 and inhibit platelets activation and aggregation [12]. DAPT is the standard therapy in the acute phase post-ACS; however, duration and type of treatment in patients with stable CAD or PAD is still subject of ongoing debate.
In the Clopidogrel for High Atherothrombotic Risk and Ischemic Stabilization Management and Avoidance (CHARISMA) trial, 15,603 patients with established CV disease or high-risk profile were randomly assigned either to DAPT with aspirin and clopidogrel or aspirin alone. Although the overall trial was neutral, in the subgroup of patients with prior MI, the addition of clopidogrel to aspirin significantly reduced the primary endpoint of CV death, MI, or stroke (HR 0.77, 95% CI: 0.62–0.98; p = 0.03) [13]. In LE-PAD patients (n = 3096), MACE were not significantly reduced (HR 0.85, 95% CI: 0.66–1.08; p = 0.18), and there was a numerically lower rate of peripheral arterial bypass surgery (p = 0.07) with similar risk of leg amputation, at the cost of increased moderate bleeding (HR 1.36, 95% CI: 1.03–1.79; p = 0.03). Asymptomatic carotid artery disease was an inclusion criterion in 7% of patients enrolled and no benefit was observed between DAPT and SAPT [14]. The use of DAPT with ticagrelor, in patients with prior MI, was examined in the Prevention of Cardiovascular Events TIMI 54 in Patients with Prior Heart Attack Using Ticagrelor Compared to Placebo on a Background of Aspirin-Thrombolysis in Myocardial Infarction 54 (PEGASUS-TIMI 54). Patients with prior MI and PAD present a double CV risk than those with PAD alone (8.4% vs. 19.3%, placebo group, p < 0.001, Figure 1, Panel A). The combination of aspirin and ticagrelor 60 mg twice daily (the approved dose) compared to aspirin alone, provided in 1143 patients with concomitant PAD an absolute risk reduction for MACE of 4.1% and a significant reduction in risk of MALE (HR 0.65, 95% CI: 0.44–0.95, p = 0.026) with no differences in TIMI major bleeding (HR 1.18, 95% CI: 0.29–4.70; p = 0.82) compared to patients without PAD [15]. Subgroup analyses from the Dual Antiplatelet Therapy (DAPT) and Prolonging Dual Antiplatelet Treatment After Grading Stent-Induced Intimal Hyperplasia Study (PRODIGY) trials provided information regarding extended use of DAPT after coronary stenting in patients with PAD. In the DAPT trial, extended DAPT (clopidogrel or prasugrel plus aspirin for 30 vs. 12 months) was associated with consistent ischemic benefit among patients with (HR: 0.63; 95% CI: 0.32–1.22) and without PAD (HR: 0.53; 95% CI: 0.42–0.66, p for interaction = 0.63) [16], while in the PRODIGY trial, there was a significant interaction based on PAD. Prolonged versus shorter DAPT duration was associated with a greater reduction in MACE in patients with PAD (16.1% vs. 27.3%; HR, 0.54; 95% CI, 0.31–0.95; p = 0.03) compared with those without PAD (9.3% vs. 7.4%; HR, 1.28; 95% CI, 0.92–1.77; p = 0.15, p for interaction 0.01) [17]. Based on this overall evidence, the current guidelines for CCS recommend a prolonged DAPT regimen in post-MI patients with a high risk of ischemic events, such as those with concomitant PAD, and without high bleeding risk, if they have tolerated the DAPT regimen for 1 year [1].
