Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Vasile Valeriu Lupu | -- | 2527 | 2023-08-22 11:23:41 | | | |
| 2 | Jason Zhu | Meta information modification | 2527 | 2023-08-23 03:27:47 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Mocanu, A.; Bogos, R.A.; Lazaruc, T.I.; Trandafir, L.M.; Lupu, V.V.; Ioniuc, I.; Alecsa, M.; Ivanov, A.; Lupu, A.; Starcea, I.M. Gut Microbiota and Chronic Kidney Disease. Encyclopedia. Available online: https://encyclopedia.pub/entry/48314 (accessed on 13 January 2026).
Mocanu A, Bogos RA, Lazaruc TI, Trandafir LM, Lupu VV, Ioniuc I, et al. Gut Microbiota and Chronic Kidney Disease. Encyclopedia. Available at: https://encyclopedia.pub/entry/48314. Accessed January 13, 2026.
Mocanu, Adriana, Roxana Alexandra Bogos, Tudor Ilie Lazaruc, Laura Mihaela Trandafir, Vasile Valeriu Lupu, Ileana Ioniuc, Mirabela Alecsa, Anca Ivanov, Ancuta Lupu, Iuliana Magdalena Starcea. "Gut Microbiota and Chronic Kidney Disease" Encyclopedia, https://encyclopedia.pub/entry/48314 (accessed January 13, 2026).
Mocanu, A., Bogos, R.A., Lazaruc, T.I., Trandafir, L.M., Lupu, V.V., Ioniuc, I., Alecsa, M., Ivanov, A., Lupu, A., & Starcea, I.M. (2023, August 22). Gut Microbiota and Chronic Kidney Disease. In Encyclopedia. https://encyclopedia.pub/entry/48314
Mocanu, Adriana, et al. "Gut Microbiota and Chronic Kidney Disease." Encyclopedia. Web. 22 August, 2023.
Copy Citation
The human intestinal microbiota is a highly intricate structure with a crucial role in promoting health and preventing disease. It consists of diverse microbial communities that inhabit the gut and contribute to essential functions such as food digestion, nutrient synthesis, and immune system development. The composition and function of the gut microbiota are influenced by a variety of factors, including diet, host genetics, and environmental features. In pediatric patients, the gut microbiota is particularly dynamic and vulnerable to disruption from endogenous and exogenous factors.
microbiota
children
gut–kidney axis
chronic kidney disease
1. Introduction
Up to 10% of the population worldwide is affected by CKD [1]. In recent years, researchers have focused on the gut–kidney interaction in CKD. Patients often experience a prevalent disturbance in their gut microbiota. This imbalance is connected to various factors, including elevated levels of gut-derived uremic toxins in the bloodstream, inflammation, and oxidative stress. These factors are closely associated with cardiovascular disease and an increased risk of morbidity and mortality. Targeting the gut microbiota through therapies could potentially prevent CKD and its associated health conditions [2][3]. Given that CKD can start in early life, even during the prenatal period in some cases such as severe obstructive malformations, Prune Belly syndrome, and polycystic diseases, it is crucial to initiate prevention and treatment as early as possible. Recent research suggests that the early disruption of the microbiota is considered significant in the onset and advancement of various diseases, as it affects crucial metabolic and immunomodulatory processes. Already established is the fact that there are differences in the quantity and quality of intestinal microflora in newborns depending on the method of delivery. [4][5][6][7].
2. Developmental Origins of Health and Disease and CKD
Developmental Origins of Health and Disease (DOHaD) is a concept proposing that early life experiences, including fetal development, can have long-term effects on an individual’s health later in life [8]. This field of research suggests that environmental factors and experiences during critical periods of development can influence the programming of physiological systems and increase susceptibility to certain diseases in adulthood. The DOHaD framework suggests that unfavorable circumstances in prenatal development, such as poor maternal nutrition, distress, exposure to toxins, or impaired placental function, can lead to alterations in fetal growth and development. The adaptations of the fetus to these adverse conditions during pregnancy may increase the risk of installing chronic diseases [9][10].
The number of nephrons a person has is determined during fetal development and remains relatively stable throughout life. Research suggests that individuals born with a lower number of nephrons, a condition known as a low nephron endowment, are more susceptible to developing kidney disease and consecutive hypertension later in life. The low nephron endowment theory proposes that individuals with a reduced nephron number have a limited capacity to compensate for age-related nephron loss or adapt to other insults such as high blood pressure or diabetes. For this reason, they may be more prone to developing kidney disease, including CKD, and hypertension, which is a leading cause of CKD [11].
