Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Gabriel da Silva Oliveira | -- | 1850 | 2023-08-23 20:48:45 | | | |
| 2 | Dean Liu | Meta information modification | 1850 | 2023-08-24 03:51:14 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Oliveira, G.D.S.; Mcmanus, C.; De Araújo, M.V.; De Sousa, D.E.R.; De Macêdo, I.L.; Castro, M.B.D.; Santos, V.M.D. Sanitizing Hatching Eggs with Essential Oils. Encyclopedia. Available online: https://encyclopedia.pub/entry/48393 (accessed on 07 February 2026).
Oliveira GDS, Mcmanus C, De Araújo MV, De Sousa DER, De Macêdo IL, Castro MBD, et al. Sanitizing Hatching Eggs with Essential Oils. Encyclopedia. Available at: https://encyclopedia.pub/entry/48393. Accessed February 07, 2026.
Oliveira, Gabriel Da Silva, Concepta Mcmanus, Maria Viviane De Araújo, Davi Emanuel Ribeiro De Sousa, Isabel Luana De Macêdo, Marcio Botelho De Castro, Vinícius Machado Dos Santos. "Sanitizing Hatching Eggs with Essential Oils" Encyclopedia, https://encyclopedia.pub/entry/48393 (accessed February 07, 2026).
Oliveira, G.D.S., Mcmanus, C., De Araújo, M.V., De Sousa, D.E.R., De Macêdo, I.L., Castro, M.B.D., & Santos, V.M.D. (2023, August 23). Sanitizing Hatching Eggs with Essential Oils. In Encyclopedia. https://encyclopedia.pub/entry/48393
Oliveira, Gabriel Da Silva, et al. "Sanitizing Hatching Eggs with Essential Oils." Encyclopedia. Web. 23 August, 2023.
Copy Citation
Increased meat and egg production leads to concomitant changes in poultry practices, including the indiscriminate use of formaldehyde to sanitize hatching eggs. Although this sanitizer aids in the increase in poultry production, its toxic potential for man and for avian embryos represents an obstacle to its long-term use.
economic gains
egg disinfection
embryological safety
egg microbiology
poultry health
1. Introduction
The large number of healthy embryos that hatch supports the hypothesis that eggs have good microbiological quality. Ensuring embryo safety in the face of microbiological challenges is not easy. The embryo’s immature status makes it insecure and defenseless against infection [1]. In this case, the eggshell can have a negative effect because it contains pathogenic microorganisms [2] and has communication routes with the embryo, favoring contact between them. Therefore, the quest for healthier poultry is increasing the need to incubate eggs with minimal microbial loads in poultry hatcheries during all incubation cycles. In this case, sanitizing hatching eggs with liquid or gas is the gold standard method of achieving this goal [3]. The sanitization of hatching eggs is nothing more than an antimicrobial resource intermediated by a simple or complex system (e.g., fumigation, spraying, or immersion) that applies a sanitizing solution to the eggshells to solve poultry losses caused by microorganisms. This step must occur within half an hour after laying or immediately collection [4][5][6].
In line with the current trend towards ecologically friendly products with minimal impact on animals, the poultry industry needs to gradually adopt sanitizers that respect safety criteria for the protection of avian life. In a previously published review, Oliveira et al. [3] showed that there are various sanitizers for hatching eggs that are available to the poultry industry which are divided into two large groups (synthetic and natural). Among the natural options recommended to the industry, the authors show that essential oils derived from volatile liquids from aromatic plants are antimicrobial and safe to use. The use of essential oils as sanitizers for hatching eggs was reviewed by Oliveira et al. [5]. They reported that essential oils compete with synthetic materials for reasons that are of interest to the poultry industry, including embryo and human safety, the ability to control microorganisms in the eggshell, and increased production rates. These effects can be seen at low concentrations, which may overcome the disadvantages of essential oils where they are more expensive than synthetic compounds that require higher concentrations for effective action. Thus, validating the potential and advantageous characteristics of essential oils in the management of hatching eggs can open an important path for their inclusion in the official list of sanitizers used in poultry routines around the world.