Finally, the inhibition thrombin-induced platelet aggregation through PAR-1 with vorapaxar has been investigated in the Thrombin Receptor Antagonist in Secondary Prevention of Atherothrombotic Ischemic Events—Thrombolysis in Myocardial Infarction (TRA-2°P-TIMI 50) which enrolled a broad population of patients with ASCVD [18]. Consistently with the other studies, patients with both CAD and PAD presented with higher risk of MACE (12.8% vs. 7.6%). More interestingly, there was significant heterogeneity in the magnitude of benefit of vorapaxar therapy in PAD patients with CAD, that experienced a greater benefit in term of MACE reduction compared to those with PAD alone (number needed to treat 45 vs. 1000) [19]. In the FDA approved population (patients with CAD or PAD without history of stroke/transient ischemic attack), the benefit of vorapaxar was associated with increased bleeding, leading to a class IIB recommendation in the current American Guidelines [20]. The overall findings highlight the clinical importance of identifying subgroups of patients that derive greater benefit from a more intense antithrombotic approach.
3. Therapy with Aspirin Combined with an Anticoagulant Drug
Beside platelets activation and aggregation, coagulation is the other key player in thrombus formation. Atherosclerotic plaque rupture exposes tissue factor, which initiates the coagulation cascade and triggers, through the activation of factor X to factor Xa, thrombin generation and fibrin formation. Both thrombin and factor Xa, through the protease-activated receptors (PARs), enhanced platelet activation, with a continuous cross-talking between the coagulation and platelet pathway. Furthermore, is now well established that in patients with acute coronary syndromes, a hypercoagulable state persists for a prolonged period after clinical stabilization and it is associated with worse CV outcome [21][22]. Finally, factor Xa and thrombin modulate a number of inflammation pathways, which further supports their contributing role in atherogenesis and its thrombotic complications [23].
Thus, hypothesizing a synergistic effect, a strategy combining an oral anticoagulant on top of a single antiplatelet agent has been investigated in studies enrolling patients with ASCVD. In particular, the combination of aspirin with a low-dose of the selective direct factor Xa inhibitor rivaroxaban at the dosage of 2.5 mg b.i.d. was shown to reduce the rates of CV death and all-cause death (2.9% vs. 4.5%; HR, 0.68; 95% CI, 0.53–0.87) compared with placebo in the ATLAS ACS 2-TIMI 51 trial (Anti-Xa Therapy to Lower Cardiovascular Events in Addition to Standard Therapy in Subjects with Acute Coronary Syndrome 2–Thrombolysis in Myocardial Infarction 51), enrolling 15 526 patients with acute coronary syndromes [24].
This strategy was, therefore, tested in the Cardiovascular Outcomes for People Using Anticoagulation Strategies (COMPASS) trial enrolling patients (n = 27,395) with established coronary or peripheral artery disease (included carotid artery disease). Participant subjects have been randomized to rivaroxaban 5 mg OD alone, rivaroxaban 2.5 mg BID plus aspirin and aspirin alone. The trial has been stopped earlier owing to overwhelming proof of efficacy of rivaroxaban 2.5 mg BID plus aspirin, which provided a 24% relative risk reduction in MACE (rivaroxaban plus aspirin vs. aspirin alone: HR 0.76, 95% CI 0.66–0.86, p < 0.001; rivaroxaban versus aspirin: HR 0.90, 95% CI 0.79–1.03, p = 0.12) driven by reductions in the rate of CV death and stroke. All causes of death were significantly reduced by 18% in the rivaroxaban 2.5 mg plus aspirin arm (HR 0.82, 95% CI 0.71–0.96; p = 0.01). Major bleeding, according to a modified International Society on Thrombosis and Haemostasis (ISTH) classification, occurred more frequently in patients in the rivaroxaban plus aspirin group than in those receiving aspirin alone (3.1% vs. 1.9%; HR 1.70, 95% CI 1.40–2.05, p < 0.001), driven by gastrointestinal bleeding, with no significant differences in fatal bleeding or intracranial bleeding [25].
A subgroup analysis of 6391 patients with LE-PAD demonstrated that aspirin plus a low-dose rivaroxaban, compared to aspirin alone, reduced peripheral vascular outcome by 24% (5.5% vs. 7.1%; p = 0.03), MALE by 43% (1.5% vs. 2.6%; p = 0.01) and amputation by 58% (0.5% vs. 1.2%; p = 0.01), at the cost of a significant increase in major bleeding (2.0% vs. 3.2%; p = 0.02), without difference in intracranial or fatal bleeding [26]. The consensus document from the ESC working group on aorta and peripheral vascular diseases recommends, therefore, rivaroxaban 2.5 mg bid (the so-called “vascular-dose”) on top of low-dose aspirin in stable patients with chronic symptomatic PAD without conditions at high risk of bleeding.