Maternal protein restriction [12][13][14] and iron and vitamin A deficiency [15][16] have been identified as factors that can disrupt normal fetal nephrogenesis—the process of kidney development. Furthermore, a recent study revealed the significance of fetal programming in nephrogenesis by demonstrating the effects of maternal fasting for 16 h per day [17].
3. Nitric Oxide (NO) Prenatal Deficiency and CKD
NO modulates a number of important physiological functions in the digestive system and appears to be a crucial mediator of tissue damage in a number of diseases. Patients with chronic kidney disease have lower levels of the antioxidant enzymes catalase and Cu-Zn superoxide dismutase [18]. This shows that inflammation and disruption of the epithelial tight junction by uremic toxins is linked to a weaker antioxidative system. Simultaneously, plasma concentrations of nitric oxide synthase, monocyte chemotactic protein 1, and COX-2 are elevated. Nitric oxide is known to significantly modulate a number of physiological processes. The mechanisms, through which NO plays various roles in organisms, include the modulation of sodium transporters, epigenetic regulation, and the influence of gut microbiota.
-
Sodium transporters: renal disease and high blood pressure have been linked to increased expression and activity of sodium transporters, leading to higher sodium reabsorption [18][19]. NO has been shown to inhibit the work of certain sodium transporters [20]. Therefore, it is thought that a deficient NO may fail to balance the impaired sodium transporters in the context of early life insults, ultimately contributing to programmed high blood pressure, as illustrated in Figure 1.
-
Epigenetic regulation: Epigenetic mechanisms, such as histone alterations, DNA methylation, and RNAs of a non-coding nature play a role in developmental programming [21]. These mechanisms can influence gene expression patterns and contribute to long-term health outcomes. It is possible that NO signaling may impact epigenetic regulation, thereby influencing programming of hypertension and renal disease.
-
Gut microbiota: The diversity of the gut microbiota is influenced by various factors, including genetics, comorbidities, and environmental factors like physical exercise, smoking, and medication use. However, it is undeniable that diet, dietary patterns, and specific components of the diet play a significant role in shaping the composition of the gut microbiota. These components refer to microorganisms that are not broken down, but can instead colonize the colon [22]. Moreover, the composition of the diet and the presence or absence of specific nutrients are crucial factors determining the rate at which these bacteria generate and the metabolic effects of the metabolites they produce [23].
Emerging evidence suggests a connection between hypertension development and dysbiosis of the gut microbiota [24][25]. Notably, insufficient NO has been proposed as a potential link between dysbiosis and hypertension [25].
Several recent investigations have elucidated potential interconnections between gut dysbiosis and the impairment of the nitric oxide pathway, as well as the dysregulation of the renin–angiotensin system, in relation to secondary hypertension [18][26]. The accumulation of uremic toxins in chronic kidney disease is a significant factor contributing to the increased risk of cardiovascular complications [27][28]. Besides asymmetric dimethylarginine (ADMA) and symmetric dimethylarginine (SDMA), endogenous inhibitors of nitric oxide synthase are significant uremic toxins that have a role in the development of cardiovascular disease during chronic kidney disease. SDMA is related to arterial hypertension in children and adolescents with CKD [29][30].
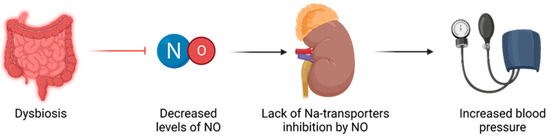
Figure 1. Relationship between dysbiosis and hypertension, mediated through NO imbalances.
4. The Kidney–Gut Axis in CKD
The relationship between gut microbiota and CKD is bidirectional and referred to as the kidney–gut axis [31]. This relationship is reciprocal: CKD can influence intestinal microbiome composition and potentially lead to dysbiosis, while dysbiosis in CKD patients can increase levels of uremic toxins, further exacerbating CKD progression, as shown in Figure 2. Recognizing the intestine as a potential factor in CKD-related complications, therapeutic approaches targeting gut microbiota will have a significant impact on CKD management.
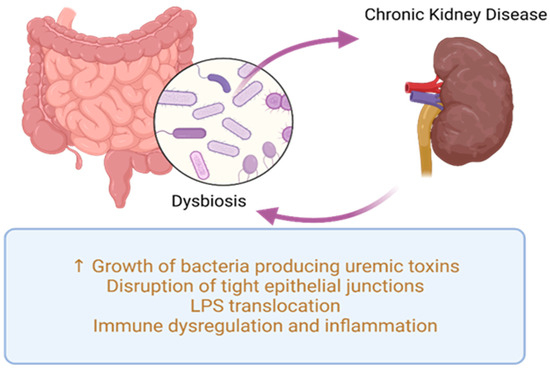
Figure 2. Common pathogenic processes in dysbiosis and CKD. LPS—lipopolysaccharides.
