Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Victor Hugo Gonzalez Sanchez | -- | 16584 | 2023-08-21 20:33:23 | | | |
| 2 | Jason Zhu | -8 word(s) | 16589 | 2023-08-22 04:59:19 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Gonzalez Sanchez, V.H.; Johnson, J.; Frausto-Martínez, O.; Mejia-Ortiz, L.M.; Pereira-Corona, A.; Blanco Parra, M.D.P.; Charruau, P.; Niño-Torres, C.A. Herpetofauna of the Insular Systems of Mexico. Encyclopedia. Available online: https://encyclopedia.pub/entry/48293 (accessed on 08 February 2026).
Gonzalez Sanchez VH, Johnson J, Frausto-Martínez O, Mejia-Ortiz LM, Pereira-Corona A, Blanco Parra MDP, et al. Herpetofauna of the Insular Systems of Mexico. Encyclopedia. Available at: https://encyclopedia.pub/entry/48293. Accessed February 08, 2026.
Gonzalez Sanchez, Victor Hugo, Jerry Johnson, Oscar Frausto-Martínez, Luis M. Mejia-Ortiz, Alberto Pereira-Corona, Maria Del Pilar Blanco Parra, Pierre Charruau, Carlos Alberto Niño-Torres. "Herpetofauna of the Insular Systems of Mexico" Encyclopedia, https://encyclopedia.pub/entry/48293 (accessed February 08, 2026).
Gonzalez Sanchez, V.H., Johnson, J., Frausto-Martínez, O., Mejia-Ortiz, L.M., Pereira-Corona, A., Blanco Parra, M.D.P., Charruau, P., & Niño-Torres, C.A. (2023, August 21). Herpetofauna of the Insular Systems of Mexico. In Encyclopedia. https://encyclopedia.pub/entry/48293
Gonzalez Sanchez, Victor Hugo, et al. "Herpetofauna of the Insular Systems of Mexico." Encyclopedia. Web. 21 August, 2023.
Copy Citation
The herpetofauna of the insular systems of Mexico is composed of 226 species, of which 14 are anurans, two are salamanders, and 210 are reptiles, comprised of two crocodilians, 195 squamates, and 13 turtles. Although the surface of the Mexican islands is only 0.26% of the Mexican territorial extension, these 226 species constitute 16.1% of Mexico’s documented herpetofauna of 1405 species.
islands
cays
archipelagos
endemism
EVS
1. Introduction
Islands have become important model systems for scientific research in ecology, evolutionary biology, and biogeography by allowing the isolation of particular ecological factors and processes and the exploration of their effects [1]. In fact, it can be said that one milestone event in the coming-of-age of ecological science as a discipline with a theoretical/conceptual base was the publication of MacArthur and Wilson’s Theory of Island Biogeography in 1967 [2]. In addition, many of the theories generated by island biogeography have been extensively used (but not without controversy) in the understanding of the dynamics of discontinuous habitats or “insular like systems” [3], and have great importance in biodiversity conservation and management, since scientists and conservationists attempt to manage the impacts of habitat loss and fragmentation [1].
Islands cover 2.7% of the Earth’s surface [4]. Despite their reduced surface compared with that of the mainland, they are hotspots for biodiversity conservation, combining the attributes of unique biodiversity, recent species extinction, and high risk of future species losses [1]. Often, islands are the refuge of lineages (relict species) that cannot survive the biotic pressure of most environments and only persist in habitats that their competitors or predators might have not reached [5]. Some remarkable examples of ancient relicts on the islands of Sonora are Aspidoscelis ceralbensis, Sceloporus angustus, and Sceloporus grandaevus [6].
Islands are also scenarios of the in-situ evolution of species with limited defensive or competitive abilities [5]. Thus, it is not surprising that islands harbor 61% of all species listed by the IUCN as extinct and 37% of species listed as critically endangered [7] and that most known animal extinctions occurred on islands [4]. This is critical for reptiles, of whom 90% of extinctions are insular species [8]. Moreover, the protection of island ecosystems constitutes a considerable challenge, not only ecologically, but also because of their fragmented nature, scattered across the globe and, generally below the horizon of media networks [1].
Mexico has a terrestrial extension of 1,964,375 km2, of which 1,959,248 km2 correspond to the continental surface, and only 5127 km2 (0.26%) to islands [9]. This surface area is barely higher than the territorial extension of the smaller Mexican entities such Ciudad de México (1485 km2), Tlaxcala (3991 km2), and Morelos (4958 km2), and barely smaller than Colima (5625 km2) and Aguascalientes (5589 km2) [10].
Remarkably, Mexico encompasses 231,813 km2 of territorial sea and 3,149,920 km2 of exclusive economic zone [9]. The easternmost and westernmost territories of Mexico are islands: Isla Mujeres and Roca Elefante (Isla Guadalupe), respectively [11]. More importantly, the archipelagos Revillagigedo and Alacranes, and Isla Guadalupe play a key role because of their remote locations; they are the farthest extensions of the Mexican exclusive economic zone. Thus, despite their discrete contribution to terrestrial extension, the insular territories of Mexico are strategic in the conformation of the country’s maritime limits [12][13].
The total of insular elements registered for Mexico, according with Instituto Nacional de Estadística y Geografía (INEGI) is 4111, most of them remaining unnamed. At least 3210 are true islands, whereas 1203 are in oceanic waters. The oceanic islands are 29.3% of the Mexican insular elements and cover 4529.7 km2, which represent more than half of the Mexican insular surface. On the other hand, the coastal elements cover 3136.7 km2. The great majority of the insular surface is in the Mexican northwest Pacific, especially in the Sea of Cortes. The islands of this interior sea constitute half of the Mexican insular surface [14] (Figure 1).
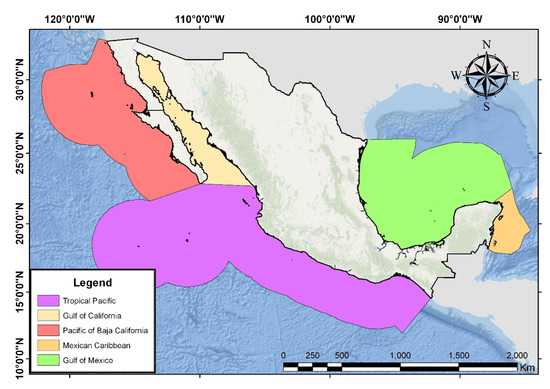
Figure 1. Mexican Insular Territory Regions, modified from Instituto Nacional de Estadística y Geografía (INEGI) (2015).
