Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Giuseppe Maggiore | -- | 3057 | 2023-08-28 11:21:48 | | | |
| 2 | Dean Liu | -2 word(s) | 3055 | 2023-08-29 02:23:08 | | | | |
| 3 | Dean Liu | Meta information modification | 3055 | 2023-09-06 03:42:28 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Nastasio, S.; Mosca, A.; Alterio, T.; Sciveres, M.; Maggiore, G. Juvenile Autoimmune Hepatitis. Encyclopedia. Available online: https://encyclopedia.pub/entry/48528 (accessed on 07 February 2026).
Nastasio S, Mosca A, Alterio T, Sciveres M, Maggiore G. Juvenile Autoimmune Hepatitis. Encyclopedia. Available at: https://encyclopedia.pub/entry/48528. Accessed February 07, 2026.
Nastasio, Silvia, Antonella Mosca, Tommaso Alterio, Marco Sciveres, Giuseppe Maggiore. "Juvenile Autoimmune Hepatitis" Encyclopedia, https://encyclopedia.pub/entry/48528 (accessed February 07, 2026).
Nastasio, S., Mosca, A., Alterio, T., Sciveres, M., & Maggiore, G. (2023, August 28). Juvenile Autoimmune Hepatitis. In Encyclopedia. https://encyclopedia.pub/entry/48528
Nastasio, Silvia, et al. "Juvenile Autoimmune Hepatitis." Encyclopedia. Web. 28 August, 2023.
Copy Citation
Juvenile autoimmune hepatitis (JAIH) is severe immune-mediated necro-inflammatory disease of the liver with spontaneous progression to cirrhosis and liver failure if left untreated. The diagnosis is based on the combination of clinical, laboratory and histological findings. Prothrombin ratio is a useful prognostic factor to identify patients who will most likely require a liver transplant by adolescence or early adulthood. JAIH treatment consists of immune suppression and should be started promptly at diagnosis to halt inflammatory liver damage and ultimately prevent fibrosis and progression to end-stage liver disease. The risk of relapse is high especially in the setting of poor treatment compliance.
autoimmune hepatitis
acute liver failure
autoimmune liver disease
active chronic hepatitis liver transplantation
1. Introduction
Juvenile autoimmune hepatitis (JAIH) is an immune-mediated necro-inflammatory disease of the liver, of unknown origin, with spontaneous progression to cirrhosis and terminal liver failure, if diagnosis is overlooked and treatment delayed [1]. A complex interaction among genetic susceptibility, exposure to triggering factors and dysregulation of the immune response to antigens expressed on the hepatocyte surface represent the basis of the pathogenesis of the disease.
Specific circulating autoantibodies along with increased concentration of serum IgG and distinctive histopathological aspects identify “classic” AIH or so called “seropositive” AIH [1][2]. Seropositive AIH is divided into two subtypes: AIH type 1 (AIH-1), positive for smooth muscle antibodies (SMA) and/or antinuclear antibodies (ANA); and type 2 (AIH-2), positive for liver kidney microsomal antibody type 1 (anti-LKM-1) and/or anti-liver cytosol type 1 (anti-LC1) [1][2][3][4].
JAIH affects all ages and races worldwide with incidence peaks between 10 and 11 years for AIH-1 and between 6 and 7 years for AIH-2. It is characterized by a female predominance, with a ratio of 3:1 for AIH-1 and up to 9:1 for AIH-2 [2][3].
The role of a genetic predisposition has been discussed for decades. To date, it is known that pediatric AIH-1, similarly to that observed in adults, is associated with human leukocyte antigen (HLA) DRB1*03. AIH-2 is associated with specific HLA class II susceptibility alleles; DQB1*0201 is considered the main determinant of susceptibility while DRB1*07/DRB1*03 is associated with the type of autoantibody present. HLA DQB1*0201 is in strong linkage disequilibrium with both HLA DRB1*03 and DRB1*07 [5].
2. Diagnosis
The diagnosis of JAIH can be difficult due to the lack of a single specific marker of the disease or gold standard testing. Rather, AIH diagnosis is based on the combination of laboratory (elevated serum IgG, positive autoantibodies) and histological (most commonly interface hepatitis and portal lymphoplasmacytic infiltrate) features in the setting of clinical suspicion and exclusions of other causes of liver disease (e.g., viral hepatitis, drug-induced liver injury, hereditary, metabolic) (Figure 1). Most patients present with non-specific symptoms such as fatigue and abdominal pain; other presentations include an acute viral hepatitis-like onset with nausea, vomiting and anorexia, or an insidious onset, in about 30% of cases, with vague symptoms up to 2 years before diagnosis. On physical examination, hepatomegaly and/or splenomegaly may be present in up to 50% of children at the time of diagnosis [1][2][6]. The clinical and laboratory presentation of JAIH varies from an asymptomatic elevation of serum aminotransferases with increased serum IgG, to acute liver failure, in about 20% of cases, requiring urgent liver transplantation. In several studies, the prevalence of cirrhosis at diagnosis ranges from 17% to 75% of children with JAIH. Acute and severe hepatitis with jaundice even without encephalopathy and with normal INR (international ratio), usually requires hospitalization and close monitoring given the risk of disease progression [7]. The lack of specific diagnostic markers makes the exclusion of other disorders a fundamental aspect of JAIH diagnosis (e.g., viral hepatitis, drug-induced liver damage, Wilson’s disease, hereditary hemochromatosis). Patients with AIH-2 tend to present at a younger median age and have lower rates of cirrhosis at presentation (38% vs. 69%) [8]. In addition, AIH-1 sometimes presents with autoimmune sclerosing cholangitis (ASC)-AIH overlapping syndrome [9].