2. Eggshell Microorganisms: Risks for Poultry Embryos and Chicks
Even as an immunologically sensitive embryo, poultry already interact with pathogenic microbes originating from any stage prior to hatching [7]. This interaction may be a consequence of horizontal transmission [8] (Figure 1) and puts the poultry’s life in danger. Fonseca et al. [9] observed that, by contaminating the eggshell, Campylobacter jejuni bacteria can penetrate it, cross the albumen and reach the yolk sac, probably resulting in embryonic mortality. This led the authors to state that the immunity conferred by breeding hens to the egg/embryo may be insufficient and inefficient for certain infections. In addition, although the eggshell is an oval antimicrobial wall formed by the fusion of membranes and mineral layers equivalent to a vital organ of a living organism (it promotes the flow of nutrients, water, oxygen and carbon dioxide to keep the embryo alive) [10], it is not totally resistant to microbial entry. The eggshell is challenged when there are microorganisms trying to move from its surface to the main target (embryo). Oliveira et al. [6] reviewed the types of microorganisms that contaminate eggshells. Among the bacterial and fungal genera cited are Alcaligenes, Enterobacter, Escherichia, Klebsiella, Proteus, Providencia, Pseudomonas, Salmonella, Clostridium, Enterococcus, Staphylococcus, Streptococcus, Aspergillus, Candida and Penicillium.
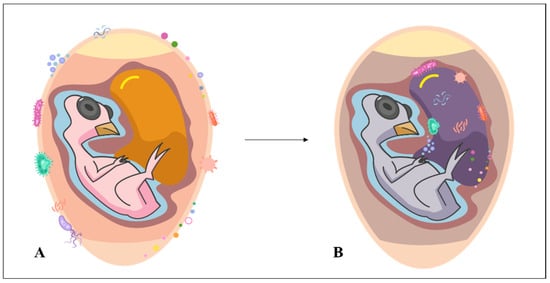
Figure 1. Horizontal contamination in hatching eggs. Microorganisms on the shell (A) penetrated and reached the yolk sac (B).
Previously published studies have reported the adverse relationship of microorganisms with embryos and/or chicks. Weil and Volentine [11] reported that contamination of the yolk sac of the chicken embryo by Shigella dysenteriae can cause lethal infection. Embryos from chickens killed by contamination with avian pathogenic Escherichia coli and Salmonella enterica subsp. enterica serovar Enteritidis showed signs of congestion and diffuse redness throughout the skin, head and neck, as well as microscopic lesions in the yolk sac, including congestion, inflammation, damaged blood vessels and abnormal endodermal epithelial cells [1]. Fungi of the genus Aspergillus, which may be responsible for causing mycoses or mycotoxicosis, have been isolated from dead chicken embryos [12]. Saleemi et al. [13] reported that aflatoxigenic fungal extracts isolated from Aspergillus fungi caused high embryonic mortality, weight reduction and severe alterations in the liver (fatty alteration and cell necrosis) and kidneys (congestion and tubular necrosis) of chicks. Karunarathna et al. [14] demonstrated that multidrug-resistant Escherichia coli and Enterococcus were recovered from the yolk of non-viable chicken embryos at hatching. Contamination by Enterococcus spp. can trigger pulmonary hypertension syndrome in chicken embryos and chicks [15]. Mortality of chicken embryos associated with Enterococcus contamination was reported by Karunarathna et al. [16]. Multidrug-resistant bacteria that cause yolk sac infection, including Escherichia coli, Salmonella, and Staphylococcus, have been recovered from dead embryos and chicks [17][18]. Far et al. [19] observed that dead ostrich embryos were contaminated with Pseudomonas spp., Klebsiella spp., Bacillus spp., Citrobacter spp., Staphylococcus spp., Proteus spp., Aeromonas spp., Enterobacter spp., as well as Escherichia coli with antimicrobial resistance profile.