Island: A natural extension of land, surrounded by water, and situated above the high tide level. It includes small portions of land permanently surrounded by water, or scarped massive structures that are permanently emerged.
Insular reef: Rocky structures, generally of coralline origin, which emerge from the sea surface or are located at a very shallow level. Usually located near the shoreline.
Cay: Extension of land surrounded by sea water, located above the high tide level, derived of the accumulation of non-consolidated materials of calcarean nature, rocky or sandy texture with a permanent tropical vegetal cover, located mainly in the warm waters of the Caribbean Sea and Gulf of Mexico, whose formation dynamics are tightly linked to the coral reef systems. Also included in this category are the insular-like bodies, formed by aggregations of hydrophilic vegetation (i.e., mangroves) surrounded by sea water, which usually grow above banks of soft sediments with muddy and waterlogged soils, sometimes barely under the high tide level, and which are common in the littoral and lagunar systems of the Gulf of Mexico and the Caribbean Sea.
2. Composition of the Herpetofauna
2.1. Families
The herpetofauna of the insular systems of Mexico comprises 40 families. Seven of these families contain amphibians (six anurans and one salamander). The reptiles comprise 33 families, including one crocodylian, 24 squamate, and 8 turtle families. The anuran families Bufonidae and Hylidae collectively contain slightly more than half of all the amphibian species (4 and 5, respectively, of a total of 16). The most speciose reptile families are the Phrynosomatidae (31), Teiidae (22), Colubridae (36), Dipsadidae (19), and Viperidae (18), comprising 66.5% of the squamates and 61.5% of all reptiles.
2.2. Genera
Ninety-five genera are represented within the herpetofauna of the insular systems of Mexico. Fourteen genera represent amphibians, including two salamander and twelve anuran genera. Except for Incilius and Eleutherodactylus, with two species each, all the other amphibian genera are monospecific for the islands of Mexico (however, one of the two species of Eleutherodactylus, E. planirostris, is an introduced species). The reptiles are arranged among 81 genera, including 1 of crocodiles, 11 of turtles, and 69 of squamates. Ranked among the most speciose reptile genera are Aspidoscelis (21 species), Crotalus (16), Sceloporus (12), Phyllodactylus (15), Uta (7), Masticophis (6), and Lampropeltis (5), all comprising squamates. In the Testudines there are 11 genera, with 13 species; Kinosternon and Lepidochelys have 2 species each.
2.3. Species
Despite the insular territories of Mexico representing only 0.26% of the country’s emerged surface, they host an outstanding total amount of 226 herpetofaunal species. This number is 16.1% of the 1405 species known for Mexico [15]. Of these 226 species, 16 are amphibians (7.4%) and 210 are reptiles (92.9%). This number of reptile species is higher than that reported for some large Mexican states, such as Chihuahua (155), Durango (138), Sonora (169), Tamaulipas (160), Jalisco (194), Puebla (184) [16], and 120 species reported within the three states that comprise the Mexican Yucatan Peninsula [17][18]. If the insular territories of Mexico constituted a state, the reptile fauna would rank fourth in size, only below that of Oaxaca, with 328, Chiapas with 254, and Veracruz with 244, and only slightly higher than Guerrero with 200, and Jalisco with 194 [16].
On the other hand, the amphibians have a low specific richness, with 16 species, 15 of which are native species. These 15 species comprise 3.5% of the 430 native amphibian species in Mexico [15]. The low specific richness of amphibians on islands is not surprising; the physiological traits of amphibians, such as thin moist skin, soft eggs, and life cycles with an aquatic phase, are not suitable for the dry conditions often found in Mexican insular ecosystems. These habitats are generally scarce also in resources, and on the great majority of the Mexican islands there are no permanent sources of freshwater available. Furthermore, the probability of amphibian colonization in such environments through rafting is low, as only a limited number of amphibians can endure prolonged dehydration and exposure to a saline environmentThis contrast in low amphibian and high reptile richness on islands is very evident if compare a Mexican state similar in size to the area of the Mexican insular territories, such as Aguascalientes, which has an area of 5680.3 km2, which represents 0.3% of the country’s land surface. This state has a herpetofaunal diversity of 20 amphibian species and 78 reptiles [15] in contrast to the 16 amphibian species (80% of the diversity in Aguascalientes) and 210 reptile species (269.2% of the diversity in Aguascalientes) on islands. Another example is Morelos (4878.9 km2, 0.25% of the Mexican territory), with 43 amphibian species and 105 reptile species [15]. Comparatively, the insular amphibians and reptiles are the 37.2 and 205.7%, respectively, of the herpetofauna of Morelos. Of particular interest is the high specific richness of insular rattlesnakes (16 spp.). Forty-five species in the genus Crotalus (counting C. caliginis) inhabit Mexico [15]. The 16 of these rattlesnake taxa (35.6%) that occur in the Mexican insular systems are restricted to the two physiographic regions associated with the peninsula of Baja California (the Gulf of California islands and the Pacific islands of Baja California). Interestingly, there are no rattlesnakes recorded from any of the other three Mexican insular systems physiographic regions. Of the sixteen insular species, eight (50.0%) are insular endemics (Crotalus angelensis, C. caliginis, C. catalinensis (Figure 2), C. estebanensis, C. lorenzoensis, C. polisi, C. thalassoporus, and C. tortuguensis), one (C. enyo) is a country endemic (6.3%), and the remaining seven species (43.8%) are Mexican–US species (C. atrox, C. cerastes, C. mitchellii, C. molossus, C. pyrrhus, C. ruber, and C. tigris). Only 3 of the 16 species (C. enyo, C. mitchelli, and C. ruber) inhabit islands on both sides of the Baja California peninsula.

Figure 2. Catalina Island Rattlesnake (Crotalus catalinensis) searching for prey. A distinctive characteristic of this insular rattlesnake is the absence of rattle, which is reduced only to a button on the tip of the tail. Perhaps an adaptation for arboreality and/or simply a loss of this structure due to the lack of large mammals in the island, which would make the rattle useless as a warning mechanism. EVS = H(19), IUCN = CT, NOM-059 = A. Photo by Ruben Alonso Carbajal-Márquez.