Recent studies focused on adults with CKD have presented various mechanisms that establish a link between gut microbiota dysbiosis and kidney disease. These mechanisms include inflammation, impaired gut barrier function, changes in microbiota composition, immune response, accumulation of trimethylamine N-oxide (TMAO), disruptions in short-chain fatty acids (SCFA) and their receptors, as well as uremic toxins [1][32]. Dysbiosis fosters the proliferation of uremic toxin-generating bacteria (illustrated in Figure 3), such as p-cresyl sulfate (p-CS), indole-3-acetic acid (IAA), IS, and TMAO, which accumulate in individuals with CKD [33]. Additionally, dysbiosis disrupts the integrity of tight junctions in the epithelium, resulting in bacterial LPS displacement, impaired immune function, and the onset of inflammation [34][35].
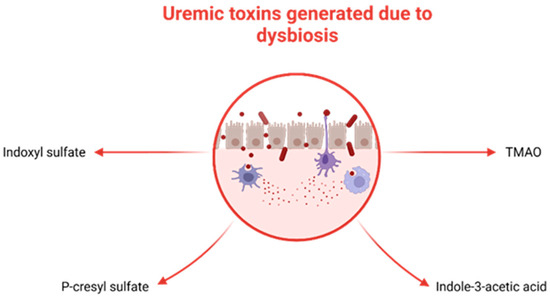
Figure 3. Uremic toxins generated by selected bacteria in the context of dysbiosis. TMAO.
CKD affects the integrity of the intestinal barrier by compromising tight epithelial junctions [36], primarily due to the presence of uremic toxins [1]. Consequently, there is an escalation in intestinal permeability, facilitating the displacement of LPS and pathogens through the digestive barrier. In individuals with CKD, intestinal flora stimulates the immune system by triggering the T-helper, which will amplify cytokine generation. Meanwhile, LPS can activate the innate immune response using the nuclear factor kappa B (NF-κB) and Toll-like receptor 4 (TLR4) pathways, leading to an inflammatory process and an immune response [1][32].
The presence of a leaky gut can lead to inflammation, malnutrition, and a faster progression of CKD [37][38]. Uremia has an important influence on the biochemical environment, favoring disruptions in gut microbiota and the intestinal barrier [39][40][41][42]. Moreover, the following also play a role in the development of dysbiosis: increased uric acid, inappropriate fiber consumption (decreased amounts of fruits and vegetables which can lead to hyperkalemia), and medication regimens (including antibiotics) [34].
In patients with CKD, the generation of uremic toxins has a negative impact on the growth of intestinal microbes. Studies have shown that individuals with end-stage kidney disease (ESKD) have lower diversity in their gut microbiota compared to healthy individuals [43]. Previous research has also suggested that CKD recipients tend to have decreased levels of good bacteria such as Bifidobacterium and Lactobacillus species [33].
Furthermore, there are various elements associated with CKD that contribute to an unbalanced gut microbiota. These factors encompass insufficient consumption of dietary fiber, malnutrition, metabolic acidosis, the utilization of antibiotics or other pharmaceuticals, augmented elimination of urea in the intestines, the buildup of uremic toxins, and reduced intestinal motility. These modifications in the uremic environment are linked to significant ramifications, including the advancement of CKD to end-stage renal disease, complications such as protein–energy wasting, and cardiovascular ailments, ultimately culminating in heightened mortality rates [44].
However, data are scarce regarding the impact of the kidney–gut axis in renal diseases of the minor population and the impact of intestinal dysbiosis on the modulation of pathological processes [1].
5. The Kidney–Gut Axis in Urinary Tract Infections
Periurethral contamination with specific uropathogenic bacteria, which are normally resident in the intestine, is recognized as playing a critical part in the pathology of urinary tract infection (UTI). This contamination is followed by the urethral colonization and the consequent ascension of the causative agent into the urinary bladder (Figure 4). Once in the bladder, the bacteria adhere to the uroepithelial cells using species-specific mechanisms, and then begin to multiply. Therefore, investigating the relationship between gut microbiota, bacteriuria, and UTI is an important area of research [45][46].
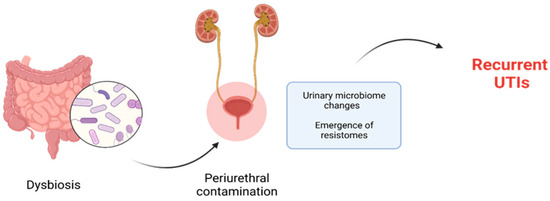
Figure 4. Dysbiosis promotes periurethral contamination with selected microorganisms involved in development of recurrent UTIs.