4. Antithrombotic Therapy after Revascularization
4.1. Lower Extremity Peripheral Revascularization
After endovascular and surgical lower extremity revascularization, the risk of limb events, particularly acute limb ischemia and amputation, remains high. However, until recently, few powered clinical trials of antithrombotic therapies in this setting have been developed, most of them with poor results. Antiplatelet therapy after endovascular revascularization has often been based on studies of patients undergoing percutaneous coronary interventions (PCI). The European guidelines suggest that DAPT should be considered after infra-inguinal stent implantation for one month (Class IIa, level of evidence C) and the AHA/ACC guidelines state that DAPT (aspirin and clopidogrel) may be reasonable to reduce the risk of limb-related events in patients with symptomatic PAD after lower extremity revascularization (level of evidence C). At the same time, based on the CHARISMA trial [14], the AHA/ACC guidelines give a Class-IIb recommendation for long-term DAPT. In the Management of peripheral arterial interventions with mono or dual antiplatelet therapy (MIRROR) study, a 6-month DAPT strategy with aspirin plus clopidogrel vs. aspirin monotherapy showed, in 80 patients, a lower rate of target lesion revascularization at 6 months (5% vs. 8%, p = 0.04), but not at 1 year (25% vs. 32%, p = 0.35). No significant differences were observed in terms of bleeding at 6 months (2.5% vs. 5.0%, p = ns) [27].
After open surgery, the guidelines recommend long-term SAPT. The Clopidogrel and acetylsalicylic acid in bypass surgery for peripheral arterial disease (CASPAR) trial showed no benefit of DAPT with aspirin and clopidogrel versus aspirin alone after lower extremity bypass, with excess of bleeding [28]. The Edoxaban Plus Aspirin vs. Dual Antiplatelet Therapy in Endovascular Treatment of Patients With Peripheral Artery Disease (ePAD) trial compared edoxaban plus aspirin to DAPT with low-dose aspirin and clopidogrel for a period of 3 months after femoro-popliteal endovascular revascularization. The trial was not powered for efficacy, and the risk of restenosis was not different between the two groups (HR 0.89; 95% CI 0.59–1.34). No significant excess in TIMI bleeding was observed with edoxaban (HR 0.56; 95% CI 0.19–1.62) [29]. The Dutch Bypass Oral anticoagulants or Aspirin (DUTCH-BOA) study compared ASA with VKA after infrainguinal bypass surgery, showing no difference for graft patency or MALE with an increased risk of major bleeding, included hemorrhagic stroke [30].
The most recent Vascular Outcomes Study of ASA (acetylsalicylic acid) Along with Rivaroxaban in Endovascular or Surgical Limb Revascularization for PAD (peripheral artery disease) (VOYAGER-PAD) is, so far, the larger, more well-powered randomized clinical trial in the peripheral revascularization setting. Overall, 6564 patients who underwent infrainguinal percutaneous or surgical revascularization were enrolled and randomized within 10 days after revascularization to rivaroxaban 2.5 mg twice daily plus aspirin or to placebo plus aspirin [31]. Low-dose rivaroxaban plus aspirin, compared to aspirin alone, significantly reduced the primary composite end point of acute limb ischemia, major vascular amputation, myocardial infarction, ischemic stroke, or CV death (HR 0.85, 95% CI 0.76–0.96; p = 0.009) without heterogeneity (p for interaction = 0.43) between surgical and endovascular LE-revascularization [32]. Therefore, the use of low-dose rivaroxaban plus aspirin is supported irrespective of the type of treatment. Rates of Thrombolysis in Myocardial Infarction (TIMI) major bleeding were not increased with rivaroxaban, whereas there was a significant excess of major bleeding as defined by the International Society on Thrombosis and Haemostasis (ISTH, HR 1.42; 95% CI 1.10–1.84; p = 0.007). The guidelines recommend, therefore, rivaroxaban at the “vascular-dose” on top of low-dose aspirin in patients not at high bleeding risk.