3. Patterns of Physiographic Distribution
The total number of species in the different biogeographic regions ranges from 40 to 108, ranked in descending order, as follows: Islands of the Gulf of California (108), Tropical islands of the Pacific (57), Mexican Caribbean islands (52), Islands of the Gulf of Mexico (46), and the Islands of the Pacific of Baja California [19]. Notably, the islands of the Gulf of Baja California are far richer in species than any the other physiographic regions, which have only 52.8% (Tropical Pacific), 48.2% (Caribbean), 42.6% (Golfo de México), and 37.0% (Pacific of Baja California) of the richness of the islands of the Sea of Cortes.
Since the insular elements of Mexico are scattered in both oceans, it is to be expected that no insular reptile or amphibian species would be distributed among all of the five physiographic regions. In addition, only three species occupy four of the five regions, all sea turtles (Caretta caretta, Chelonia mydas and Eretmochelys imbricata). Only 9 species of the total 226 (~4%) occupy three regions, including two anurans (Rhinella horribilis and Smilisca baudini), three lizards (Iguana iguana, Marisora brachypoda, Sceloporus clarkii, Aspidoscelis deppii, and the non-native gecko Hemidactylus frenatus), three snakes (Hypsiglena slevini, Rena umilis and the non-native blindsnake Indotyphlops braminus). In addition, among these six terrestrial species, five species are restricted to the tropical regions (Tropical Pacific, Gulf of Mexico, and the Mexican Caribbean), and the sixth one, Hypsiglena slevini, occurs along the regions of the Pacific (Pacific of Baja California, Tropical Pacific, and Tropical islands of the Gulf).
The rest of the species are distributed in either two (51 species or 22.5%) or one (163 species or 72.1%) physiographic region(s). The mean value for insular distribution is 93.3. Thus, almost three-quarters of the 226 insular species are limited to single-island regions.
There are 78 single-region species limited to the islands of the Gulf of California (as indicated below). Of these 78 species, 44 (56.4%) are insular endemics, 10 (12.8%) are country endemics, and 24 (30.8%) are non-endemics. As perhaps expected, almost half (47.8%) of the single-region species are found in the Gulf of California region. Remarkable examples of endemicity are the species of the genus Crotalus and Uta. (Figure 3 and Figure 4) (No asterisk = Non-endemic; * = country endemic; ** = regional endemic; and ‡ = non-native).
| Scaphiopus couchii | Aspidoscelis bacata ** |
| Crotaphytus dickersonae ** | Aspidoscelis cana ** |
| Crotaphytus insularis ** | Aspidoscelis carmenensis ** |
| Gambelia wislizenii | Aspidoscelis catalinensis ** |
| Coleonyx gypsicolus ** | Aspidoscelis celeripes ** |
| Ctenosaura conspicuosa ** | Aspidoscelis ceralbelsis ** |
| Ctenosaura hemilopha * | Aspidoscelis danheimae ** |
| Ctenosaura nolascensis ** | Aspidoscelis espiritensis ** |
| Dipsosaurus catalinensis ** | Aspidoscelis franciscensis ** |
| Sauromalus ater | Aspidoscelis martyris ** |
| Sauromalus hispidus * | Aspidoscelis pictus ** |
| Sauromalus klauberi ** | Bogertophis rosaliae |
| Sauromalus slevini * | Lampropeltis californiae |
| Sauromalus varius * | Lampropeltis catalinensis ** |
| Petrosaurus mearnsi | Masticophis barbouri |
| Petrosaurus repens * | Masticophis bilineatus |
| Petrosaurus slevini ** | Masticophis slevini ** |
| Petrosaurus thalassinus * | Phyllorhynchus decurtatus |
| Phrynosoma solare | Rhinocheilus etheridgei ** |
| Sceloporus angustus ** | Sonora savagei * |
| Sceloporus grandaevus ** | Sonora semiannulata |
| Sceloporus hunsakeri * | Tantilla planiceps |
| Sceloporus lineatulus ** | Trimorphodon lyrophanes |
| Sceloporus magister | Hypsiglena catalinae ** |
| Sceloporus orcutti | Hypsiglena chlorophaea |
| Uta encantadae ** | Micruroides euryxanthus |
| Uta lowei ** | Crotalus angelensis ** |
| Uta nolascensis ** | Crotalus atrox |
| Uta palmeri ** | Crotalus catalinensis |
| Uta squamata | Crotalus cerastes |
| Uta tumidarostra ** | Crotalus estebanensis ** |
| Phyllodactylus angelensis ** | Crotalus lorenzoensis ** |
| Phyllodactylus apricus ** | Crotalus molossus |
| Phyllodactylus bugastrolepis ** | Crotalus polisi ** |
| Phyllodactylus coronatus ** | Crotalus pyrrhus |
| Phyllodactylus homolepidurus * | Crotalus thalassoporus ** |
| Phyllodactylus partidus ** | Crotalus tigris |
| Phyllodactylus santacruzensis ** | Crotalus tortuguensis ** |
| Phyllodactylus unctus * | Gopherus morafkai |

Figure 3. Horsehead Island Speckled Rattlesnake (Crotalus polisi), only known from Isla Cabeza de Caballo (Gulf of California). The islands of the Sea of Cortes are home to fifteen Crotalus species, this number alone is superior to the Crotalus species diversity of many mainland Mexican states and represents the 32.6% of all Crotalus species in Mexico (46). Five of them are insular endemics (11% of Mexican Crotalus) and, consequently only known in their type locality. Distributional status = IE, EVS = H (18), IUCN = NE, NOM-059 = NS. Photo by Tania Pérez-Fiol.

Figure 4. Enchanted Side-blotched Lizard (Uta Encantadae) from Encantada. Phrynosomatid lizards are diverse in the islas of the Sea of Cortes with 23 species. Interestingly, five of the six Uta species in these islands are insular endemics. Distributional status = IE, EVS = H (17), IUCN = VU, NOM-059 = NS. Photo by Jorge H Valdez.