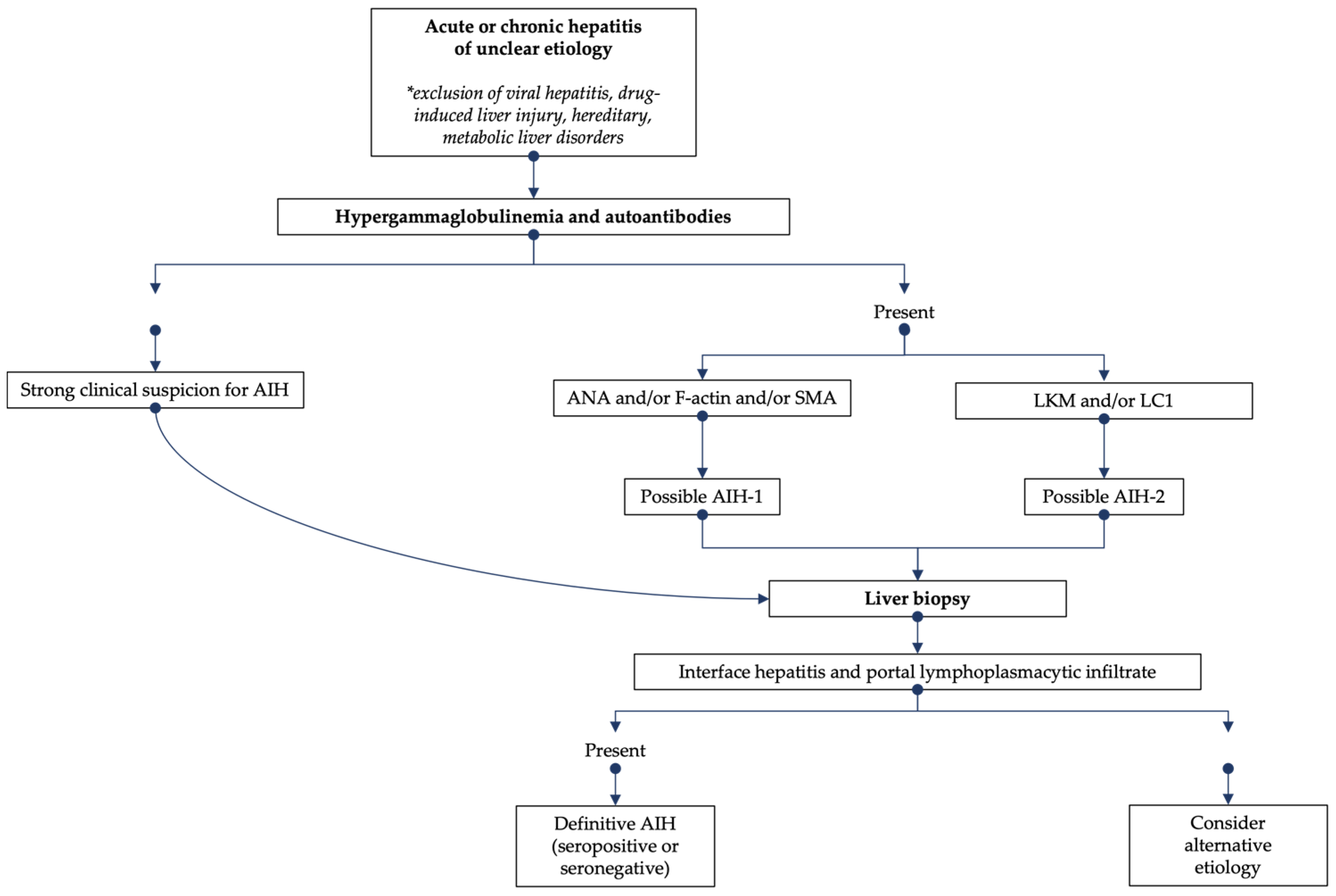
Figure 1. Diagnostic algorithm for the evaluation of suspected AIH.