4.2. Carotid Revascularization
Antithrombotic therapy is recommended for secondary prevention of carotid artery disease after carotid artery stenting (CAS) and carotid endarterectomy (CEA). DAPT is recommended in patients undergoing CAS for 1 month, based on two small randomized clinical trials that were terminated earlier due to higher 30-days rate of stent thrombosis and neurological events in the aspirin-alone group, compared to the DAPT (aspirin plus clopidogrel) group, without increase of bleeding [33][34]. The optimal duration of DAPT after CAS should be based on the ischemic-bleeding risk balance. For instance, the occurrence of periprocedural ischemic brain lesions on magnetic resonance imaging has been associated with increased risk for recurrent cerebrovascular events and suggest a potential benefit of more aggressive and prolonged antiplatelet therapy in this scenario [35]. However, the ischemic benefit of a prolonged DAPT strategy must be counterbalanced by the potential increased in intracranial hemorrhage, particularly in those patients with recent stroke. DAPT may, therefore, be prolonged beyond 1 month after CAS if low-bleeding risk or in patients with concomitant CAD, particularly if at high ischemic risk, such as patients with a recent MI.
A meta-analysis involving 36,881 patients after CEA and 150 after CAS showed no differences in the composite of stroke, transient ischemic attack or death between SAPT and DAPT after CEA, but there was a significant risk of major bleeding and neck hematoma with DAPT [36]. The current guidelines and consensus document recommend, therefore, at least 1-month DAPT after CAS and life-long SAPT after CEA [1][2].
4.3. Coronary Revascularization in Chronic Coronary Syndrome
A recent consensus document recognized the heterogeneity of the population of CCS patients, recommending the stratification of patients based on the presence or absence of a previous MI and on different revascularization strategies. Specifically for patients without a previous MI who underwent a PCI, the evidence for the optimal antithrombotic strategy emerged from RCTs, comparing a shorter versus a longer DAPT regimen, that often enrolled a mixed population represented by both CCS and ACS patients. Overall, the benefit of a prolonged DAPT duration in terms of MACE reduction was counterbalanced by an increase in bleeding, which tended to neutralize the net benefit [37]. Particularly in high bleeding risk patients, a recent meta-analysis of 11 randomized clinical trials involving 9006 patients at high bleeding risk undergoing PCI, a shorter DAPT regimen (1–3 months) was associated with lower bleeding and CV mortality, without increasing ischemic events, compared with a longer (≥6-months) DAPT regimen [38]. Therefore, also in this setting, the balance between ischemic and bleeding risk is a key issue. After PCI, 1- to 6-month DAPT, followed by long-life SAPT monotherapy is the default approach; however, patients at higher bleeding risk may benefit from shortening DAPT with aspirin and clopidogrel to 1 to 3 months with transition to SAPT with either ASA or a P2Y12 receptor inhibitor. On the other hand, in patients with a prevalent ischemic risk, the intensity of the antithrombotic therapy can be increased with a prolonged DAPT duration (>6 months) or by adding to aspirin a low-dose rivaroxaban. The latter strategy is particularly recommended in patients at low bleeding risk and at high risk for stroke, including patients with concomitant PAD [39]. For patients who underwent a coronary artery bypass grafting (CABG), a SAPT strategy with aspirin or clopidogrel is recommended. In the prespecified subgroup analysis form the COMPASS trial in CABG patients, aspirin plus rivaroxaban 2.5 mg did not affect graft patency compared with aspirin alone, with a consistent benefit in MACE as seen in the overall population [40]. Therefore, similarly to patients undergoing PCI, this strategy may be considered when in patients in whom concerns over ischemic events prevail over bleeding, precisely as patients with polyvascular disease.