The previously well-established diagnostic and therapeutic approach for UTI has become less prominent with the discovery of a complex symbiotic microbiome in the healthy urogenital tract. Specifically, current evidence suggests that vaginal dysbiosis may lead to colonization by Escherichia coli and recurrent UTIs. Moreover, disruptions in the microbial flora of the urinary tract favor the onset of UTIs and other diseases of the urinary system, such as urinary lithiasis [47].
The recognition of the urinary microbiome’s role in urinary system health has sparked numerous research studies aiming to identify various classifications, including classes, orders, families, genera, and specific species. In the past decade, research involving the microbiome has sought to establish correlations between dysbiosis (microbial imbalance) in the gut bacterial community and a wide range of medical conditions, including gastrointestinal, respiratory, cardiac, neurological, psychiatric (and autism), metabolic, and cancer diseases [48]. One example of a disorder associated with gut–brain interactions is irritable bowel syndrome, which has shown an increasing incidence in recent years [49][50]. Furthermore, the human microbiome plays a role in impaired nutrient absorption, as seen in conditions such as celiac disease. This disorder is characterized by a microbial imbalance, with increased amounts of certain genera of Gram-negative bacteria, like Bacteroides, Escherichia, and Prevotella, and decreasing concentrations of good bacteria, such as lactobacilli and bifidobacteria. Moreover, individuals with celiac disease also exhibit dysbiosis involving viruses and fungi [51].
Although the gut microbiota of adults is considered to be relatively stable, the gut microbiota of developing infants and children is highly dynamic and susceptible to disturbances caused by external factors, such as exposure to antibiotics. It is well known that antibiotic therapy is one of the most commonly prescribed treatments in neonatal and pediatric populations.
The disruption caused by antibiotic therapy during the crucial development of children’s intestinal microbiota has significant implications for their health, leading to metabolic and immune disruptions [52]. Equally concerning is the possibility of enriching the reservoir of antibiotic resistance genes (“resistomes”), which can be transferred to pathogens [53][54], posing challenges in the treatment of infections, especially in vulnerable populations. This fact holds particular importance in the therapy of recurrent urinary tract infections.
Antibiotics used to treat UTIs can affect the intestinal microbiota, leading to a reduction in abundance and diversity, known as dysbiosis [55]. Similarly, recurrent exposure of the urinary microbiome to antibiotics could potentially result in alterations in the urinary microbial community, contributing to the emergence of resistomes [56]. Dysbiosis of the urinary microbiome is one of the main causes of recurrent UTIs.
6. The Kidney–Gut Axis in Urinary Lithiasis
Although urinary lithiasis is uncommon in children, the connection between intestinal flora and renal lithiasis has been described [57]. A meta-analysis revealed that the intestinal flora in patients with stone formation is marked by lower levels of Prevotella, Prevotellaceae, and Roseburia, and increased levels of Enterobacteriaceae and Streptococcaceae [57]. Urease-producing bacteria, such as Proteus mirabilis, Klebsiella pneumoniae, Staphylococcus aureus, Pseudomonas aeruginosa, Providentia stuartii, Serratia, and Morganella morganii, are always associated with the formation and recurrence of struvite stones [58].
Since urinary levels of oxalate, calcium, and uric acid are significant factors in the formation of kidney stones, an increased overall absorption in the gastrointestinal tract resulting from bacterial decomposition of these constituents of calculi could potentially influence their elimination through urine. Kidney stones composed of calcium oxalate are the most prevalent type. The absence of microbes capable of breaking down oxalate, such as Oxalobacter formigenes, has been associated with the formation of these stones [59]. The microbiota may also play an important role in the development of uric acid lithiasis, as under normal conditions one third of it is eliminated through the gut. Various data have demonstrated differences in the profile of intestinal flora between individuals with kidney stones and those without, further suggesting the crucial involvement of the intestinal microbiota in the formation of stones [57].
7. The Kidney–Gut Axis in Kidney Transplantation
Renal transplantation stands as the most effective treatment option for individuals diagnosed with end-stage renal disease. Despite the enhanced quality of life experienced by renal transplant recipients (RTRs) compared to individuals undergoing dialysis, they may encounter various challenges in the years following transplantation [60]. Recent studies have revealed that one in five RTRs experiences chronic diarrhea, which is associated with reduced quality of life, increased morbidity and mortality, and intestinal dysbiosis [61][62][63].
The use of immunosuppressive medications and frequent reliance on antibiotic therapy not only impacts the intestinal microbiome but also affects the urobiome [64]. These factors contribute to excessive growth of Escherichia coli strains and increased colonization by opportunistic pathogens [65].