The findings mentioned above raise concerns, especially in relation to the health of poultry and humans, since multiresistant microorganisms can spread and cause massive irreversible damage. In addition, the undue, exacerbated use of sanitizers without proven scientific tests and without the prescription of trained professionals can contribute to even worse health and economic instability. Therefore, collective efforts within the poultry industry should focus on antimicrobial interventions that involve the controlled use of broad-spectrum sanitizers focused on hatching egg sanitization.
3. Essential Oils and Their In Vitro Antimicrobial Activity
Essential oils are any aromatic, viscous and volatile oils belonging to plants. Syzygium aromaticum, Allium sativum, Ocimum basilicum, Thymus vulgaris, Lavandula angustifolia, Eucalyptus globulus, Citrus sinensis, Citrus aurantifolia, Cinnamomum cassia, Rosmarinus officinalis, Origanum vulgare, Allium cepa, Cymbopogon winterianus, Cymbopogon flexuosus, Piper nigrum, Zingiber officinale, Protium pallidum, Litsea citrata, Satureja hortensis, Salvia officinalis, Mentha piperita, Cedrus deodara, and Cuminum cymincum are examples of plant species that provide commercially available essential oils that may have promising futures in poultry nutrition and production such as egg coating additives and sanitizers for hatching eggs. This is because essential oils have a chemical configuration that triggers their biological properties. For example, hydrocarbons, esters, lactones, alcohols, oxides, phenols, ketones, and aldehydes are present in the chemical composition of essential oils with similar or distinct bioactive functions. Depending on the compound, these functions include antimicrobial, antiviral, antitumoral, antibacterial, stimulant, anesthetic, anti-inflammatory, anti-fungal, antipyretic, and spasmolytic [20]. The content, quality, and effectiveness of essential oil compounds depend on factors such as extraction, which can be by hydro distillation, steam distillation, supercritical CO2 extraction, ultrasonic extraction, and cold pressing [21][22][23][24].
In vitro antimicrobial screenings initially detect the potential viability of essential oils before they are used as in vivo antimicrobial agents. These screenings demonstrated that essential oils are effective against standard Gram-negative and positive bacterial strains and avian isolates, as well as standard and avian-isolated fungi. Among the bacteria are Salmonella enterica subsp. enterica serovar Enteritidis, Salmonella enterica subsp. enterica serovar Typhimurium, Salmonella enterica subsp. enterica serovar Infantis and avian pathogenic Escherichia coli (APEC), which are important pathogenic bacteria for poultry and public health. The antimicrobial effectiveness of essential oils ranges from mild to very strong. In fact, some of them have been shown to be more effective than conventional antibiotics [25][26]. Thymol, eugenol, carvacrol, linalool, citral, limonene, trans-cinnamaldehyde, geraniol and citronellal are some compounds that are part of the composition of some essential oils that can act as protagonists in antimicrobial action. The main mechanisms responsible for making the bacterial [27] and fungal [28] cells unfeasible are listed below:
-
Bacteria:
-
Cell membrane alteration and increased permeability.
-
Stops energy production.
-
Blocks active transport.
-
Fungi:
-
Cell membrane disruption, alteration, and inhibition of cell wall formation.
-
Dysfunction of fungal mitochondria.
-
Inhibition of efflux pumps.
Essential oils can promote beneficial actions for human health by reducing pain and inflammation, protecting and healing wounds, neutralizing or stopping the development of carcinogens, neutralizing oxidative stress and possessing antiviral, antibacterial, antifungal, cardioprotective, antidiabetic, and insect-repellent properties; among other benefits, they can also potentially treat central-nervous-system-based disorders [29][30][31][32]. The safety of a stock of essential oils including Ocimum basilicum, Zingiber officinale, Lavandula officinalis, Cymbopogon citratus, Mentha piperita, Rosmarinus officinalis, Thymus vulgaris, Eugenia caryophyllata, and Allium sativum has been documented and they received the generally recognized as safe (GRAS) seal [33]. However, the intake of essential oils needs to be monitored, as they can, like any other edible food, cause an inappropriate effect.