3. Seronegative AIH
Historically, the presence of autoantibodies with elevated serum immunoglobulin in adult patients with active chronic hepatitis (ACH) was able to predict the favorable response to immunosuppression and ultimately lead to the description of AIH [10][11][12]. Soon after it was noted that some patients with autoantibodies-negative ACH or with cryptogenic chronic hepatitis also had a comparable response to immunosuppression [13]. A systematic review of patients successfully treated with immunosuppression led to the full description of the so-called seronegative AIH (sAIH). Patients with sAIH were found to be undistinguishable from those with “seropositive” AIH in terms of demographic, laboratory and histological features as well as in terms of treatment outcomes [14]. Moreover, this group of patients shared several features of immune dysregulation with seropositive-AIH including hypergammaglobulinemia, association with characteristic HLA haplotypes, propensity to relapse and seroreactivity against investigational autoantigens such as auto-antibodies against the liver membrane lipoprotein preparation known as liver-specific membrane lipoprotein (LSP) or against the hepatic asialoglycoprotein receptor (ASGP-R) [15]. Frequency of sAIH varied from 5 to 35% of a reported cohort of AIH being higher in those presenting with acute or fulminant hepatitis [16]. Criteria for diagnosis were limited to the finding of suggestive histological features in patients with acute or chronic hepatitis once viral, toxic or metabolic causes had been excluded [3]. In the 2019 AASLD guidelines, sAIH was finally endorsed and its prevalence was estimated as 20% of AIH [3]. More recently sporadic cases of antibody-negative disease were reported in children and adolescents with concomitant celiac disease [17][18].
In 2016 researchers reported a series of 38 children with sAIH representing about 17% of 223 children with autoimmune hepatitis (AIH), diagnosed at two paediatric liver centres in Europe, over a 22-year period [16]. In 2021 two further smaller case series were published for a total of 64 paediatric patients described to date [19][20]. Interestingly the proportion of sAIH was similar in all series varying from 17% to 27% even though, in one series, up to 50% of patients developed autoantibodies a few weeks after clinical onset.
The large majority of children with sAIH presented with acute liver failure [16][19]. Criteria for diagnosis of sAIH in children are the same as in adults: negative virological studies, absence of serum AIH-related autoantibodies, exclusion of others causes of liver disease and liver histology compatible with AIH [16]. It is noteworthy that sAIH was not included in the more recent ESPGHAN/NASPGHAN position paper [2] despite the increasing availability of data, mainly in adult patients.
Seronegative AIH is probably not a unique entity; the first distinction relies on presence or not of hematological abnormalities. Children without hematological disease have, almost in equal proportion, increased or normal immunoglobulin G levels. Seronegative AIH with increased IgG is similar to classical seropositive AIH. Patients often present with symptomatic disease but with signs of chronicity such as splenomegaly and half of them have severe fibrosis or cirrhosis at liver biopsy. In the series, all patients achieved biochemical remission with conventional immunosuppressive treatment, however relapses were frequent as in seropositive-AIH [16]. Withdrawal of therapy was attempted in 50% of children and successful in all. Among patients with hematological comorbidities, defined as the combination of thrombocytopenia, with or without neutropenia and with a normal bone marrow examination, a common (66%) finding was a lymphocyte count < 1000/mm3 with a decreased CD4/8 ratio. Anti-platelets or anti-neutrophils antibodies were found in two thirds of cases. In this group of patients, no relapse of hepatitis was observed under or after the withdrawal of therapy when attempted [16].
A subgroup of 10 patients with sAIH had associated severe aplastic anemia (SAA) defined as transfusion-dependent pancytopenia. SAA was present at diagnosis in 30% of cases and occurred 1 to 14 months later in the others. Interestingly, these children were older, with median age of 11 years, all presented with acute severe liver disease and most often liver failure. Fibrosis was absent or mild, but centrilobular necrosis, panlobular infiltrate and bridging necrosis were present and often accompanied by a characteristic portal lymphomonocytic infiltrate and interface activity. Lymphocytopenia with low CD4/8 ratio was noted in 80% of cases of SAA-sAIH at diagnosis. In this subgroup of patients, immunosuppression led to remission of sAIH in all but one child who underwent liver transplant. Noteworthy immunosuppression did not prevent the emergence of SAA, which was managed with cyclosporine and anti-lymphocyte globulins. Four of these patients required bone marrow transplant [16]. Ultimately, all patients, except the transplanted one, were able to stop immunosuppression without relapse [16].
Hepatitis-associated aplastic anemia is a well-known clinical syndrome with poor prognosis, often leading to end-stage liver disease and refractory bone marrow failure requiring hematopoietic stem cell transplant [21][22][23]. Humoral signs of increased systemic inflammation and immune dysregulation characterize this condition also characterized by low NK cell activity, high-perforin-expressing CD8 T-cells and increased levels of soluble IL-2 receptor as well as sporadic signs of hemophagocytosis at bone marrow examination [22]. It partially overlaps the clinical picture of hemophagocytic lymphohistiocytosis (HLH) even though these patients do not meet criteria for a definite diagnosis of HLH. It seems reasonable that patients with sAIH and SAA described to date represent the milder side of the spectrum of disease as most of them could be successfully cured with immunosuppression [24].
In summary, sAIH is probably a clinical syndrome, which recognizes different mechanisms of disease. Researchers previously proposed stratification in homogeneous groups of patients, which could be the basis for further research [19]. Immunosuppression should be tailored to the specific needs of each subgroup in terms of quality and duration. Larger retrospective series or ideally prospective studies are necessary to establish the best treatment protocol.
