1. Introduction
The levels of adropin in blood circulation have been proposed to direct the metabolic state in skeletal muscle by influencing fuel selection preference towards glucose oxidation in the fed state [1]. Studies have shown that adropin regulates the expression of hepatic lipogenic genes and the PPARγ receptor (peroxisome proliferator-activated receptor gamma), the major regulator of lipogenesis [2]. Moreover, adropin regulates angiogenesis, increases blood flow, boosts capillary density and has a protective role for endothelial cells [3]. Apparently, the tissue level of adropin varies in several physiological and biological conditions such as multiple sclerosis [4], COVID-19 [5], gestational diabetes [6] obstructive sleep apnea [7], rheumatoid arthritis [8], coronary artery ectasia [9], acute mesenteric ischemia [10] and diabetic nephropathy [11].
2. Diabetes Mellitus
When adropin was first discovered, most of the attention was given to its role in lipid and carbohydrate metabolism and insulin resistance. Interestingly, some studies show that adropin deficiency plays a role in the development and progression of chronic diseases, such as diabetes mellitus. Zang et al. reported that serum concentrations of adropin were significantly decreased in Chinese type 2 diabetic patients, compared to control subjects
[12]. Other studies confirmed this finding by reporting the downregulation in circulating adropin in adults with type 2 diabetes mellitus
[13], liver disease
[14] and children with type 1 diabetes mellitus
[15]. In contrast, it was elucidated that higher insulin resistance and higher fasting plasma glucose positively correlated with serum adropin levels in patients with type 2 diabetes mellitus
[16].
Additionally, low adropin levels have been shown to correlate with a risk of developing diabetic complications such as diabetic retinopathy
[17], diabetic nephropathy
[18] and gestational diabetes mellitus
[19].
As it was reported that serum adropin levels vary between diabetic and normal subjects, several investigations tried to understand the mechanisms underlying these variations. For instance, it was demonstrated that hyperglycemia was associated with increased adropin expression, as well as the signal transducer and activator of transcription 3 (STAT3) activation in the liver of streptozotocin-induced diabetic rats. The mechanism underlying the elevation of adropin levels and
Enho gene expression in the diabetic rats was suggested to be through STAT3 activation
[20].
During the discovery of adropin, its physiological role was linked to glucose homeostasis. It is thus important to understand the potential role of adropin in controlling hyperglycemia and its effect on insulin-sensitive tissues.
In skeletal muscle, a study that was performed by Gao et al. showed that adropin played a crucial role in modulating glucose utilization in DIO mice with insulin resistance
[1]. Adropin was able to promote glucose oxidation and diminish fatty acid oxidation in skeletal muscle, and that led to an increase in glucose uptake and enhanced mitochondrial function. The metabolic actions for enhancing mitochondrial function were mediated by suppressing the activity of peroxisome proliferator-activated receptor gamma coactivator-1a (PGC-1a), a transcription co-activator that regulates the expression of the genes that are involved in fatty acid oxidation. Moreover, adropin promoted skeletal muscle sensitization to insulin signaling actions by increasing insulin-induced Akt phosphorylation and the cell surface expression of glucose transporter 4 (GLUT4)
[1].
In contrast, a study utilizing insulin-resistant hepatocytes showed that adropin could reduce glucose production in the liver. Adropin treatment downregulated the transcription of hepatic gluconeogenesis genes by inhibiting the binding site of transcription factors forkhead box protein O1(FoxO1) and cAMP-response element binding protein (CREB), along with their co-activators, peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC1α) and CREB regulated transcription coactivator 2 (CRTC2), respectively, to the promoter of gluconeogenesis genes
[21][22]. FoxO1-PGC1α and CREB-CRTC2 complex promoter binding is required to activate the transcription of genes that are involved in hepatic glucose production
[23][24].
Interestingly, this effect of adropin was not observed in insulin-sensitive hepatocytes
[22]. This research team earlier reported that a downregulation in adropin expression led to systemic insulin resistance in mice that were introduced to a high-fat diet for a long period
[25].
Furthermore, it was reported that adropin was associated with incretins in obese males with type 2 diabetes receiving a 3-month treatment of liraglutide
[26]. There was an increase in plasma adropin levels in those subjects. Liraglutide is an anti-diabetic agent, a specific glucagon-like peptide-1 receptor (GLP-1) agonist, also known as incretin mimetic molecule. Incretins are endogenous peptide hormones that are secreted by the GI tract to stimulate insulin secretion from pancreatic β-cells after meals
[27][28][29][30].