4. Comparing Essential Oils and Formaldehyde for Sanitizing Hatching Eggs
Formaldehyde is still preferably used in the practice of sanitizing hatching eggs [3][34][35]. Antimicrobial effectiveness and cost are two of the main reasons why formaldehyde remains in use in the poultry industry. Even its strong toxicity to poultry embryos [34][36][37] and humans [38][39][40] has not managed to have it removed from the practice of sanitizing hatching eggs. However, researchers are strongly committed to continuing to alert the poultry industry that, from a health point of view, formaldehyde is not compatible with a sustainable and safe poultry chain.
The sanitization of hatching eggs with natural sanitizers is based on a sanitary practice of microbial control of eggshells without synthetic chemical treatments, which aims to contribute to the production of healthy chicks free of pathogenic microorganisms using exclusively substances derived from plants and friends of living organisms [5][41][42][43]. Comparing natural sanitizers made from essential oils with synthetic sanitizers made from formaldehyde, there should be conscious support for the transition from sanitization systems that involve aggressive products to those that use green and responsible products. In addition to the antibiotic profile capable of significantly reducing the microbial count of hatching eggshells, one of the main advantages of using essential oils as sanitizers for hatching eggs is the productive results promoted in terms of hatchability, which, on average, are not inferior to those of sanitization with formaldehyde (Table 1). Thus, the application of essential oils to hatching eggs does not require additional or different practices to promote the production of the same number of poultry than is routine in the conventional poultry sector. The prioritized use of synthetic chemicals in hatching egg management can be minimized by replacing them with essential oils.
Table 1. Comparison between the efficiency of essential oils and formaldehyde after application in hatching eggs.
| Compounds | Bacterial Count (log) a | Hatchability (%) a | Significance b | Most Efficient | Study |
|---|---|---|---|---|---|
| Origanum onites | <0.47 | >1.98 | * TBC ns Hatchability | Essential oil | [44] |
| Formaldehyde | <0.06 | >1.89 | |||
| Thymus vulgaris | <1.68 | >6.95 | * | Formaldehyde | [45] |
| Formaldehyde | <1.81 | >9.70 | |||
| Syzygium aromaticum | <1.19 | >10.66 | ns | Similar | [41] |
| Paraformaldehyde | <1.26 | >7.84 | |||
| Origanum vulgare | <6.33 | >12.05 | * | Essential oils | [36] |
| Cuminum cyminum | <6.13 | >11.70 | |||
| Formaldehyde | <3.03 | <2.01 |
a Comparison of essential oils and formaldehyde with non-sanitized eggs; b Comparison between essential oil and formaldehyde; * Significant; ns non-significant, TBC, Total bacteria count.
References
- Rezaee, M.S.; Liebhart, D.; Hess, C.; Hess, M.; Paudel, S. Bacterial Infection in Chicken Embryos and Consequences of Yolk Sac Constitution for Embryo Survival. Vet. Pathol. 2021, 58, 71–79.
- Fardows, J.; Shamsuzzaman, S.M. Detection of Potential Pathogenic Aerobic Bacteria from Egg Shell and Egg Contents of Hen Collected from Poultry. Bangladesh Med. Res. Counc. Bull. 2015, 41, 67–72.
- Oliveira, G.d.S.; McManus, C.; Salgado, C.B.; dos Santos, V.M. Effects of Sanitizers on Microbiological Control of Hatching Eggshells and Poultry Health during Embryogenesis and Early Stages after Hatching in the Last Decade. Animals 2022, 12, 2826.
- Araújo, W.A.G.; Albino, L.F.T. Incubação Comercial ; Transworld Research Network: Trivandrum, India, 2011.