The number of single-region species in the tropical Pacific islands is 36 (22.1%). Eight of these 36 species (22.2%) are insular endemics, 20 (55.5%) are country endemics, 7 (19.5.3%) are non-endemics, and one (2.8%) is a non-native species (See below). Lampropeltis and Phyllodactylus are typical examples of herpetofaunistical diversity within the Tres Marias Archipielago (Figure 5). (No asterisk = Non-endemic; * = country endemic; ** = insular endemic; and ‡ = non-native):
| Incilius mazatlanensis * | Aspidoscelis lineatissima * |
| Eleutherodactylus pallidus * | Boa sigma * |
| Norops nebulosus * | Drymarchon melanurus |
| Gehyra mutilata ‡ | Lampropeltis polyzona * |
| Ctenosaura pectinata | Leptophis diplotropis * |
| Marisora aquilonaria * | Masticophis anthonyi ** |
| Urosaurus auriculatus ** | Oxybelis microphtalmus * |
| Urosaurus bicarinatus * | Tantilla bocourti * |
| Urosaurus clarionensis ** | Tantilla calamarina * |
| Phyllodactylus benedetii * | Conophis vittatus |
| Phyllodactylus cloefasensis ** | Geophis annuliferus * |
| Phyllodactylus isabelae ** | Hypsiglena torquata |
| Phyllodactylus lanei * | Hypsiglena unaocularis ** |
| Phyllodactylus lupitae ** | Imantodes gemmistratus |
| Phyllodactylus tuberculosus | Rhadinaea hesperia * |
| Aspidoscelis communis * | Epictia bakewelli * |
| Aspidoscelis costata * | Agkistrodon bilineatus |
| Aspidoscelis guttatus * | Kinosternon integrum * |

Figure 5. West Mexican Milksnake (Lampropeltis polyzona) from Isla Isabel, Tropical Pacific. Milksnakes are especially vulnerable to depredation from introduced mammals, such as the black rat (Rattus rattus) and feral cats (Felis silvestris catus). Thus, the population’s number of milksnakes can constitute a reference indicator of impact from introduced mammals in islands. Distributional status = CE, EVS = M (11), IUCN = NE, NOM-059 = A. Photo by Edgar Alvarado-Rodríguez.
The number of single-region species in the Caribbean islands is 19 (11.7%). Three of these species (15.8%) are country endemics, fourteen species (73.7%) are non-endemics, and the remaining two (10.5%) are non-natives (see below). As expected, the Gekkonidae are conspicuous in most of these islands (Figure 6) (No asterisk = Non-endemic; * = country endemic; ** = insular endemic; and ‡ = non-native).
| Eleutherodactylus planirostris ‡ | Aspidoscelis rodecki * |
| Dendrosophus microcephalus | Leptophis mexicanus |
| Trachycephalus vermiculatus | Oxybelis fulgidus |
| Anolis allisoni ‡ | Tantilla moesta |
| Norops lemurinus | Epictia magnamaculata |
| Coleonyx elegans | Thamnophis proximus |
| Sceloporus cozumelae * | Agkistrodon russeolus * |
| Mesoscincus schwartzei | Trachemys venusta |
| Aristelliger georgeensis | Rhinoclemmys areolata |
| Sphaerodactylus continentalis |

Figure 6. Spotted least gecko (Sphaerodactylus continentalis) from Cozumel. Little is known from this gecko in the Mexican Yucatan Peninsula with Cozumel being the location of its northernmost population. Distributional status = NE, EVS= M (10), IUCN = NE, NOM-059 = NS. Photo by Luis Díaz-Gamboa.
In the Pacific islands of Baja California, there are seventeen (10.4%) single-region species, of these seventeen species, four (23.5%) are insular endemics, another four (23.5%) are country endemics, and nine (52.9%) are non-endemics (See below). This is the only insular region in which worm lizards can be found (Figure 6) (No asterisk = Non-endemic; * = country endemic; ** = insular endemic; and ‡ = non-native).
| Hyliola rejilla | Gambelia copeii |
| Aneides lugubris | Phrynosoma cerroense * |
| Batrachoseps major | Sceloporus occidentalis |
| Elgaria cedrosensis * | Plestiodon skiltonianus |
| Elgaria multicarinata | Lampropeltis herrerae ** |
| Elgaria nana ** | Pituophis insularis ** |
| Anniella geronimensis * | Diadophis punctatus |
| Anniella pulchra | Crotalus caliginis ** |
| Bipes biporus * |

Figure 7. The Baja California Legless lizard (Aniella geronimensis) is one of the only two species of legless lizards inhabiting Mexican insular systems. Its range goes along the Pacific coast of the Mexican state of Baja California, and the islands San Martin and San Jerónimo. Although it is of secretive habits, it is common to find between the roots of the introduced iceplant (Mesembrianthemum cristallynum) that overpopulates the sandunes of that island (VHGS pers. Observ.) EVS = M (13), IUCN = EN, NOM-059 = Pr.
Finally, in the islands of the Gulf of Mexico, there are 13 (~8%) single-region species and all 13 of these species are non-endemics, as are enlisted below (No asterisk = Non-endemic; * = country endemic; ** = insular endemic; and ‡ = non-native):
| Crocodylus moreletii | Dipsas brevifacies |
| Sceloporus variabilis | Imantodes cenchoa |
| Drymobius margaritiferus | Ninia sebae |
| Lampropeltis abnormal | Tropidodipsas sartorii |
| Pseudelaphe flavirufa | Dermatemys mawii |
| Spilotes pullatus | Staurotypus triporcatus |
| Coniophanes imperialis |
In summary, of the 163 single-region species documented in the insular systems herpetofauna of Mexico, 56 (34.3%) are insular endemics, 37 (22.7%) are country endemics, 67 (41.1%) are non-endemics, and 3 (1.8%) are non-natives. Insular endemic species constitute the largest proportion of these species’ categories only in the Gulf of California physiographic region (44 of 78 species, or 56.4%). Country endemics comprise the greatest proportion only in the Tropical Pacific Islands physiographic region (20 of 36 species or 55.5%). Non-endemic species make up the highest proportion in the Baja California Pacific Islands (9 of 17 species or 52.9%), Gulf of Mexico Islands (all 13 of 13 species or 100%), and Caribbean Islands physiographic regions (14 of 19 species or 73.7%). Non-native species are in the Tropical Pacific Islands, Gulf of Mexico, and Mexican Caribbean Islands physiographic regions (3, 4, and 6, respectively). Hemidactylus frenatus and Indotyphlops braminus are within these three regions.