4. Management of AIH
Treatment of AIH is based on immune suppression and should be started promptly at diagnosis to halt inflammatory liver damage and ultimately prevent fibrosis and progression to end stage liver disease. Treatment aims to obtain complete biochemical remission defined as strict normalization of serum activities of ALT/AST, and of immunoglobulin G values, negative or very low-titre autoantibodies as well as normalization of the liver function (albumin, clotting factors). The combination of prednisolone or prednisone and azathioprine constitutes the so-called first-line conventional treatment and allows remission to be achieved in most patients. Alternative therapies are used in cases of initial treatment failure or multiple relapses during tapering or discontinuation attempts [4][25] (Table 1).
Table 1. Treatments for juvenile autoimmune hepatitis: regimens and dosages reported in literature.
| Medication | Class of Drug | Regimens | Dose | Side Effects | Additional Information |
|---|---|---|---|---|---|
| Prednisone | Corticosteroid | First line | 1–2 mg/kg/day | Hyperphagia, weight gain, fat redistribution, cutaneous striae, acne, impaired height growth, severe growth retardation, cataract, osteoporosis, hyperglycemia, arterial hypertension, psychosis | Constitutes the so-called conventional treatment in combination with azathioprine. |
| Azathioprine | Purine antagonist | First line | 1–2.5 mg/kg/day | Cytopenia, pancreatitis, nausea, vomiting, abdominal pain, hepatotoxicity, malignancy | Steroid sparing effect. |
| Cyclosporine | Calcineurin inhibitor | First line Second line |
4–10 mg/kg/day Target trough levels: 100–300 ng/mL |
Hypertrichosis, gingival hypertrophy, hypertension, nephrotoxicity | Most effective drug for conventional treatment-refractory JAIH |
| Mycophenolate mofetil | Purine antagonist | Second line | 20–40 mg/kg/day | Leukopenia, headache, diarrhea, dizziness, hair loss | Independent from the thiopurine methyltransferase pathway of catabolism. Teratogenic. |
| Budesonide | Corticosteroid | First line | 6–9 mg/day | Weight gain, fat redistribution, cutaneous striae, acne | Contraindicated in cirrhotic patients. |
| Tacrolimus | Calcineurin inhibitor | Second line | Target trough levels: 1–10 ng/mL | Bone marrow toxicity, neurotoxicity, nephrotoxicity, opportunistic infections | Unknown optimal through level. |
| Rituximab | Monoclonal anti CD-20 antibody | Third line | 375 mg/m2 weekly for 4 weeks, repeated depending on response | Infections, progressive multifocal encephalitis | Only limited data available. |
5. Long-Term Follow-Up
JAIH is a severe disease with high-risk of relapse due to poor compliance [26][27] and wide variability of reported sustained remission rates after treatment withdrawal ranging from 3 to 87%. [28][29]. In children, the official recommendations prior to attempting treatment withdrawal include normalization of transaminases for 2 to 3 years, normal serum IgG, negative or low titres of serum autoantibodies and no inflammation on liver histology [2]. In adults, on the other hand, liver biopsy is no longer considered a mandatory prerequisite for treatment withdrawal [3]. In addition, there are only limited pediatric reports on the long-term follow-up beyond 10 years of management [29][30].
Researchers recently published data on a long-term observational study of 117 children with AIH, excluding fulminant, seronegative, drug-induced hepatitis and sclerosing cholangitis, who were diagnosed and treated in a single pediatric hepatology center in France [31]. Cirrhosis was present in 80 children at diagnosis. Management consisted of immunosuppression with prednisone with/without azathioprine in most. Attempts at treatment withdrawal under medical supervision were carried out in one of two ways: before 1981, withdrawal was performed after liver histology with a combination of normal aminotransferase activity and serum gamma globulins. Later, having found that liver histology did not reliably predict the lack of relapse after withdrawal [32], another approach was undertaken: when alanine aminotransferase activity remained normal on therapy for at least 1 year, no liver biopsy was performed, and prednisone was progressively decreased over one year, while checking aminotransferases for normalization before each new decrease. Once prednisone was stopped, azathioprine was continued for another 6 months and then stopped if aminotransferases remained normal. A complete biochemical response was defined as normalization of serum alanine aminotransferase and gammaglobulins no later than 6 months after initiation of treatment following the recent recommendations of the IAIHG [33]. Data were available until death and for 8 to 38 years (median, 20 years) after starting treatment in surviving patients. [31].