It is worth mentioning that irisin, which is a peptide hormone involved in glucose homeostasis
[31][32], has a similar effect on incretins, specifically GLP-1. Both adropin and irisin can enhance glucose-stimulated insulin secretion
[33]. In addition, the peptide apelin increased plasma GLP-1 levels in rats that were intraperitoneally injected with apelin-13
[34]. However, the role of adropin in inducing incretin secretion and augmenting incretin effect remains unclear and needs more study to elucidate the mechanism by which it regulates these GI tract hormones
Table 1.
3. Obesity
Obesity is a major health problem worldwide
[39][40]. Studies performed on humans and animal models suggest that adropin may play a role in lipid metabolism and obesity.
C57BL/6J mice that were fed a high-fat diet exhibited a rapid increase in
Enho gene expression, while fasting reduced the expression of this gene, when compared to the control mice. However, liver
Enho gene expression declined when a high-fat diet was introduced to the mice for a longer period of time, suggesting a regulatory role of the
Enho gene in nutrition, but the expression of adropin is diet-dependent
[2]. Moreover, Kumar et al. generated an adropin knockout mice, which exhibited increased adiposity
[41].
In humans, adropin level was negatively correlated with body mass index (BMI)
[42] in diabetic patients, as overweight and obese patients had considerably reduced levels of adropin, compared to lean patients
[12]. Moreover, when adropin levels were measured in plasma samples that were obtained from healthy subjects, it was found that the peptide levels correlated negatively with BMI and aging
[43]. This observation was supported by studying patients who underwent bariatric surgery and monitoring serum adropin levels before and after surgery
[44]. Serum adropin levels were higher 6 months after bariatric surgery than at baseline, leading to the conclusion that, in some patients, body mass reduction may restore the impaired production of adropin. Another study was conducted to investigate the role of adropin in children with obesity or metabolic syndrome
[45]. The results of this research showed that—there was no significant difference between the plasma level of adropin in obese children, and those individuals with normal weight
Table 1.
4. Cardiovascular Diseases
There is overwhelming evidence that cardiovascular diseases are common in patients suffering from diabetes mellitus
[35][46][47][48][49][50][51]. Several studies and reports indicate the involvement of adropin in the functioning of the cardiovascular system. As mentioned previously, the immunoreactivity of adropin has been detected in many tissues, including the three layers of the heart
[52].
High cardiac fatty acid oxidation rates and impaired cardiac insulin signaling are associated with decreased cardiac efficiency and various cardiac diseases
[53]. Altamimi et al. investigated the effect of adropin on cardiac energy metabolism, insulin signaling and cardiac efficiency
[54]. C57Bl/6 mice were injected with a secretable form of adropin (450 nmol/kg, i.p.) three times over 24 h, then they were fasted, and the hearts were isolated and perfused. Altamimi et al. demonstrated that adropin administration improved cardiac function, cardiac efficiency and coronary flow, compared to the untreated mice. Moreover, by measuring glucose and palmitate contribution in catabolic pathways for ATP production, the important role of adropin on the preference of cardiac glucose oxidation and the inhibition of cardiac fatty acid oxidation were reported. In cardiomyocytes, adropin regulates cell bioenergetics through GPR19 activation. The receptor activation leads to stimulation of p44/42 phosphorylation and, consequently, the downregulation of pyruvate dehydrogenase kinase 4 (PDK4) and pyruvate dehydrogenase (PDH) phosphorylation
[55][56][57][58].
Hyperlipidemia is a risk factor that is associated with cardiovascular diseases
[59]. Akcilar et al. demonstrated the role of a low dose of adropin in reducing hyperlipidemia in rats that were fed a high-fat diet. A reduction in the levels of serum triglycerides, total cholesterol and low-density lipoprotein cholesterol (LDL-C), as well as an increment in the level of high-density lipoprotein cholesterol (HDL-C) were reported
[60]. Additionally, adropin administration reduced the mRNA expression of pro-inflammatory cytokines, tumor necrosis factor alpha (TNF-α) and interleukin-6 (IL-6). This suggests that adropin may have an anti-inflammatory role in the liver and possibly in other organs, including the heart.