- Oliveira, G.d.S.; dos Santos, V.M.; Nascimento, S.T. Essential Oils as Sanitisers for Hatching Eggs. Worlds Poult. Sci. J. 2021, 77, 605–617.
- Oliveira, G.d.S.; dos Santos, V.M.; McManus, C. Propolis: Effects on the Sanitisation of Hatching Eggs. Worlds Poult. Sci. J. 2022, 78, 261–272.
- Oliveira, G.d.S.; McManus, C.; dos Santos, V.M. Garlic as Active Principle of Sanitiser for Hatching Eggs. Worlds Poult. Sci. J. 2022, 78, 1037–1052.
- Jahantigh, M. Bacteriological Study of Dead-in-Shell Embryos of Ostrich. Iran J. Vet. Res. 2010, 11, 88–90.
- Fonseca, B.B.; Beletti, M.E.; Melo, R.T.; Mendonça, E.P.; Vieira, C.U.; Levenhagen, M.A.; Rossi, D.A. Transfer, Viability and Colonisation of Campylobacter jejuni in the Chicken Vitellus and in Embryos. Br. Poult. Sci. 2011, 52, 279–286.
- Hincke, M.; Gautron, J.; Rodriguez-Navarro, A.B.; McKee, M.D. The Eggshell: Structure and Protective Function. In Improving the Safety and Quality of Eggs and Egg Products: Egg Chemistry, Production and Consumption; Woodhead Publishing: Sawston, UK, 2011; pp. 151–182.
- Weil, A.J.; Volentine, J.A. Infection of the Developing Chick Embryo with Dysentery Bacilli. Proc. Soc. Exp. Biol. Med. 1940, 44, 160–161.
- Kwanashie, C.N.; Kazeem, H.M.; Umoh, J.U.; Abdu, P.A. Aspergillus Species Associated with Dead-in-Shell Chick Embryo in Some Hatcheries in Northwest Nigeria. Eurasian J. Vet. Sci. 2014, 30, 11–13.
- Saleemi, M.K.; Khan, M.Z.; Khan, A.; Hassan, Z.U.; Khan, W.A.; Rafique, S.; Fatima, Z.; Sultan, A. Embryotoxic and Histopathological Investigations of In-Ovo Inoculation of Aflatoxigenic Fungal Extracts in Chicken Embryos. Pak. Vet. J. 2015, 35, 403–408.
- Karunarathna, R.; Ahmed, K.A.; Liu, M.; Yu, C.; Popowich, S.; Goonewardene, K.; Gunawardana, T.; Kurukulasuriya, S.; Gupta, A.; Ayalew, L.E.; et al. Non-Viable Chicken Embryos: An Overlooked Niche Harbouring a Significant Source of Multidrug Resistant Bacteria in the Poultry Production. Int. J. Vet. Sci. Med. 2020, 8, 9–17.
- Kizerwetter-Świda, M.; Binek, M. Bacterial Microflora of the Chicken Embryos and Newly Hatched Chicken. J. Anim. Feed Sci. 2008, 17, 224–232.
- Karunarathna, R.; Popowich, S.; Wawryk, M.; Chow-Lockerbie, B.; Ahmed, K.A.; Yu, C.; Liu, M.; Goonewardene, K.; Gunawardana, T.; Kurukulasuriya, S.; et al. Increased Incidence of Enterococcal Infection in Nonviable Broiler Chicken Embryos in Western Canadian Hatcheries as Detected by Matrix-Assisted Laser Desorption/Ionization-Time-of-Flight Mass Spectrometry. Avian. Dis. 2017, 61, 472–480.
- Nasrin, S.; Islam, M.; Khatun, M.; Akhter, L.; Sultana, S. Characterization of Bacteria Associated with Omphalitis in Chicks. Bangladesh Vet. 2012, 29, 63–68.