6. JAIH and Future Pregnancies
JAIH affects a number of young women of childbearing age, some of whom have a long-lasting history of disease and immunosuppression [34]. Moreover, with at least one third of patients having cirrhosis at diagnosis, the population of pregnant women with AIH is very heterogeneous in terms of severity of disease and treatment regimens. There are many aspects of JAIH that may affect pregnancy: presence or not of cirrhosis and related complications; presence of potentially dangerous comorbidities due to concomitant autoimmune diseases such as type 1 diabetes or antiphospholipid syndrome; biochemical remission or active disease at the beginning of pregnancy; ongoing immunosuppression regimen and its potential toxicity or teratogenicity for the fetus. The literature addressing this issue can be roughly divided into two categories: population-based cohort studies [35][36][37] and single-center retrospective reports [38][39][40][41][42]. In population-based studies data, are retrieved from health registries via the ICD code, in one case matched with histopathology report data [35]. The major pitfalls of these studies are the limited amount of clinical information available and possible coding errors. On the other hand, accurate epidemiological analysis could be conducted thanks to the huge size of the study cohort and possibility to build a control group. Single-centers experiences are valuable because diagnosis is well-documented and clinical details are usually available. Limited size and retrospective nature are the limitations. Fertility in AIH depends on disease activity and it is decreased in poorly controlled AIH [40][42] and in cirrhotic patients, 10% of whom are reported to use assisted reproductive technology [40]. During pregnancy, the increase in estrogen and progesterone induces maternal immunity to enter a tolerogenic status to safely host the semi-allograft fetus. Th0 cells are stimulated to differentiate into Treg and Th2 phenotypes that produce high levels of IL-10, IL-4, IL-5 and TGFβ, anti-inflammatory and tolerogenic cytokines [43]. However, after delivery, pregnancy hormones levels abruptly fall and the reactivity of immune system is rapidly restored. Average transaminase levels in AIH patients are significantly lower during gestation, as reported in a recent metanalysis [44].
Loss of remission is rare during pregnancy, although it has been reported in as many as 10–15% of cases in the same studies [38][40]. Relapse after delivery, on the other hand, has been reported to occur in up to 50% of cases [40]. More recent studies, however, report lower post-partum relapse rates, which are likely related to the improved knowledge on drugs’ safety and general AIH management during pregnancy. It is known that patients who are in remission before conception have a lower risk of post-partum relapses [44] and that poorly controlled AIH during pregnancy is associated with a greater incidence of adverse outcomes [38]. In terms of maternal outcomes, more recent population studies have not shown an increased rate of caesarean sections in AIH women [35][36]. No difference was found in the incidence of maternal death excluding patients with decompensated cirrhosis [44][45], while almost all studies report an increased risk of gestational diabetes [35][36][37]. Even patients who are off treatment during pregnancy have a higher risk of diabetes [36], suggesting that it is not related to use of steroids but rather to the implicit association of diabetes with other autoimmune diseases. An increased risk of gestational hypertension, preeclampsia, eclampsia and HELLP syndrome was reported in many single-center studies and population-based studies in the US and Sweden [35][36]. It is still debated whether to consider AIH patients as belonging to the high-risk group for other inflammatory diseases and whether low-dose aspirin prophylaxis should be recommended. In terms of fetal outcomes, preterm birth was more common in AIH than in the general population, with a rate of 9–20% [35][36][37]; interestingly, immunosuppression or cirrhosis did not increase this risk. Conversely, the occurrence of a relapse during pregnancy was associated with an increased risk of preterm birth and higher rate of admission to a neonatal intensive care unit [36]. Birth weight < 2500 g but not small-for-gestational-age newborns were associated with AIH in the US and Sweden [35][36] suggesting that low weight is related only to the greater proportion of preterm. No differences in terms of risk of congenital malformations, neonatal mortality and stillbirth were found in any recent population-based studies [35][36][37][44], but only in older ones, which report a rate of fetal loss of up to 27% [40]. The presence of anti-phospholipids and anti-Ro/SSA antigens must be monitored for they carry the risk of adverse outcomes. Only the presence of cirrhosis was also associated with an increased risk of miscarriage in more recent studies [38][41]. There is increasing evidence that remission of disease should be achieved before conception and maintained during pregnancy. A safe pregnancy should be programmed; immunosuppression should not be weaned during gestation with low-dose steroids and azathioprine being safe during pregnancy [42][46][47]. Breastfeeding is also considered safe. A small amount of both drugs can be detected in breast milk but should not have any effect on the infant; however, it is recommended to wait four hours before nursing [48].
Although ciclosporin exposure has been associated with hypertension and intrauterine growth retardation and tacrolimus with nephrotoxicity and gestational diabetes when used during pregnancy, calcineurin-inhibitors are now considered safe both during pregnancy and the breastfeeding period. Data are mainly derived from studies on transplanted patients and rheumatologic ones [49]. It must be considered that pregnancy increases the unbound and active form of tacrolimus, resulting in the underestimation of the whole blood level. Consequently, the dosage should not be increased to avoid toxicity.
Mycophenolate is strongly discouraged due to its known teratogenicity and replacement with azathioprine should be considered [50].
In conclusion, in the majority of women with AIH, pregnancy does not carry substantial risks but remission of disease is strongly recommended. Preconception counseling and multidisciplinary care during gestation and after delivery must be provided.