To further improve personalization in antithrombotic approaches, bleeding and ischemic risk should be integrated with the individual responsiveness to an antiplatelet agent [37]. On-treatment platelet reactivity assessed by platelet function test or genetic testing in order to assess genetic polymorphisms of the hepatic cytochrome P450 (CYP) system in patients undergoing PCI to guide the selection of P2Y12 inhibitor may be useful to inform the clinician to either escalation (from more potent to less potent P2Y12 inhibitor) or de-escalation of therapy (from more potent P2Y12 inhibitor to clopidogrel), in order to reduce interindividual variability and consequently bleeding and thrombotic complications [41].
References
- Knuuti, J.; Wijns, W.; Saraste, A.; Capodanno, D.; Barbato, E.; Funck-Brentano, C.; Prescott, E.; Storey, R.F.; Deaton, C.; Cuisset, T.; et al. ESC Scientific Document Group. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur. Heart J. 2020, 41, 407–477.
- Aboyans, V.; Ricco, J.B.; Bartelink, M.E.L.; Björck, M.; Brodmann, M.; Cohnert, T.; Collet, J.P.; Czerny, M.; De Carlo, M.; Debus, S.; et al. ESC Scientific Document Group. 2017 ESC Guidelines on the Diagnosis and Treatment of Peripheral Arterial Diseases, in collaboration with the European Society for Vascular Surgery (ESVS): Document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteries Endorsed by: The European Stroke Organization (ESO)The Task Force for the Diagnosis and Treatment of Peripheral Arterial Diseases of the European Society of Cardiology (ESC) and of the European Society for Vascular Surgery (ESVS). Eur. Heart J. 2018, 39, 763–816.
- Berger, J.S.; Kranty, M.J.; Kittelson, J.M.; Hiatt, W.R. Aspirin for the prevention of cardiovascualr events in patients with peripheral artery disease: A meta-analysis of randomized trials. JAMA 2009, 301, 1909–1919.
- Collaboration, A.T. Antithrombotic Trialists’ Collaboration. Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high-risk patients. BMJ 2002, 324, 71–86.
- CAPRIE Steering Committee. A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE). CAPRIE Steering Committee. Lancet 1996, 348, 1329–1339.
- Ferreiro, J.L.; Bhatt, D.L.; Ueno, M.; Bauer, D.; Angiolillo, D.J. Impact of smoking on long-term outcomes in patients with atherosclerotic vascular disease treated with aspirin or clopidogrel: Insights from the CAPRIE trial (Clopidogrel versus Aspirin in Patients at Risk of Ischemic Events). J. Am. Coll. Cardiol. 2014, 63, 769–777.
- Gerhard-Herman, M.D.; Gornik, H.L.; Barrett, C.; Barshes, N.R.; Corriere, M.A.; Drachman, D.E.; Fleisher, L.A.; Fowkes, F.G.R.; Hamburg, N.M.; Kinlay, S.; et al. 2016 AHA/ACC guideline on the management of patients with lower extremity peripheral artery disease: Executive summary: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guide- lines. J. Am. Coll. Cardiol. 2017, 69, 1465–1508.
- Bauersachs, R.; Wu, O.; Briere, J.B.; Bowrin, K.; Borkowska, K.; Jakubowska, A.; Taieb, V.; Toumi, M.; Huelsebeck, M. Antithrombotic Treatments in Patients with Chronic Coronary Artery Disease or Peripheral Artery Disease: A Systematic Review of Randomised Controlled Trials. Cardiovasc. Ther. 2020, 2020, 3057168.
- Hess, C.N.; Hiatt, W.R. Antithrombotic Therapy for Peripheral Artery Disease in 2018. JAMA 2018, 319, 2329–2330.