In order to analyze the herpetofaunal similarity relationships among the five insular physiographic regions, researchers constructed a Coefficient of Biogeographic Resemblance (CBR) matrix using the algorithm of Duellman [20]. As mentioned above, the greatest species richness of 108 species is found in the Gulf of California region and the least of 40 species in the Pacific islands of Baja California. The mean species richness for the five regions is 60.6. The number of shared species between all the regional pairs ranges from 0 in two instances to 23 between the Gulf of California islands and those in the Pacific region of Baja California, and the same value between the islands of the Gulf of Mexico and those of the Caribbean Sea (Table 1). The mean value of shared species among all five regions is 7.0. As expected, the greatest similarity exists between those regions in closest proximity to one another, i.e., between the islands of the Gulf of Mexico and those of the Caribbean (also 27 species), closely followed by the shared species between the islands of the Pacific regions of Baja California and those of the Gulf of California (23 species) and, also expected, and here demonstrated, was the complete lack of species shared between the two regions associated with Baja California and the two on the eastern coast of Mexico (Figure 7).
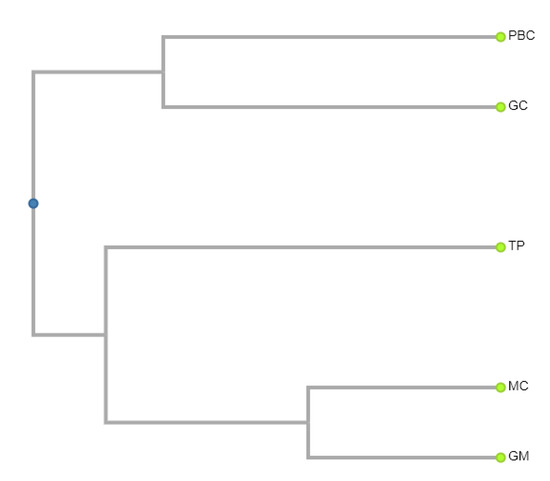
Figure 7. UPGMA-generated dendrogram illustrating the similarity relationships of species richness among the herpetofauna in the five physiographic regions of the Mexican Insular Systems (based on the data in Table 1). researchers calculated the similarity values using Duellman’s (1990) Coefficient of Biogeographic Resemblance (CBR). PBC = Pacific of Baja California; GC = Gulf of California (Sea of Cortés), TP = Tropical Pacific, GM = Gulf of Mexico, MC= Mexican Caribbean ((GC:0.345, PBC:0.345):0.133,(TP:0.404,(GM:0.197,MC:0.197):0.207):0.074).
Table 1. Pair-wise comparison matrix of Coefficient of Biogeographic Resemblance (CBR). Bold/Underlined values = number of species in each region; upper triangular matrix values = species in common between two regions; and lower triangular matrix values = CBR values. The formula for this algorithm is CBR = 2C/(N1 + N2), where C is the number of species in common to both regions, N1 is the number of species in the first region, and N2 is the number of species in the second region.
| Gulf of California | Pacific Baja California | Tropical Pacific | Gulf of Mexico | Mexican Caribbean | |
|---|---|---|---|---|---|
| Gulf of California | 108 | 23 | 10 | 3 | 3 |
| Pacific Baja California | 0.31 | 40 | 3 | 0 | 0 |
| Tropical Pacific | 0.123 | 0.064 | 54 | 9 | 10 |
| Gulf of Mexico | 0.04 | 0 | 0.187 | 42 | 27 |
| Mexican Caribbean | 0.0387 | 0 | 0.198 | 0.607 | 47 |
The herpetofauna of the tropical Pacific islands exhibits a limited overlap of species (ranging from three to ten) with both the Baja California regions and the two east coast Mexican regions. The species that are part of the Pacific Tropical Islands herpetofauna shared with those in the Gulf of California are as follows: Sceloporus clarkia; Urosaurus ornatus; Hypsiglena slevini; Hydrophis platurus; Rena humilis; Caretta caretta; Chelonia mydas; Eretmochelys imbricata; Lepidochelys olivacea; and Dermochelys coriacea. Of these ten species, only four are terrestrial, the first two listed are lizards and the other two are snakes; the remainder are the six marine species occurring along the Pacific shores of Mexico, one a snake and five turtles (Figure 8). The species that are part of the Pacific tropical islands herpetofauna and also those of the islands of the Gulf of Mexico (eleven species) and the islands of the Caribbean (twelve species) are as follows: Rhinella horribilis; Smilisca baudinii; Hypopachus variolosus (only the islands of the Gulf of Mexico); Crocodylus acutus (only the Caribbean islands); Hemidactylus frenatus; Iguana iguana; Aspidoscelis deppii; Mastigodryas melanolomus (only the Caribbean islands); Oxybelis aeneus (only the Caribbean islands); Sibon nebulatus (only the islands of the Gulf of Mexico); Indotyphlops braminus and three sea turtles (Caretta caretta, Chelonia mydas, and Eretmochelys imbricata). The pattern on the eastern coast of Mexico is distinct from that seen on the Pacific coast. Taking apart the sea turtles, the species involved are all terrestrial, except for C. acutus, which can occupy both fresh and saltwater habitats, in addition to occurring on land. Most of the species are native to the area they occupy in the insular systems, except for two non-native species, H. frenatus and I. braminus. Three of the species are anurans, one is a crocodilian, four are lizards (including the non-native gecko H. frenatus), and four are snakes (including the non-native blindsnake I. braminus). The terrestrial species involved are among some of the most widespread species both inside and outside Mexico.

Figure 8. Green sea turtle (Chelonia agassizi) Isla Clarión (Tropical Pacific). As expected, the different physiographic regions used in this paper show little biogeographic resemblance among them. This is because of the characteristic isolation of insular systems along with the almost inexistent species turnover. It is not surprising that marine reptiles are the only species shared among several different physiographic insular regions. The sea turtles occur in the surrounding waters of many Mexican islands, but only spawn or nest in few of them. Distributional status = NE, IUCN = EN, NOM-059 = P (EVS do not apply for marine species). Photo by Humberto Almanza.
4. Patterns of Distribution within Physiograph Regions
Cedros is the fourth largest island in Mexico, after Isla Tiburón, Isla Ángel de la Guarda, and Cozumel (wikipedia.com; accessed on 3 March 2020). The herpetofauna of this island comprises 15 species, including one anuran, eight lizards (Including the horned lizard Phrynosoma cerroense, Figure 9), and six snakes. Six of the seventeen islands support only one recorded species, which is the same species in all cases (Uta stansburiana). Not surprisingly, this phrynosomatid lizard is found on all 17 islands in this region. The remainder of the 39 species in this region occur in from one to six islands. The next most widely distributed species is Aspidoscelis tigris, which occupies six islands in this region. The rest of the 39 species occur in these islands as follows: one island (13 species); two islands (17 species); three islands (three species); four islands (three species); and five islands (one species).