References
- Maggiore, G.; Nastasio, S.; Sciveres, M. Juvenile autoimmune hepatitis: Spectrum of the disease. World J. Hepatol. 2014, 6, 464–476.
- Mieli-Vergani, G.; Vergani, D.; Baumann, U.; Czubkowski, P.; Debray, D.; Dezsofi, A.; Fischler, B.; Gupte, G.; Hierro, L.; Indolfi, G.; et al. Diagnosis and Management of Pediatric Autoimmune Liver Disease: ESPGHAN Hepatology Committee Position Statement. J. Pediatr. Gastroenterol. Nutr. 2018, 66, 345–360.
- Mack, C.L.; Adams, D.; Assis, D.N.; Kerkar, N.; Manns, M.P.; Mayo, M.J.; Vierling, J.M.; Alsawas, M.; Murad, M.H.; Czaja, A.J. Diagnosis and Management of Autoimmune Hepatitis in Adults and Children: 2019 Practice Guidance and Guidelines from the American Association for the Study of Liver Diseases. Hepatology 2020, 72, 671–722.
- Sciveres, M.; Nastasio, S.; Maggiore, G. Novel Diagnostic and Therapeutic Strategies in Juvenile Autoimmune Hepatitis. Front. Pediatr. 2019, 7, 382.
- Lapierre, P.; Alvarez, F. Type 2 autoimmune hepatitis: Genetic susceptibility. Front. Immunol. 2022, 13, 1025343.
- Kemme, S.; Mack, C.L. Pediatric Autoimmune Liver Diseases. Pediatr. Clin. N. Am. 2021, 68, 1293–1307.
- Radhakrishnan, K.R.; Alkhouri, N.; Worley, S.; Arrigain, S.; Hupertz, V.; Kay, M.; Yerian, L.; Wyllie, R.; Feldstein, A.E. Autoimmune hepatitis in children—Impact of cirrhosis at presentation on natural history and long-term outcome. Dig. Liver Dis. 2010, 42, 724–728.
- Homberg, J.-C.; Abuaf, N.; Bernard, O.; Islam, S.; Alvarez, F.; Khalil, S.H.; Poupon, R.; Darnis, F.; Lévy, V.-G.; Grippon, P.; et al. Chronic active hepatitis associated with antiliver/kidney microsome antibody type 1: A second type of “autoimmune” hepatitis. Hepatology 1987, 7, 1333–1339.
- Roberts, E.A. Autoimmune hepatitis from the paediatric perspective. Liver Int. 2011, 31, 1424–1431.
- Czaja, A.J. Behavior and significance of autoantibodies in type 1 autoimmune hepatitis. J. Hepatol. 1999, 30, 394–401.
- Soloway, R.D.; Summerskill, W.; Baggenstoss, A.H.; Geall, M.G.; Gitnick, G.L.; Elveback, L.R.; Schoenfield, L.J. Clinical, Biochemical, and Histological Remission of Severe Chronic Active Liver Disease: A Controlled Study of Treatments and Early Prognosis. Gastroenterology 1972, 63, 820–833.
- Czaja, A.J.; Davis, G.L.; Ludwig, J.; Baggenstoss, A.H.; Taswell, H.F. Autoimmune features as determinants of prognosis in ster-oid-treated chronic active hepatitis of uncertain etiology. Gastroenterology 1983, 85, 713–717.
- Gassert, D.J.; Garcia, H.; Tanaka, K.; Reinus, J.F. Corticosteroid-Responsive Cryptogenic Chronic Hepatitis: Evidence for Seronegative Autoimmune Hepatitis. Dig. Dis. Sci. 2007, 52, 2433–2437.
- Czaja, A.J. Autoantibody-Negative Autoimmune Hepatitis. Dig. Dis. Sci. 2012, 57, 610–624.
- Johnson, P.J.; Mcfarlane, I.G.; Mcfarlane, B.M.; Williams, R. Auto-immune features in patients with idiopathic chronic active hepatitis who are seronegative for conventional auto-antibodies. J. Gastroenterol. Hepatol. 1990, 5, 244–251.
- Maggiore, G.; Socie, G.; Sciveres, M.; Roque-Afonso, A.-M.; Nastasio, S.; Johanet, C.; Gottrand, F.; Fournier-Favre, S.; Jacquemin, E.; Bernard, O. Seronegative autoimmune hepatitis in children: Spectrum of disorders. Dig. Liver Dis. 2016, 48, 785–791.
- Quail, M.A.; Russell, R.K.; Bellamy, C.; Mieli-Vergani, G.; Gillett, P.M. Seronegative autoimmune hepatitis presenting after diagnosis of coeliac disease: A case report. Eur. J. Gastroenterol. Hepatol. 2009, 21, 576–579.
- Caprai, S.; Vajro, P.; Ventura, A.; Sciveres, M.; Maggiore, G.; SIGENP Study Group for Autoimmune Liver Disorders in Celiac Disease. Autoimmune liver disease associated with celiac disease in childhood: A multicenter study. Clin. Gastroenterol. Hepatol. 2008, 6, 803–806.