8. The Kidney–Gut Axis in Other Kidney Diseases
Renal tubular impairment (acute tubular necrosis, tubulointerstitial nephritis, or idiopathic interstitial nephritis) is frequently observed in patients with inflammatory bowel disease, and the morbidity associated with renal manifestations in these cases can be as high as 20% [66]. Furthermore, deficiencies in intestinal immune tolerance lead to antigen absorption and activation of mucosa-associated lymphoid tissue, resulting in excessive deposition of abnormal IgA1 in the glomerular region, ultimately leading to IgA nephropathy [67]. The gut microbiota, through the B-cell activating factor of the TNF family, plays a key role in the development of IgA nephropathy [68].
References
- Hsu, C.-N.; Tain, Y.-L. Chronic Kidney Disease and Gut Microbiota: What Is Their Connection in Early Life? Int. J. Mol. Sci. 2022, 23, 3954.
- Yang, T.; Richards, E.M.; Pepine, C.J.; Raizada, M.K. The gut microbiota and the brain–gut–kidney axis in hypertension and chronic kidney disease. Nat. Rev. Nephrol. 2018, 14, 442–456.
- Al Khodor, S.; Shatat, I.F. Gut microbiome and kidney disease: A bidirectional relationship. Pediatr. Nephrol. 2017, 32, 921–931.
- Lupu, V.V.; Miron, I.C.; Raileanu, A.A.; Starcea, I.M.; Lupu, A.; Tarca, E.; Mocanu, A.; Buga, A.M.L.; Lupu, V.; Fotea, S. Difficulties in adaptation of the mother and newborn via cesarean section versus natural birth—A narrative review. Life 2023, 13, 300.
- Hajjo, R.; Sabbah, D.A.; Al Bawab, A.Q. Unlocking the Potential of the Human Microbiome for Identifying Disease Diagnostic Biomarkers. Diagnostics 2022, 12, 1742.
- Bozomitu, L.; Miron, I.; Raileanu, A.A.; Lupu, A.; Paduraru, G.; Marcu, F.M.; Buga, A.M.L.; Rusu, D.C.; Dragan, F.; Lupu, V.V. The Gut Microbiome and Its Implication in the Mucosal Digestive Disorders. Biomedicines 2022, 10, 3117.
- Słabuszewska-Jóźwiak, A.; Szymański, J.K.; Ciebiera, M.; Sarecka-Hujar, B.; Jakiel, G. Pediatrics Consequences of Caesarean Section—A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2020, 17, 8031.
- Gluckman, P.D.; Hanson, M.A. The developmental origins of health and disease. In Early Life Origins of Health and Disease; Springer: New York, NY, USA, 2006; pp. 1–7.
- Gillman, M.W. Developmental origins of health and disease. N. Engl. J. Med. 2005, 353, 1848.
- Gluckman, P.D.; Hanson, M.A.; Buklijas, T. A conceptual framework for the developmental origins of health and disease. J. Dev. Orig. Health Dis. 2010, 1, 6–18.
- Charlton, J.R.; Baldelomar, E.J.; Hyatt, D.M.; Bennett, K.M. Nephron number and its determinants: A 2020 update. Pediatr. Nephrol. 2021, 36, 797–807.
- Welham, S.J.; Wade, A.; Woolf, A.S. Protein restriction in pregnancy is associated with increased apoptosis of mesenchymal cells at the start of rat metanephrogenesis. Kidney Int. 2002, 61, 1231–1242.
- Woods, L.L.; Weeks, D.A.; Rasch, R. Programming of adult blood pressure by maternal protein restriction: Role of nephrogenesis. Kidney Int. 2004, 65, 1339–1348.
- Hoppe, C.C.; Evans, R.G.; Bertram, J.F.; Moritz, K.M. Effects of dietary protein restriction on nephron number in the mouse. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 292, R1768–R1774.
- Lisle, S.J.; Lewis, R.M.; Petry, C.J.; Ozanne, S.E.; Hales, C.N.; Forhead, A.J. Effect of maternal iron restriction during pregnancy on renal morphology in the adult rat offspring. Br. J. Nutr. 2003, 90, 33–39.
- Lelievre-Pegorier, M.; Vilar, J.; Ferrier, M.L.; Moreau, E.; Freund, N.; Gilbert, T.; Merlet-Benichou, C. Mild vitamin A deficiency leads to inborn nephron deficit in the rat. Kidney Int. 1998, 54, 1455–1462.
- Mohany, M.; Ashton, N.; Harrath, A.H.; Nyengaard, J.R.; Alomar, S.Y.; Alwasel, S. A new model for fetal programming: Maternal Ramadan-type fasting programs nephrogenesis. J. Dev. Orig. Health Dis. 2018, 9, 287–298.
- Schlender, J.; Behrens, F.; McParland, V.; Müller, D.; Wilck, N.; Bartolomaeus, H.; Holle, J. Bacterial metabolites and cardiovascular risk in children with chronic kidney disease. Mol. Cell. Pediatr. 2021, 8, 17.