Figure 9. Cedros island horned lizard (Phrynosoma cerroense). The only horned lizard on the Mexican islands, despite its common name, it is not exclusive of Cedros Island, but is endemic from the Baja California Peninsula (as is this individual photographed in San Fernando, in the Vizcaino desert in mainland Baja California). Distributional Status = CE, EVS = H (16), IUCN = NE, NOM-059 = A. Photo from the Amphibian and Reptile Atlas of Peninsular California ((herpatlas.sdnhm.org) San Diego Natural History Museum), courtesy of Bradford Hollingsworth.
The total number of islands occupied by the 54 resident species (five anurans, one crocodilian, forty-one squamates, and six turtles) vary from 1 to 13 (mean, 2.9). The greatest number of species (22) is recorded from Isla María Madre, the largest of the four principal islands making up the Islas Marías (wikipedia.com; accessed 4 March 2020). The second and third next-largest herpetofaunal segments are found on the second and third largest islands in the archipelago, i.e., Isla María Magdalena (18 species) and Isla María Cleofas (14 species). The remaining island in this archipelago is Isla San Juanito, with seven species. Of the total number of species (30, including Dermochelys coriacea, recorded only from the archipelago in general without documentation on a specific island) inhabiting the Islas Marías archipelago, including four anurans, one crocodylian, twenty squamates, and four turtles, none are regional endemics, whereas eleven are country endemics (with P. cleofasensis as the most reciente description of an insular endemic species, Figure 10); no non-native species are recorded and nineteen are non-endemics. Species found on all four of the major islands in this archipelago are Ctenosaura pectinata and Aspidoscelis communis; otherwise, there are 11 species inhabiting three of the four islands, 3 on two, and 13 on a single island (D. coriacea has to be excused from this accounting). Lying between the Islas Marías archipelago and the mainland of Nayarit is Isla Isabela, which is designated as a national park. Eleven species are known to occur on this small island, including one anuran and ten squamates. Of these eleven species, three are the non-native species Gehyra mutilata, Hemidactylus frenatus, and Indotyphlops braminus, five are non-endemics, two are country endemics, and one is a regional endemic. In summary, the numbers of species found on the 17 islands in this region range from 4 to 22. The most broadly distributed species in this region is Ctenosaura pectinata, which occupies 13 islands. The next most widely ranging species is Norops nebulosus on 10 islands. The remainder of the 53 species occur on the islands of this region as follows: one island (22 species); two islands (5 species); three islands (12 species); four islands (6 species); five islands (1 species); six islands (2 species); and seven islands (3 species). Another group of notable islands in this physiographic region is the Las Marietas, which are uninhabited and located a few kilometers off Punta Mita in extreme southwestern Nayarit. The known herpetofauna of these islands comprises ten species, including eight squamates and two sea turtles. These ten species include nine non-endemics and one country endemic.

Figure 10. María Cleofas leaf-toed Gecko (Phyllodactyllys cleofasensis) from Isla María Cleofas (Tres Marías Archipielago), Tropical Pacific. In the past it was assumed that insular amphibians and reptiles from the Western Mexican Pacific were representatives of taxa found on the adjacent mainland. However, recent molecular studies had revealed that many Phylldactylus geckos dwelling within the Pacific Insular Systems are, in fact, distinct and independent species. One notable example is Phyllodactylus cleofasensis, marking the identification of the third gecko species residing on the islands off the coast of Nayarit. This finding is accompanied by the recognition of P. isabelae as an endemic species to the Marietas Islands and P. lupitae as an endemic species to El Coral Island. Photo by José Rafael Nolasco-Luna.
5. Patterns of Distribution within Physiograph Regions
The largest number of species (118 or 52.2% of 226 species) is allocated to the non-endemic category. The ordinal proportion of non-endemic species is as follows: Anura (11 of 14 species or 78.6%); Caudata (2 of 2 species or 100%); Crocodylia (2 of 2 or 100%); Squamata (91 of 195 species or 46.7%); and Testudines (12 of 13 species or 92.3%).
The next largest group of species comprises the regional or insular endemics, amounting to 56 species (24.8% of 226 species). The ordinal proportions are as follows: Anura (0 of 14 species or 0%); Caudata (0 of 2 species or 0%); Crocodylia (0 or 2 species or 0%); Squamata (56 of 195 species or 28.7%); and Testudines (0 of 13 species or 0%).
The third largest group of species consists of the country endemics of which there are 43 species (19.0% of 226 species). The ordinal proportions are as follows: Anura (2 of 14 species or 14.3%); Caudata (0 of 2 species or 0%); Crocodylia (0 of 2 species or 0%); Squamata (42 of 195 species or 21.1%); and Testudines (1 of 13 species or 7.7%).
The total number of endemic species (country endemic plus regional or insular endemics) is 99 or 43.8% of the total insular herpetofauna of 226 species. This proportional endemicity is less than that reported for Mexican states such as Jalisco (64.6%;[16]); Michoacán (63.7%; [21]), Nayarit (57.1%; [22]), Oaxaca (58.1%; [23]), or Puebla [24], but higher than that documented for Chiapas (17.6%;[25]), Coahuila (28.0%; [26]), Nuevo León (28.1%; [27]), and Tamaulipas (32.1%; [28]). This dichotomy is principally due to the positioning of the state relative to either the Mexico–US border or the Mexico–Central American border, with states lying in the vicinity of one or the other of these borders having endemic species proportions lower than that of the insular regions and of those states lying relatively remotely from those two borders having higher proportions.
The smallest group of species constitutes the non-native species, of which there are seven species (3.2%). These seven species consist of one anuran (Eleutherodactylus planirostris) (Figure 11), five lizards (Anolis allisoni, Norops sagrei, Gehyra mutilata, Hemidactylus frenatus, and H. turcicus), and one snake (Indotyphlops braminus).