- Islek, A.; Keskin, H. Seronegative autoimmune hepatitis in children: A single-center experience. Acta Gastro Enterol. Belg. 2021, 84, 305–310.
- Khedr, M.A.; Salem, T.A.; Boghdadi, G.M.; Elharoun, A.S.; El-Shahaway, A.A.; Atallah, H.R.; Sira, M.M. Seronegative autoimmune hepatitis in children: A real diagnostic challenge. Wien. Klin. Wochenschr. 2022, 134, 195–201.
- Gonzalez-Casas, R.; Garcia-Buey, L.; Jones, E.A.; Gisbert, J.P.; Moreno-Otero, R. Systematic review: Hepatitis-associated aplastic anaemia—A syndrome associated with abnormal immunological function. Aliment. Pharmacol. Ther. 2009, 30, 436–443.
- Kemme, S.; Stahl, M.; Brigham, D.; Lovell, M.A.; Nakano, T.; Feldman, A.G.; Mack, C. Outcomes of Severe Seronegative Hepatitis-associated Aplastic Anemia: A Pediatric Case Series. J. Pediatr. Gastroenterol. Nutr. 2021, 72, 194–201.
- Gonnot, M.; Neumann, F.; Huet, F.; Maudinas, R.; Leblanc, T.; Lacaille, F. Hepatitis-associated Aplastic Anemia. J. Pediatr. Gastroenterol. Nutr. 2022, 75, 553–555.
- Osugi, Y.; Yagasaki, H.; Sako, M.; Kosaka, Y.; Taga, T.; Ito, T.; Yamamoto, M.; Ohara, A.; Sato, T.; Mimaya, J.; et al. Antithymocyte globulin and cyclosporine for treatment of 44 children with hepatitis associated aplastic anemia. Haematologica 2007, 92, 1687–1690.
- Nastasio, S.; Sciveres, M.; Matarazzo, L.; Maggiore, G. Old and New Treatments for Pediatric Autoimmune Hepatitis. Curr. Pediatr. Rev. 2018, 14, 187–195.
- Kirstein, M.M.; Metzler, F.; Geiger, E.; Heinrich, E.; Hallensleben, M.; Manns, M.P.; Vogel, A. Prediction of short- and long-term outcome in patients with autoimmune hepatitis. Hepatology 2015, 62, 1524–1535.
- Kerkar, N.; Annunziato, R.A.; Liberty, F.; Schmeidler, J.; Rumbo, C.; Emre, S.; Shneider, B.; Shemesh, E. Prospective Analysis of Nonadherence in Autoimmune Hepatitis: A Common Problem. J. Pediatr. Gastroenterol. Nutr. 2006, 43, 629–634.
- van Gerven, N.M.; Verwer, B.J.; Witte, B.I.; van Hoek, B.; Coenraad, M.J.; van Erpecum, K.J.; Beuers, U.; van Buuren, H.R.; de Man, R.A.; Drenth, J.P.; et al. Relapse is almost universal after withdrawal of immunosuppressive medication in patients with autoimmune hepatitis in remission. J. Hepatol. 2013, 58, 141–147.
- Deneau, M.; Book, L.S.; Guthery, S.L.; Jensen, M.K. Outcome after Discontinuation of Immunosuppression in Children with Autoimmune Hepatitis: A Population-Based Study. J. Pediatr. 2014, 164, 714–719.e2.
- Di Giorgio, A.; Hadzic, N.; Dhawan, A.; Deheragoda, M.; Heneghan, M.A.; Vergani, D.; Mieli-Vergani, G.; Samyn, M. Seamless Management of Juvenile Autoimmune Liver Disease: Long-Term Medical and Social Outcome. J. Pediatr. 2020, 218, 121–129.e3.
- Maggiore, G.; Bernard, O.; Mosca, A.; Ballot, E.; Johanet, C.; Jacquemin, E. Long-term outcomes of patients with type 1 or 2 autoimmune hepatitis presenting in childhood. J. Hepatol. 2023, 78, 979–988.
- Maggiore, G.; Bernard, O.; Hadchouel, M.; Hadchouel, P.; Odievre, M.; Alagille, D. Treatment of autoimmune chronic active hepatitis in childhood. J. Pediatr. 1984, 104, 839–844.
- Lee, W.S.; Lum, S.H.; Lim, C.B.; Chong, S.Y.; Khoh, K.M.; Ng, R.T.; Teo, K.M.; Boey, C.C.M.; Pailoor, J. Characteristics and outcome of autoimmune liver disease in Asian children. Hepatol. Int. 2015, 9, 292–302.
- Grønbæk, L.; Otete, H.; Ban, L.; Crooks, C.; Card, T.; Jepsen, P.; West, J. Incidence, prevalence and mortality of autoimmune hepatitis in England 1997-2015. A population-based cohort study. Liver Int. 2020, 40, 1634–1644.