- Chong, E.; Yosypiv, I.V. Developmental Programming of Hypertension and Kidney Disease. Int. J. Nephrol. 2012, 2012, 760580.
- Satoh, N.; Nakamura, M.; Suzuki, A.; Tsukada, H.; Horita, S.; Suzuki, M.; Moriya, K.; Seki, G. Effects of Nitric Oxide on Renal Proximal Tubular Na(+) Transport. Biomed. Res. Int. 2017, 2017, 6871081.
- Bianco-Miotto, T.; Craig, J.M.; Gasser, Y.P.; van Dijk, S.J.; Ozanne, S.E. Epigenetics and DOHaD: From basics to birth and beyond. J. Dev. Orig. Health Dis. 2017, 8, 513–519.
- Lobach, A.R.; Roberts, A.; Rowland, I.R. Assessing the in vivo data on low/no-calorie sweeteners and the gut microbiota. Food Chem. Toxicol. 2019, 124, 385–399.
- Sonnenburg, J.L.; Bäckhed, F. Diet–microbiota interactions as moderators of human metabolism. Nature 2016, 535, 56–64.
- Al Khodor, S.; Reichert, B.; Shatat, I.F. The microbiome and blood pressure: Can microbes regulate our blood pressure? Front. Pediatr. 2017, 5, 138.
- Ma, J.; Li, H. The Role of Gut Microbiota in Atherosclerosis and Hypertension. Front. Pharmacol. 2018, 9, 1082.
- Tain, Y.-L.; Hsu, C.-N. Role of the Gut Microbiota in Children with Kidney Disease. Children 2023, 10, 269.
- Wehedy, E.; Shatat, I.F.; Al Khodor, S. The Human Microbiome in Chronic Kidney Disease: A Double-Edged Sword. Front. Med. 2022, 8, 790783.
- Holle, J.; Bartolomaeus, H.; Löber, U.; Behrens, F.; Bartolomaeus, T.U.; Anandakumar, H.; Wimmer, M.I.; Vu, D.L.; Kuhring, M.; Brüning, U.; et al. Inflammation in Children with CKD Linked to Gut Dysbiosis and Metabolite Imbalance. J. Am. Soc. Nephrol. 2022, 33, 2259–2275.
- Jezierska, M.; Stefanowicz, J. Asymmetric and Symmetric Dimethylarginines as Renal Function Parameters in Paediatric Kidney Diseases: A Literature Review from 2003 to 2022. Children 2022, 9, 1668.
- Tain, Y.-L.; Hsu, C.-N. Toxic Dimethylarginines: Asymmetric Dimethylarginine (ADMA) and Symmetric Dimethylarginine (SDMA). Toxins 2017, 9, 92.
- Evenepoel, P.; Poesen, R.; Meijers, B. The gut-kidney axis. Pediatr. Nephrol. 2017, 32, 2005–2014.
- Andersen, K.; Kesper, M.S.; Marschner, J.A.; Konrad, L.; Ryu, M.; Kumar Vr, S.; Kulkarni, O.P.; Mulay, S.R.; Romoli, S.; Demleitner, J.; et al. Intestinal Dysbiosis, Barrier Dysfunction, and Bacterial Translocation Account for CKD-Related Systemic Inflammation. J. Am. Soc. Nephrol. 2017, 28, 76–83.
- Zhao, J.; Ning, X.; Liu, B.; Dong, R.; Bai, M.; Sun, S. Specific alterations in gut microbiota in patients with chronic kidney disease: An updated systematic review. Ren. Fail. 2021, 43, 102–112.
- Vaziri, N.D.; Zhao, Y.-Y.; Pahl, M.V. Altered intestinal microbial flora and impaired epithelial barrier structure and function in CKD: The nature, mechanisms, consequences and potential treatment. Nephrol. Dial. Transplant. 2015, 31, 737–746.
- Mafra, D.; Borges, N.; Alvarenga, L.; Esgalhado, M.; Cardozo, L.; Lindholm, B.; Stenvinkel, P. Dietary Components That May Influence the Disturbed Gut Microbiota in Chronic Kidney Disease. Nutrients 2019, 11, 496.
- Vaziri, N.D.; Yuan, J.; Rahimi, A.; Ni, Z.; Said, H.; Subramanian, V.S. Disintegration of colonic epithelial tight junction in uremia: A likely cause of CKD-associated inflammation. Nephrol. Dial. Transplant. 2012, 27, 2686–2693.
- Anders, H.-J.; Andersen, K.; Stecher, B. The intestinal microbiota, a leaky gut, and abnormal immunity in kidney disease. Kidney Int. 2013, 83, 1010–1016.