- Clavijo, L.C.; Al-Asady, N.; Dhillon, A.; Matthews, R.V.; Caro, J.; Tun, H.; Rowe, V.; Shavelle, D.M. Prevalence of high on-treatment (aspirin and clopidogrel) platelet reactivity in patients with critical limb ischemia. Cardiovasc. Revasc. Med. 2018, 19, 516–520.
- Hiatt, W.R.; Fowkes, F.G.; Heiyer, G.; Berger, J.S.; Baumgartner, I.; Held, P.; Katona, B.G.; Mahaffey, K.W.; Norgren, L.; Jones, W.S.; et al. EUCLID Trial Steering Committee and Investigators. Ticagrelor versus Clopidogrel in Symptomatic Peripheral Artery Disease. N. Engl. J. Med. 2017, 376, 32–40.
- Capodanno, D.; Bhatt, D.L.; Eikelboom, J.W.; Fox, K.A.A.; Geisler, T.; Michael Gibson, C.; Gonzalez-Juanatey, J.R.; James, S.; Lopes, R.D.; Mehran, R.; et al. Dual-pathway inhibition for secondary and tertiary antithrombotic prevention in cardiovascular disease. Nat. Rev. Cardiol. 2020, 17, 242–257.
- Bhatt, D.L.; Fox, K.A.; Hacke, W.; CHARISMA Investigators. Clopidogrel and aspirin versus aspirin alone for the prevention of atherothrombotic events. N. Engl. J. Med. 2006, 354, 1706–1717.
- Cacoub, P.P.; Bhatt, D.L.; Steg, P.G.; Topol, E.J.; Creager, M.A. CHARISMA Investigators. Patients with peripheral arterial disease in the CHARISMA trial. Eur. Heart J. 2009, 30, 192–201.
- Bonaca, M.P.; Bhatt, D.L.; Storey, R.F.; Steg, P.G.; Cohen, M.; Kuder, J.; Goodrich, E.; Nicolau, J.C.; Parkhomenko, A.; López-Sendón, J.; et al. Ticagrelor for Prevention of Ischemic Events after Myocardial Infarction in Patients with Peripheral Artery Disease. J. Am. Coll. Cardiol. 2016, 67, 2719–2728.
- Secemsky, E.A.; Yeh, R.W.; Kereiakes, D.J.; Dual Antiplatelet Therapy Study Investigators. Extended Duration Dual Antiplatelet Therapy after Coronary Stenting Among Patients with Peripheral Arterial Disease: A Subanalysis of the Dual Antiplatelet Therapy Study. JACC Cardiovasc. Interv. 2017, 10, 942–954.
- Franzone, A.; Piccolo, R.; Gargiulo, G.; Ariotti, S.; Marino, M.; Santucci, A.; Baldo, A.; Magnani, G.; Moschovitis, A.; Windecker, S.; et al. Prolonged vs Short Duration of Dual Antiplatelet Therapy after Percutaneous Coronary Intervention in Patients with or without Peripheral Arterial Disease: A Subgroup Analysis of the PRODIGY Randomiyed Clinical Trial. JAMA Cardiol. 2016, 1, 795–803.
- Bonaca, M.P.; Creager, M.A.; Olin, J.; Scirica, B.M.; Gilchrist, I.C., Jr.; Murphy, S.A.; Goodrich, E.L.; Braunwald, E.; Morrow, D.A. Peripheral Revascularization in Patients with Peripheral Artery Disease with Vorapaxar: Insights from the TRA 2°P-TIMI 50 Trial. JACC Cardiovasc. Interv. 2016, 9, 2157–2164.
- Qamar, A.; Morrow, D.A.; Creager, M.A.; Scirica, B.M.; Olin, J.W.; Beckman, J.A.; Murphy, S.A.; Bonaca, M.P. Effect of vorapaxar on cardiovascular and limb outcomes in patients with peripheral artery disease with and without coronary artery disease: Analysis from the TRA 2°P-TIMI 50 trial. Vasc. Med. 2020, 25, 124–132.