- Sharma, R.; Simon, T.G.; Stephansson, O.; Verna, E.C.; Emond, J.; Söderling, J.; Roelstraete, B.; Hagström, H.; Ludvigsson, J.F. Pregnancy Outcomes in Women with Autoimmune Hepatitis—A Nationwide Population-based Cohort Study with Histopathology. Clin. Gastroenterol. Hepatol. 2023, 21, 103–114.e10.
- Wang, C.W.; Grab, J.; Tana, M.M.; Irani, R.A.; Sarkar, M. Outcomes of pregnancy in autoimmune hepatitis: A population-based study. Hepatology 2022, 75, 5–12.
- Grønbaek, L.; Vilstrup, H.; Jepsen, P. Pregnancy and birth outcomes in a Danish nationwide cohort of women with autoimmune hepatitis and matched population controls. Aliment. Pharmacol. Ther. 2018, 48, 655–663.
- Fischer, S.E.; de Vries, E.S.; Tushuizen, M.E.; de Boer, Y.S.; van der Meer, A.J.P.; de Man, R.A.; Brouwer, J.T.; Kuyvenhoven, J.P.; Klemt-Kropp, M.; Gevers, T.J.G.; et al. Importance of complete response for outcomes of pregnancy in patients with autoimmune hepatitis. Liver Int. 2023, 43, 855–864.
- Llovet, L.-P.; Horta, D.; Eliz, M.G.; Berenguer, M.; Fábrega, E.; Sáez-Royuela, F.; García-Retortillo, M.; Torrijos, Y.S.; Romero-Gómez, M.; Fernández, C.; et al. Presentation and Outcomes of Pregnancy in Patients with Autoimmune Hepatitis. Clin. Gastroenterol. Hepatol. 2019, 17, 2819–2821.
- Westbrook, R.H.; Yeoman, A.D.; Kriese, S.; Heneghan, M.A. Outcomes of pregnancy in women with autoimmune hepatitis. J. Autoimmun. 2012, 38, J239–J244.
- Terrabuio, D.R.B.; Abrantes-Lemos, C.P.; Carrilho, F.J.; Cançado, E.L.R. Follow-up of Pregnant Women with Autoimmune Hepatitis: The disease behavior along with maternal and fetal outcomes. J. Clin. Gastroenterol. 2009, 43, 350–356.
- Matarazzo, L.; Nastasio, S.; Sciveres, M.; Maggiore, G. Pregnancy outcome in women with childhood onset autoimmune hepatitis and autoimmune sclerosing cholangitis on long-term immunosuppressive treatment. Eur. J. Obstet. Gynecol. Reprod. Biol. 2022, 268, 7–11.
- Graham, J.J.; Longhi, M.S.; Heneghan, M.A. T helper cell immunity in pregnancy and influence on autoimmune disease progression. J. Autoimmun. 2021, 121, 102651.
- Si, T.; Huang, Z.; Hegarty, R.; Ma, Y.; Heneghan, M.A. Systematic review with meta-analysis: Outcomes of pregnancy in patients with autoimmune hepatitis. Aliment. Pharmacol. Ther. 2022, 55, 1368–1378.
- Schramm, C.; Herkel, J.; Beuers, U.; Kanzler, S.; Galle, P.R.; Lohse, A.W. Pregnancy in Autoimmune Hepatitis: Outcome and Risk Factors. Am. J. Gastroenterol. 2006, 101, 556–560.
- Centers for Disease Control and Prevention. National Birth Defects Prevention Study (NBDPS). Available online: https://www.cdc.gov/ncbddd/birthdefects/nbdps.html (accessed on 21 August 2023).
- Akbari, M.; Shah, S.; Velayos, F.S.; Mahadevan, U.; Cheifetz, A.S. Systematic Review and Meta-analysis on the Effects of Thiopurines on Birth Outcomes from Female and Male Patients with Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2013, 19, 15–22.
- Werner, M.; Björnsson, E.; Prytz, H.; Lindgren, S.; Almer, S.; Broomé, U.; Wallerstedt, S.; Sandberg-Gertzén, H.; Hultcrantz, R.; Sangfeldt, P.; et al. Autoimmune hepatitis among fertile women: Strategies during pregnancy and breastfeeding? Scand. J. Gastroenterol. 2007, 42, 986–991.
- Reggia, R.; Bazzani, C.; Andreoli, L.; Motta, M.; Lojacono, A.; Zatti, S.; Ramazzotto, F.; Nuzzo, M.; Tincani, A. The Efficacy and Safety of Cyclosporin A in Pregnant Patients with Systemic Autoimmune Diseases. Am. J. Reprod. Immunol. 2016, 75, 654–660.
- Le, H.L.; Francke, M.I.; Andrews, L.M.; de Winter, B.C.M.; van Gelder, T.; Hesselink, D.A. Usage of Tacrolimus and Mycophenolic Acid During Conception, Pregnancy, and Lactation, and Its Implications for Therapeutic Drug Monitoring: A Systematic Critical Review. Ther. Drug Monit. 2020, 42, 518–531.
More
Information
Subjects:
Gastroenterology & Hepatology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
637
Revisions:
3 times
(View History)
Update Date:
06 Sep 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