- Amer, M.M.; Elbayoumi, K.M.; Amin Girh, Z.M.S.; Mekky, H.M.; Rabie, N.S. A Study On Bacterial Contamination of Dead in Shell Chicken Embryos and Culled One Day Old Chicks. Int. J. Pharm. Clin. Res. 2017, 7, 5–11.
- Far, R.; Peighambari, S.M.; Sadrzadeh, A.; Badouei, A. Bacterial Contamination of Dead-in-Shell Embryos in Ostrich Hatcheries and Antimicrobial Resistance Patterns of Isolated Escherichia coli. Iran. J. Vet. Med. 2013, 7, 169–175.
- Ni, Z.J.; Wang, X.; Shen, Y.; Thakur, K.; Han, J.; Zhang, J.G.; Hu, F.; Wei, Z.J. Recent Updates on the Chemistry, Bioactivities, Mode of Action, and Industrial Applications of Plant Essential Oils. Trends Food Sci. Technol. 2021, 110, 78–89.
- Tu, X.F.; Hu, F.; Thakur, K.; Li, X.L.; Zhang, Y.S.; Wei, Z.J. Comparison of Antibacterial Effects and Fumigant Toxicity of Essential Oils Extracted from Different Plants. Ind. Crops Prod. 2018, 124, 192–200.
- Hatami, T.; Johner, J.C.F.; Zabot, G.L.; Meireles, M.A.A. Supercritical Fluid Extraction Assisted by Cold Pressing from Clove Buds: Extraction Performance, Volatile Oil Composition, and Economic Evaluation. J. Supercrit. Fluids 2019, 144, 39–47.
- Shukla, A.; Naik, S.N.; Goud, V.V.; Das, C. Supercritical CO2 Extraction and Online Fractionation of Dry Ginger for Production of High-Quality Volatile Oil and Gingerols Enriched Oleoresin. Ind. Crops Prod. 2019, 130, 352–362.
- Jadhav, N.L.; Garule, P.A.; Pinjari, D.V. Comparative Study of Ultrasound Pretreatment Method with Conventional Hydrodistillation Method for Extraction of Essential Oil from Piper betle L. (Paan). Indian Chem. Eng. 2022, 64, 132–140.
- El Atki, Y.; Aouam, I.; El Kamari, F.; Taroq, A.; Nayme, K.; Timinouni, M.; Lyoussi, B.; Abdellaoui, A. Antibacterial Activity of Cinnamon Essential Oils and Their Synergistic Potential with Antibiotics. J. Adv. Pharm. Technol. Res. 2019, 10, 63–67.
- Selles, S.M.A.; Kouidri, M.; Belhamiti, B.T.; Ait Amrane, A. Chemical Composition, In Vitro Antibacterial and Antioxidant Activities of Syzygium aromaticum Essential Oil. J. Food Meas. Charact. 2020, 14, 2352–2358.
- Swamy, M.K.; Akhtar, M.S.; Sinniah, U.R. Antimicrobial Properties of Plant Essential Oils against Human Pathogens and Their Mode of Action: An Updated Review. Evid.-Based Complement. Altern. Med. 2016, 2016, 3012462.
- Nazzaro, F.; Fratianni, F.; Coppola, R.; De Feo, V. Essential Oils and Antifungal Activity. Pharmaceuticals 2017, 10, 86.
- Elshafie, H.S.; Camele, I. An Overview of the Biological Effects of Some Mediterranean Essential Oils on Human Health. BioMed Res. Int. 2017, 2017, 9268468.
- Haro-González, J.N.; Castillo-Herrera, G.A.; Martínez-Velázquez, M.; Espinosa-Andrews, H. Clove Essential Oil (Syzygium aromaticum L. Myrtaceae): Extraction, Chemical Composition, Food Applications, and Essential Bioactivity for Human Health. Molecules 2021, 26, 6387.
- Soares, G.A.B.E.; Bhattacharya, T.; Chakrabarti, T.; Tagde, P.; Cavalu, S. Exploring Pharmacological Mechanisms of Essential Oils on the Central Nervous System. Plants 2022, 11, 21.