Interestingly, a recent meta-analysis found an association between serum adropin and coronary artery disease (CAD)
[61]. The study stated that the serum adropin level in patients with CAD was lower than in healthy individuals, indicating that the decrease in adropin concentration might play an important role in the development of CAD. Another cardiovascular disease, which has been correlated with adropin, is atrial fibrillation. Atrial fibrillation is a condition of abnormal heart rhythm
[62][63]. Decreased serum adropin concentrations were found in atrial fibrillation patients compared with healthy controls. Patients with chronic atrial fibrillation had a significantly reduced serum adropin concentration compared with control patients. Hence, adropin deficiency may contribute to the development and progression of atrial fibrillation
[64] Table 1.
5. Inflammation
Researchers and scientists have also investigated the role of adropin in inflammation and related diseases such as atherosclerosis. Atherosclerosis is a chronic inflammatory disease in response to injury of the arterial wall and the formation of plaque
[38]. Vascular inflammation stimulates the expression of certain adhesion molecules, such as intercellular adhesion molecule 1, vascular cell adhesion molecule 1 in endothelial cells. These adhesion molecules stimulate monocyte adhesion to endothelial cells and monocyte infiltration into the subendothelial space, causing an accumulation of macrophage foam cells. Chronic inflammation of the cardiovascular system is a common feature of chronic diseases including diabetes mellitus, hyperlipidemia and nosocomial conditions
[65][66][67]. Hormones such as adropin and plant-based antioxidants have been used to mitigate the adverse effects of these vascular lesions. Adropin is expressed in human endothelial cells
[3], and it has been shown earlier that adropin inhibits tumor necrosis factor α (TNFα). Sato et al. investigated the relationship between TNFα, monocyte adhesion, human endothelial cells, atherosclerosis and adropin. The experiment involved incubating human endothelial cells with adropin and TNFα and assessing the expression of the adhesion molecules that are involved in atherosclerosis. The results showed that an incubation of adropin alone had no significant effect on the mRNA expression of these adhesion molecules, which are usually stimulated by TNFα. However, when adropin and TNFα were both incubated with human endothelial cells, adropin suppressed the TNFα-induced mRNA expression of adhesion molecules, suggesting a role for adropin in the anti-atherosclerosis process by inhibiting endothelial cells’ adhesion molecules via the suppression of TNFα
[68].
In the case of obesity, the infiltration of macrophages into adipose tissues causes chronic inflammation. Adipocytes secrete cytokines such as TNFα and MCP-1 that attract macrophages and regulatory T cells, leading to fat inflammation. Adropin regulates the expression of PPAR-γ by activating the AKT pathway, thus inhibiting the differentiation of 3T3-L1 preadipocytes into mature adipocytes and consequently reducing fat accumulation and fat inflammation
[69].
In another study, lower adropin plasma levels and increased inflammation markers such as TNFα and interleukin-6 (IL-6) were reported in male patients with moderate and severe obstructive sleep apnea, compared to healthy individuals
[70].
Furthermore, in order to investigate how adropin could affect hepatocyte inflammation and injury in nonalcoholic steatohepatitis (NASH), immunohistochemistry using the inflammation markers F4/80, CD45 and MCP-1, and a gene expression analysis for TNFα and IL-6 genes, were performed using liver tissues from adropin knockout C57BL/6J mice and the control wild-type, which were fed a methionine-choline deficient diet
[71]. Methionine-choline deficient diet is the classic dietary model for studying NASH, and usually, rodents consuming this diet develop steatohepatitis, necroinflammation, and fibrosis, similar to human NASH
[36]. The pathohistological analysis showed a higher signal of F4/80, CD45 and MCP1 and a substantial induction of genes TNFα and IL-6 in adropin knockout mice. These results indicate the presence of elevated inflammatory responses in adropin knockout mice, when compared to that of the wild-type mice
[71] (
Table 1).
6. Cell Proliferation and Differentiation
Far beyond the classical action, adropin can stimulate cell proliferation and differentiation. Lovren et al. showed that adropin has the ability to induce the proliferation and capillary-like tube formation of endothelial cells that stimulate angiogenesis
[3]. Adropin upregulated endothelial NO synthase expression through VEGFR2 2-PI3K-Akt and VEGFR2-extracellular signal-regulated kinase pathways to reduce inflammation.
Furthermore, in rat primary preadipocytes and 3T3-L1 cells, preadipocyte proliferation was increased by adropin treatment, while the differentiation of those preadipocytes into mature adipocytes was reduced
[37]. The suppression of adipogenic markers and lipid accumulation demonstrate the important role of adropin in the fight against obesity
[69]. The same research team reported a similar effect of adropin on primary brown preadipocytes that were isolated from the interscapular region in rat
[72].