- Criqui, M.H.; Matsushita, K.; Aboyans, V.; Hess, C.N.; Hicks, C.W.; Kwan, T.W.; McDermott, M.M.; Misra, S.; Ujueta, F.; American Heart Association Council on Epidemiology and Prevention; et al. Lower Extremity Peripheral Artery Disease: Contemporary Epidemiology, Management Gaps, and Future Directions: A Scientific Statement from the American Heart Association. Circulation 2021, 144, e171–e191.
- Merlini, P.A.; Bauer, K.A.; Oltrona, L.; Ardissino, D.; Cattaneo, M.; Belli, C.; Mannucci, P.M.; Rosenberg, R.D. Persistent activation of coagulation mechanism in unstable angina and myocardial infarction. Circulation 1994, 90, 61–68.
- Ardissino, D.; Merlini, P.A.; Bauer, K.A.; Galvani, M.; Ottani, F.; Franchi, F.; Bertocchi, F.; Rosenberg, R.D.; Mannucci, P.M. Coagulation activation and long-term outcome in acute coronary syndromes. Blood 2003, 102, 2731–2735.
- Weitz, J.I. Insights into the role of thrombin in the pathogenesis of recurrent ischaemia after acute coronary syndrome. Thromb. Haemost. 2014, 112, 924–931.
- Mega, J.L.; Braunwald, E.; Wiviott, S.D.; Bassand, J.P.; Bhatt, D.L.; Bode, C.; Burton, P.; Cohen, M.; Cook-Bruns, N.; Fox, K.A.; et al. ATLAS ACS 2–TIMI 51 Investigators. Rivaroxaban in patients with a recent acute coronary syndrome. N. Engl. J. Med. 2012, 366, 9–19.
- Eikelboom, J.W.; Connolly, S.J.; Bosch, J.; Dagenais, G.R.; Hart, R.G.; Shestakovska, O.; Diaz, R.; Alings, M.; Lonn, E.M.; Anand, S.S.; et al. COMPASS Investigators. Rivaroxaban with or without Aspirin in Stable Cardiovascular Disease. N. Engl. J. Med. 2017, 377, 1319–1330.
- Anand, S.S.; Caron, F.; Eikelboom, J.W.; Bosch, J.; Dyal, L.; Aboyans, V.; Abola, M.T.; Branch, K.R.H.; Keltai, K.; Bhatt, D.L.; et al. Major adverse limb events and mortality in patients with peripheral artery disease: The COMPASS trial. J. Am. Coll. Cardiol. 2018, 71, 2306.
- Tepe, G.; Bantleon, R.; Brechtel, K.; Schmehl, J.; Zeller, T.; Claussen, C.D.; Strobl, F.F. Management of peripheral arterial interventions with mono or dual antiplatelet therapy--the MIRROR study: A randomised and double-blinded clinical trial. Eur. Radiol. 2012, 22, 1998–2006.
- Belch, J.J.; Dormandy, J.; Biasi, G.M.; Cairols, M.; Diehm, C.; Eikelboom, B.; Golledge, J.; Jawien, A.; Lepäntalo, M.; Norgren, L.; et al. Results of the randomized, placebo-controlled clopidogrel and acetylsalicylic acid in bypass surgery for peripheral arterial disease (CASPAR) trial. J. Vasc. Surg. 2010, 52, 825–833.e2.
- Moll, F.; Baumgartner, I.; Jaff, M.; Nwachuku, C.; Tangelder, M.; Ansel, G.; Adams, G.; Zeller, T.; Rundback, J.; Grosso, M.; et al. ePAD Investigators. Edoxaban Plus Aspirin vs Dual Antiplatelet Therapy in Endovascular Treatment of Patients with Peripheral Artery Disease: Results of the ePAD Trial. J. Endovasc. Ther. 2018, 25, 158–168.
- Efficacy of oral anticoagulants compared with aspirin after infrainguinal bypass surgery (The Dutch Bypass Oral Anticoagulants or Aspirin Study): A randomised trial. Lancet 2000, 355, 346–351.