- Vaziri, N.D. CKD impairs barrier function and alters microbial flora of the intestine: A major link to inflammation and uremic toxicity. Curr. Opin. Nephrol. Hypertens. 2012, 21, 587–592.
- Vaziri, N.D.; Wong, J.; Pahl, M.; Piceno, Y.M.; Yuan, J.; DeSantis, T.Z.; Ni, Z.; Nguyen, T.-H.; Andersen, G.L. Chronic kidney disease alters intestinal microbial flora. Kidney Int. 2013, 83, 308–315.
- Wong, J.; Piceno, Y.M.; DeSantis, T.Z.; Pahl, M.; Andersen, G.L.; Vaziri, N.D. Expansion of Urease- and Uricase-Containing, Indole- and p-Cresol-Forming and Contraction of Short-Chain Fatty Acid-Producing Intestinal Microbiota in ESRD. Am. J. Nephrol. 2014, 39, 230–237.
- Sampaio-Maia, B.; Simoes-Silva, L.; Pestana, M.; Araujo, R.; Soares-Silva, I.J. The Role of the Gut Microbiome on Chronic Kidney Disease. Adv. Appl. Microbiol. 2016, 96, 65–94.
- Lozupone, C.A.; Stombaugh, J.I.; Gordon, J.I.; Jansson, J.K.; Knight, R. Diversity, stability and resilience of the human gut microbiota. Nature 2012, 489, 220–230.
- Koppe, L.; Mafra, D.; Fouque, D. Probiotics and chronic kidney disease. Kidney Int. 2015, 88, 958–966.
- Pisano, A.; D’Arrigo, G.; Coppolino, G.; Bolignano, D. Biotic Supplements for Renal Patients: A Systematic Review and Meta-Analysis. Nutrients 2018, 10, 1224.
- Meštrović, T.; Matijašić, M.; Perić, M.; Čipčić Paljetak, H.; Barešić, A.; Verbanac, D. The Role of Gut, Vaginal, and Urinary Microbiome in Urinary Tract Infections: From Bench to Bedside. Diagnostics 2021, 11, 7.
- Cumpanas, A.A.; Bratu, O.G.; Bardan, R.T.; Ferician, O.C.; Cumpanas, A.D.; Horhat, F.G.; Licker, M.; Pricop, C.; Cretu, O.M. Urinary Microbiota—Are We Ready for Prime Time? A Literature Review of Study Methods’ Critical Steps in Avoiding Contamination and Minimizing Biased Results. Diagnostics 2020, 10, 343.
- Hanage, W.P. Microbiology: Microbiome science needs a healthy dose of scepticism. Nature 2014, 512, 247–248.
- Lupu, V.V.; Adam Raileanu, A.; Mihai, C.M.; Morariu, I.D.; Lupu, A.; Starcea, I.M.; Frasinariu, O.E.; Mocanu, A.; Dragan, F.; Fotea, S. The Implication of the Gut Microbiome in Heart Failure. Cells 2023, 12, 1158.
- Lupu, V.V.; Ghiciuc, C.M.; Stefanescu, G.; Mihai, C.M.; Popp, A.; Sasaran, M.O.; Bozomitu, L.; Starcea, I.M.; Adam Raileanu, A.; Lupu, A. Emerging role of the gut microbiome in post-infectious irritable bowel syndrome: A literature review. World J. Gastroenterol. 2023, 29, 3241–3256.
- Ford, A.C.; Sperber, A.D.; Corsetti, M.; Camilleri, M. Irritable bowel syndrome. Lancet 2020, 396, 1675–1688.
- Lupu, V.V.; Trandafir, L.M.; Raileanu, A.A.; Mihai, C.M.; Morariu, I.D.; Starcea, I.M.; Mocanu, A.; Butnariu, L.I.; Stoleriu, G.; Salaru, D.L.; et al. Advances in Understanding the Human Gut Microbiota and Its Implication in Pediatric Celiac Disease—A Narrative Review. Nutrients 2023, 15, 2499.
- Cox, L.M.; Yamanishi, S.; Sohn, J.; Alekseyenko, A.V.; Leung, J.M.; Cho, I.; Kim, S.G.; Li, H.; Gao, Z.; Mahana, D.; et al. Altering the Intestinal Microbiota during a Critical Developmental Window Has Lasting Metabolic Consequences. Cell 2014, 158, 705–721.
- Moore, A.M.; Patel, S.; Forsberg, K.J.; Wang, B.; Bentley, G.; Razia, Y.; Qin, X.; Tarr, P.I.; Dantas, G. Pediatric Fecal Microbiota Harbor Diverse and Novel Antibiotic Resistance Genes. PLoS ONE 2013, 8, e78822.