Figure 11. The Greenhouse Frog (Eleutherodactylus planirostris) from Cozumel (Mexican Caribbean) is extremely small-sized (adults < 30 mm in length). Native to Cuba, the Bahamas, and Cayman Islands. It is the only introduced amphibian inhabiting any Mexican insular system. Apparently, the populations colonizing the Mexican Yucatan Peninsula are related to the Greenhouse Frog’s populations from Panama and/or the Philippines. Environmental impacts produced by the diminutive Greenhouse Frogs need to be determined, since there has been no direct evidence for it being particularly harmful [29]. Islands are hostile environments for most amphibians, so it is not rare that there are not other examples of introduced amphibians in Mexican islands. But Caribbean, and some Tropical Pacific islands (like Tres Marias Archipielago) may be humid enough to allow colonization by the Greenhouse Frog, Cane Toad (Rhinella marina), Bullfrog (Lithobates catesbeianus) or Cuban tree frog (Osteopilus septentrionalis). Distribution status = NN, IUCN = LC. Photo by Carlos Pavón-Vázquez.
None of the amphibians are exclusively distributed on any of the islands, and just two (12.5%) are country endemics, i.e., Eleutherodactylus pallidus and Incilius mazatlanensis. The remainder are either non-endemics (thirteen or 81.3%) or non-natives (one or 6.3%).
Only the squamates have taxa occupying all four of the distributional categories. Of the 195 squamate species, 91 (46.6%) are non-endemic species, 42 (21.54%) are country endemic species, 56 (28.7%) are regional or insular endemic species, and 6 (3%) are non-native species. Among the 84 snake species, 14 are country endemics (16.6%) and 17 are regional or insular endemics (20.2%); a single species is non-native (1.2%). Of the 17 regional or insular endemic snake species, eight or 47.1% are rattlesnakes. Among the 111 lizard species, 28 are country endemics (25.2%) and 39 are insular endemics (35.1%), and 5 species are non-natives (4.5%). Among the 39 insular endemic lizard species, 11 are phrynosomatids (28.2%), 11 are teiids (28.2%), and 17 belong to five other families. No turtle species is exclusive to the islands and only one (Kinosternon integrum) is a country endemic. Most likely, molecularly based taxonomic studies of insular populations of Phyllodactylus, Sceloporus, and Uta will lead to the uncovering of new species.
6. Comparison of Distributional Categorizations and Physiographic Regional Categorizations
Researchers compared the distributional categorizations and the physiographic regional categorizations. The data in this table indicate that the largest proportion of species in each physiographic region consists of the non-endemic species, as follows: Gulf of California (49 of 108 species or 45.4%); Pacific of Baja California (27 of 40 species or 67.5%); Tropicales from the Pacific (25 of 57 species or 43.9%); Gulf of México (39 of 46 species or 84.8%); and Mexican Caribbean (39 of 46 species or 84.8%). A second conclusion is that the country endemic species are represented most evidently in the physiographic regions on the Pacific side of Mexico, as opposed to those on the Atlantic side. The proportions range from 13.9% (15 of 108 species) in the Gulf of California to 36.7% (21 of 57 species) in the Tropicals of the Pacific, respectively. On the Atlantic side the proportions are 6.5% (3 of 46 species) and 11.3% (6 of 53 species) in the Gulf of Mexico and Mexican Caribbean, respectively. The insular endemics are restricted in distribution to the physiographic regions on the Pacific side of Mexico and are most prominently a part of the herpetofauna of the Gulf of California, i.e., 44 of 108 species or 40.7%. The numbers of such species in the other two Pacific coastal regions are 8 of 57 species in the Tropical of the Pacific (14.0%) and 4 of 40 species in the Pacific of Baja California (10.0%). The non-native species are found only in three of the five regions, i.e., the Tropicals of the Pacific (3 of 57 species or 5.2%), the Gulf of Mexico (3 of 46 species or 6.5%), and the Mexican Caribbean (6 of 53 species or 11.3%). Interestingly, six of the seven non-native species in the insular regions occur in the Mexican Caribbean (all except for the gecko Gehyra mutilata), with three each in the Tropicals of the Pacific and four in the Gulf of Mexico.
7. Principal Environmental Threats
7.1. Deforestation, Agriculture, and Urban Development
Most Mexican islands are of a relatively small size, with most of them also uninhabited, but many are large enough to support temporary settlements of fishermen or navy bases, and all the larger islands have permanent towns, with the notable exception of Isla Tiburón, which is only occupied by a Mexican Navy outpost and the settlements of the Seri people [30][31], and Isla Ángel de la Guarda, which is uninhabited due to the lack of fresh water on the island [32]. Urban development is intense on the tourist islands of the Caribbean, such as Isla Mujeres and Cozumel, where the properties can have a very high economic value. Fortunately, the decree of several protected areas has limited considerably the space available for tourist development. Nevertheless, the demand for land continues on those islands to such a degree that the economic interest has involved even senior officials of the Mexican government in acts of land price speculation and land use changes. Cancún is the paradigmatic example of the almost complete destruction of an insular territory due to urban development. The process of urbanization and the transformation of Cancún into a peninsula is discussed by González-Sánchez et al. [17].
Another interesting case is that of María Madre Island, which supported a penitentiary colony since 1905. At its population peak (approximately 1986), it hosted nearly 5000 people. Environmental impact has occurred on the island due to the operation of the colony, such an open-air dump, the establishment of a sawmill, the introduction of cattle and other livestock, the proliferation of feral cats, and the furtive capture and consumption of fauna such as boas, iguanas, and sea turtles by the colonists. Even so, several conservation achievements exist, such as the decree of the Islas Marías Archipelago as a Protected Area and World Heritage site [33]. By federal decree, the penitentiary colony was closed in 2019 and, at the moment of this redaction, these installations are under the transition to become a cultural center. This change opens the Tres Marias Archipielago to biodiversity conservation and ecological restoration programs, since in the past, due security restrictions, the Tres Marías islands were hardly accessible for conservationists. However, the access, management, and operation of this touristic and cultural center is under the control of the Mexican Navy, and this raises concerns about the accessibility these islands will have in the near future.