- Bonaca, M.P.; Bauersachs, R.M.; Anand, S.S.; Debus, E.S.; Nehler, M.R.; Patel, M.R.; Fanelli, F.; Capell, W.H.; Diao, L.; Jaeger, N.; et al. Rivaroxaban in Peripheral Artery Disease after Revascularization. N. Engl. J. Med. 2020, 382, 1994–2004.
- Hess, C.N.; Debus, E.S.; Nehler, M.R.; Anand, S.S.; Patel, M.R.; Szarek, M.; Capell, W.H.; Hsia, J.; Beckman, J.A.; Brodmann, M.; et al. Reduction in Acute Limb Ischemia with Rivaroxaban versus Placebo in Peripheral Artery Disease after Lower Extremity Revascularization: Insights from VOYAGER PAD. Circulation 2021, 144, 1831–1841.
- McKevitt, F.M.; Randall, M.S.; Cleveland, T.J.; Gaines, P.A.; Tan, K.T.; Venables, G.S. The Benefits of Combined Anti-platelet Treatment in Carotid Artery Stenting. Eur. J. Vasc. Endovasc. Surg. 2005, 29, 522–527.
- Dalainas, I.; Nano, G.; Bianchi, P.; Stegher, S.; Malacrida, G.; Tealdi, D.G. Dual Antiplatelet Regime versus Acetyl-acetic Acid for Carotid Artery Stenting. Cardiovasc. Interv. Radiol. 2006, 29, 519–521.
- Gensicke, H.; van der Worp, H.B.; Nederkoorn, P.J.; Macdonald, S.; Gaines, P.A.; van der Lugt, A.; Mali, W.P.; Lyrer, P.A.; Peters, N.; Featherstone, R.L.; et al. ICSS-MRI Substudy Investigators. Ischemic brain lesions after carotid artery stenting increase future cerebrovascular risk. J. Am. Coll. Cardiol. 2015, 65, 521–529.
- Barkat, M.; Hajibandeh, S.; Hajibandeh, S.; Torella, F.; Antoniou, G.A. Systematic Review and Meta-analysis of Dual versus Single Antiplatelet Therapy in Carotid Interventions. Eur. J. Vasc. Endovasc. Surg. 2017, 53, 53–67.
- Angiolillo, D.A.; Galli, M.; Collet, J.P.; Kastrati, A.; O’Donoghue, M.L. Antiplatelet therapy after percutaneous coronary intervention. EuroIntervention 2022, 17, e1371–e1396.
- Costa, F.; Montalto, C.; Branca, M.; Hong, S.J.; Watanabe, H.; Franzone, A.; Vranckx, P.; Hahn, J.Y.; Gwon, H.C.; Feres, F.; et al. Dual antiplatelet therapy duration after percutaneous coronary intervention in high bleeding risk: A meta-analysis of randomized trials. Eur. Heart J. 2023, 44, 954–968.
- Capodanno, D.; Greco, A. Dual antiplatelet therapy in patients at high bleeding risk: Less is more-more or less. Eur. Heart J. 2023, 44, 969–971.
- Lamy, A.; Eikelboom, J.; Sheth, T.; Connolly, S.; Bosch, J.; Fox, K.A.A.; Zhu, J.; Lonn, E.; Dagenais, G.; Widimsky, P.; et al. Rivaroxaban, aspirin, or both to prevent early coronary bypass graft occlusion: The COMPASS-CABG study. J. Am. Coll. Cardiol. 2019, 73, 121–130.
- Galli, M.; Franchi, F.; Rollini, F.; Cavallari, L.H.; Capodanno, D.; Crea, F.; Angiolillo, D.J. Genetic testing in patients undergoing percutaneous coronary intervention: Rationale, evidence and practical recommendations. Expert Rev. Clin. Pharmacol. 2021, 14, 963–978.
More
Information
Subjects:
Cardiac & Cardiovascular Systems
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
691
Revisions:
2 times
(View History)
Update Date:
31 Aug 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