- Moore, A.M.; Ahmadi, S.; Patel, S.; Gibson, M.K.; Wang, B.; Ndao, M.I.; Deych, E.; Shannon, W.; Tarr, P.I.; Warner, B.B.; et al. Gut resistome development in healthy twin pairs in the first year of life. Microbiome 2015, 3, 27.
- Elvers, K.T.; Wilson, V.J.; Hammond, A.; Duncan, L.; Huntley, A.L.; Hay, A.D.; van der Werf, E.T. Antibiotic-induced changes in the human gut microbiota for the most commonly prescribed antibiotics in primary care in the UK: A systematic review. BMJ Open 2020, 10, e035677.
- Perez-Carrasco, V.; Soriano-Lerma, A.; Soriano, M.; Gutiérrez-Fernández, J.; Garcia-Salcedo, J.A. Urinary microbiome: Yin and yang of the urinary tract. Front. Cell. Infect. Microbiol. 2021, 11, 617002.
- Wang, Z.; Zhang, Y.; Zhang, J.; Deng, Q.; Liang, H. Recent advances on the mechanisms of kidney stone formation (Review). Int. J. Mol. Med. 2021, 48, 149.
- Espinosa-Ortiz, E.J.; Eisner, B.H.; Lange, D.; Gerlach, R. Current insights into the mechanisms and management of infection stones. Nat. Rev. Urol. 2019, 16, 35–53.
- Wiener, S.V.; Ho, S.P.; Stoller, M.L. Beginnings of nephrolithiasis: Insights into the past, present and future of Randall’s plaque formation research. Curr. Opin. Nephrol. Hypertens. 2018, 27, 236–242.
- Swarte, J.C.; Douwes, R.M.; Hu, S.; Vich Vila, A.; Eisenga, M.F.; van Londen, M.; Gomes-Neto, A.W.; Weersma, R.K.; Harmsen, H.J.M.; Bakker, S.J.L. Characteristics and Dysbiosis of the Gut Microbiome in Renal Transplant Recipients. J. Clin. Med. 2020, 9, 386.
- Ekberg, H.; Kyllönen, L.; Madsen, S.; Grave, G.; Solbu, D.; Holdaas, H. Clinicians Underestimate Gastrointestinal Symptoms and Overestimate Quality of Life in Renal Transplant Recipients: A Multinational Survey of Nephrologists. Transplantation 2007, 84, 1052–1054.
- Bunnapradist, S.; Neri, L.; Wong, W.; Lentine, K.L.; Burroughs, T.E.; Pinsky, B.W.; Takemoto, S.K.; Schnitzler, M.A. Incidence and Risk Factors for Diarrhea Following Kidney Transplantation and Association With Graft Loss and Mortality. Am. J. Kidney Dis. 2008, 51, 478–486.
- Lee, J.R.; Magruder, M.; Zhang, L.; Westblade, L.F.; Satlin, M.J.; Robertson, A.; Edusei, E.; Crawford, C.; Ling, L.; Taur, Y.; et al. Gut microbiota dysbiosis and diarrhea in kidney transplant recipients. Am. J. Transplant. 2019, 19, 488–500.
- Tourret, J.; Willing, B.P.; Dion, S.; MacPherson, J.; Denamur, E.; Finlay, B.B. Immunosuppressive Treatment Alters Secretion of Ileal Antimicrobial Peptides and Gut Microbiota, and Favors Subsequent Colonization by Uropathogenic Escherichia coli. Transplantation 2017, 101, 74–82.
- Lee, J.R.; Muthukumar, T.; Dadhania, D.; Toussaint, N.C.; Ling, L.; Pamer, E.; Suthanthiran, M. Gut microbial community structure and complications after kidney transplantation: A pilot study. Transplantation 2014, 98, 697–705.
- Ambruzs, J.M.; Larsen, C.P. Renal Manifestations of Inflammatory Bowel Disease. Rheum. Dis. Clin. N. Am. 2018, 44, 699–714.
- Lei, J.; Xie, Y.; Sheng, J.; Song, J. Intestinal microbiota dysbiosis in acute kidney injury: Novel insights into mechanisms and promising therapeutic strategies. Ren. Fail. 2022, 44, 571–580.
- Yang, J.; Kim, C.J.; Go, Y.S.; Lee, H.Y.; Kim, M.-G.; Oh, S.W.; Cho, W.Y.; Im, S.-H.; Jo, S.K. Intestinal microbiota control acute kidney injury severity by immune modulation. Kidney Int. 2020, 98, 932–946.
More
Information
Subjects:
Pediatrics
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
683
Revisions:
2 times
(View History)
Update Date:
23 Aug 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