On the other hand, it was reported that adropin downregulated the proliferation and migration of human aortic smooth muscle cells (HASMCs) in vitro, providing evidence that the peptide is also protective against atherosclerosis
[68] (
Table 1). The role of adropin in different tissues and organ systems is depicted in
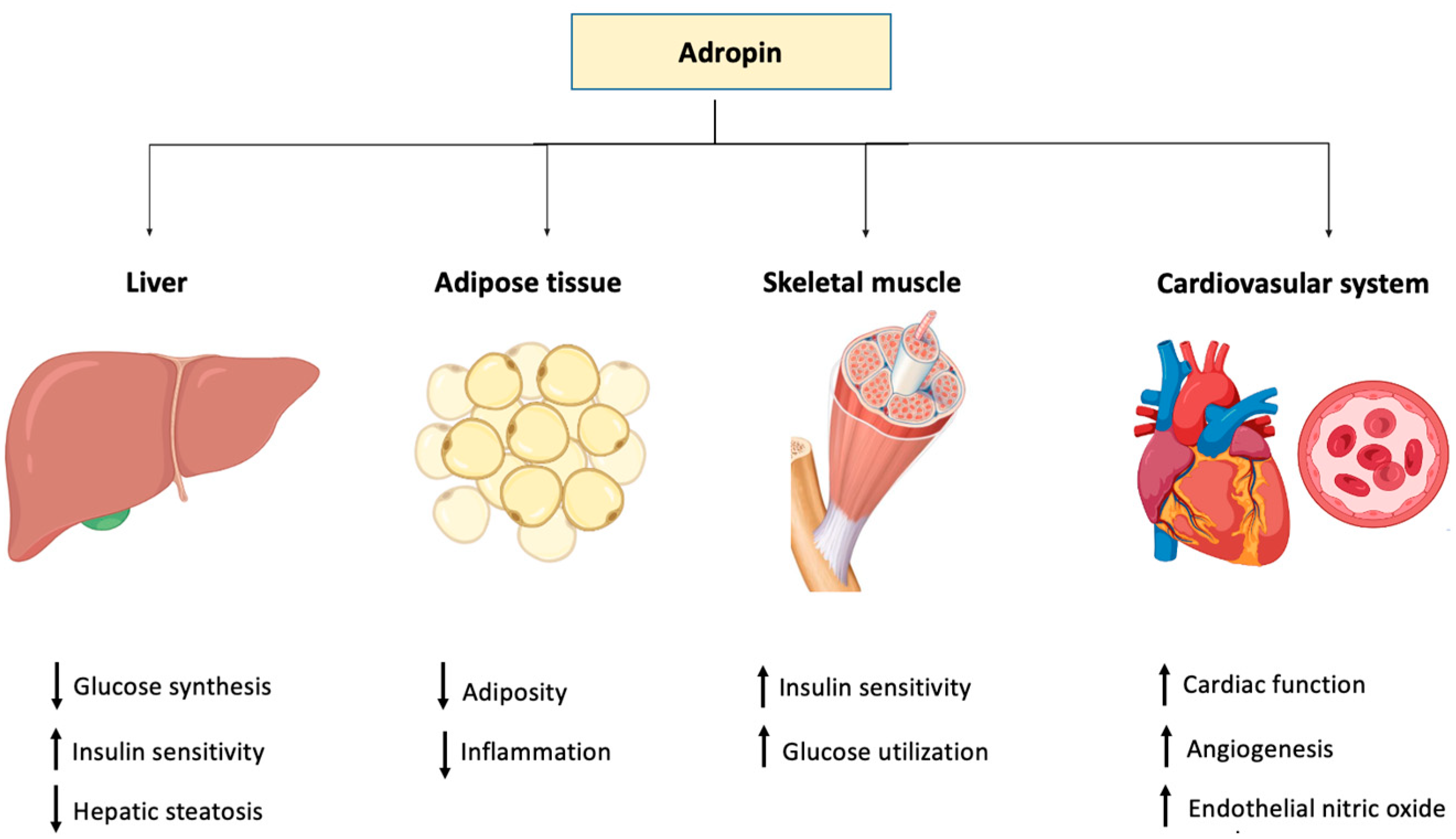
Figure 1.
Figure 1. Graphical representation of the function of adropin in different body tissues.