- Ezeorba, T.P.C.; Chukwudozie, K.I.; Ezema, C.A.; Anaduaka, E.G.; Nweze, E.J.; Okeke, E.S. Potentials for Health and Therapeutic Benefits of Garlic Essential Oils: Recent Findings and Future Prospects. Pharmacol. Res. Mod. Chin. Med. 2022, 3, 100075.
- Code of Federal Regulations (CFR). Part 182.20 Essential Oils, Oleoresins (Solvent-Free), and Natural Extractives (including Distillates). Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/cfrsearch.cfm?fr=182.20. (accessed on 16 May 2023).
- Cadirci, S. Disinfection of Hatching Eggs by Formaldehyde Fumigation—A Review. Eur. Poult. Sci. 2009, 73, 116–123.
- Melo, E.F.; Clímaco, W.L.S.; Triginelli, M.V.; Vaz, D.P.; de Souza, M.R.; Baião, N.C.; Pompeu, M.A.; Lara, L.J.C. An Evaluation of Alternative Methods for Sanitizing Hatching Eggs. Poult. Sci. 2019, 98, 2466–2473.
- Bekhet, G.; Khalifa, A.Y.Z. Essential Oil Sanitizers to Sanitize Hatching Eggs. J. Appl. Anim. Res. 2022, 50, 695–701.
- Hayretdaǧ, S.; Kolankaya, D. Investigation of the Effects of Pre-Incubation Formaldehyde Fumigation on the Tracheal Epithelium of Chicken Embryos and Chicks. Turk. J. Vet. Anim. Sci. 2008, 32, 263–267.
- Qu, M.; Lu, J.; He, R. Formaldehyde from Environment. In Formaldehyde and Cognition; He, H., Ed.; Springer Science + Business Media B.V.: Berlin, Germany, 2017; Volume 1, pp. 1–19.
- Dan, S.; Pant, M.; Kaur, T.; Pant, S. Toxic effect of formaldehyde: A systematic review. Int. Res. J. Mod. Eng. Technol. Sci. 2020, 2, 179–189.
- Bernardini, L.; Barbosa, E.; Charão, M.F.; Brucker, N. Formaldehyde Toxicity Reports from In Vitro and in vivo Studies: A Review and Updated Data. Drug Chem. Toxicol. 2020, 45, 972–984.
- Oliveira, G.D.S.; Nascimento, S.T.; dos Santos, V.M.; Silva, M.G. Clove Essential Oil in the Sanitation of Fertile Eggs. Poult. Sci. 2020, 99, 5509–5516.
- Fouad, W.; Abdel-Hafez, M.S. Effect of Spraying Hatching Eggs of Japanese Quails by Live Yeast on Physiological Changes in the Embryonic Development, Hatchability and Total Bacterial Count. Egypt. Poult. Sci. J. 2017, 37, 1303–1321.
- Toghyani, P.; Shahzamani, S.; Gholami Ahangaran, M.; Ali Mousavi Firouzabadi, S. Comparison of Eucalyptus Extract and Formaldehyde on Hatchability and Survival Rate of Chicks in Disinfection of Fertile Eggs. Int. J. Pharm. Res. Allied Sci. 2020, 9, 105–109.
- Copur, G.; Arslan, M.; Duru, M.; Baylan, M.; Canogullari, S.; Aksan, E. Use of Oregano (Origanum onites L.) Essential Oil as Hatching Egg Disinfectant. Afr. J. Biotechnol. 2010, 8, 2531–2538.
- Shahein, E.H.A.; Sedeek, E.K. Role of Spraying Hatching Eggs with Natural Disinfectants on Hatching Characteristics and Eggshell Bacterial Counts. Egypt. Poult. Sci. J. 2014, 34, 213–230.
More
Information
Subjects:
Microbiology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
687
Revisions:
2 times
(View History)
Update Date:
24 Aug 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