Also, Isla de Términos supports the important Ciudad del Carmen, which has been an important hub of the nationalized Mexican petroleum industry since 1971 [34][35] and has hosted a population of approximately 27% of the total population of the state of Campeche [35]. By far, it is the most highly populated island in Mexico [19]. The island used to be covered extensively by mangroves (Rhizophora mangle, Avicennia germinans, Laguncularia racemosa, and in a much lesser proportion Conocarpus erecta), with the shallow water on the lagunar side of the island being dominated by beds of seagrasses (Thalassia testudinum, Halodule wrightii, and Siringodium filiforme [36]). Most of this vegetation has been replaced by coconut and rice plantations and by the expansion of Ciudad del Carmen [37], an urban area of approximately 2700 ha that supports 170,000 inhabitants [34]. Several changes in land use and population dynamics of Ciudad del Carmen have occurred in recent years, since the oil industry of the region has faced great uncertainty since the mid-2010s [35]. Even so, Isla del Carmen, along with the entire Laguna de Términos system, is one of the most important biodiversity areas in Mesoamerica [37]. The associated effects of urbanization on that island are summarized in the other portions of this section.
Other islands with important settlements and a growing population are Isla Cedros, Holbox, San Marcos, and Natividad [19]. Of special concern is the growing population in Holbox, where, in the last few years, uncontrolled development and a lack of a proper waste management had led to an environmental crisis [38][39] (Figure 12).

Figure 12. A Morelet’s Crocodile (C. moreletti) taking a sunbath on a disposed spring in one of the many marshes on Holbox island. A major problem in populated islands is the waste disposal, which is a major logistical challenge for municipal authorities. This is more severe in those islands subject to rapid urban development due to tourism activities, such as in Holbox, Mujeres, and Cozumel. Photo courtesy of Eduardo Pacheco Cetina.
7.2. Agriculture and Cattle
The practice of agriculture is almost absent from the islands, but is extensive in Isla del Carmen, Campeche. In the past, the dominant vegetation consisted of mangroves and sea grasses, but urban development and fields of coconuts and rice [17] have replaced most of this vegetation.
The beginning of the introduction of cattle can be traced to the colonial times, when Spaniard navigators left on many islands several animals, such as goats, sheep, and pigs, with the aim of having food stocks on their navigation routes. Generally, however, this activity was limited only to the release of these animals with no particular interest in nurturing. The outcome of this activity was the establishment of feral populations. The problems associated with such populations are discussed in the section on invasive species [40] (Figure 13).

Figure 13. Eroded Landscape (18°45′06″ N y 110°58′07″ W) in Socorro Island (Revillagigedo Archipielago). In one of the few cases in which the introduction of an invasive species can be traced to one single event and/or specific date, approximately 100 sheep were introduced to Socorro in approximately 1869 with the intention of them becoming food sources for fishermen and boaters. They became feral and roamed free in the southern and eastern sides of the island and, by, 1989, they had an estimated population of 3000 individuals. They were eradicated completely by 2010. But by then, several portions of this island had been overgrazed. As is shown in this picture, the whole Horizon 0 and A are absent. The removal of the canopy and vegetation exposed the soil to the elements, since Socorro is from volcanic origin, several portions of the island are of pronounced slides, which aggravated the effects of hydric erosion, cleared all the superior Horizons of the soil, and resulted in this guilled landscape in which only parent material remains.
The existence of formal cattle raising as a productive economic activity Is present on few islands, most notoriously on Isla del Carmen, where extensive areas of cattle pasture exist. In the last few decades, the area devoted to agriculture and cattle raising on the islands has decreased [41] because of the increase of oil industry activities [42].
7.3. Hurricanes and Other Tropical Storms
Hurricanes and tropical storms are a major force that shape the structure of the insular and coastal ecosystems of Mexico, and are particularly important on the Mexican Yucatán Peninsula, where tropical storms occur with relatively high frequency. Hurricanes can be highly destructive for those species who spawn in beaches or sand dunes, like crocodiles or sea turtles. For instance, based on internal reports from CONANP (accessed through request to the Instituto Nacional de Acceso a la Información), Hurricane Delta flooded 40% of Chelonia mydas nests in Ixpalbarco (Cozumel) (7 October 2020). Almost one year later (19 August 2021), on that same beach, Hurricane Grace destroyed approximately 90% of the nests of Chelonia mydas and 100% of Caretta caretta nests (totaling ~400 nests). That year, approximately 80% of nests from APFF Isla de Cozumel and PN Arrecifes de Cozumel were lost (~1300 nests of both species) by flooding and/or erosion. This means that in 2021, a whole reproductive season of marine turtles from Cozumel was almost lost (inserter cita de reporte interno). However, recent studies on the effect of such intense climatic events on American crocodiles’ population of Isla Cozumel and Banco Chinchorro Atoll are highlighting the negative impacts of hurricanes on the short-term but also positive impacts on the longer term on the ecology and health of these reptiles [43][44][45]. Then, the high frequency of tropical storms surely is a threat but also an important factor in the evolution of crocodiles and other reptiles in the Caribbean islands (Figure 14).

Figure 14. Severe devastation observed in the nesting grounds of the American crocodile (C. acutus) within Cayo Centro, Banco Chinchorro (Mexican Caribbean), following the impact of a hurricane. This nesting area is highly susceptible to erosion, flooding, and canopy loss (resulting in increased exposure to intense solar radiation). The escalating frequency of tropical storms, driven by the effects of climate change, intensifies the vulnerability of this location, and amplifies the profound risk of losing an entire cohort or even causing extinction of this isolated population. Photo by Pierre Charruau.
Those meteorological phenomena, however, are not exclusive to the Atlantic; in the tropical Pacific, researchers found that CONANP identified three hurricanes that impacted Isla Isabel and Isla Marietas, and although there is no information on the damage of turtles’ nesting sites CONANP identified Hurricane Kena (5 in Saffir Simpson) as being severely damaging for birds and vegetation, with almost all trees of Isla Isabel being defoliated. It is important to notice that vegetation loss can be an important factor affecting the nesting success, since the canopy loss may result in major exposure to sunlight by the nesting sites, and thus changing the temperature of the nesting site.
In October 2015, Hurricane Patricia became the most intense (by central pressure) and powerful (by wind speed) tropical storm ever measured in history, and impacted the coasts of Jalisco and Colima [46]. The trajectory of the storm passed near the islas Marietas and impacted the region of Chamela in Jalisco. Apparently, in the Marietas the damages were minimal (https://www.gob.mx/semarnat/prensa/reporta-sector-ambiental-danos-menores-en-zonas-costeras-por-paso-de-huracan-patricia accessed on 1 June 2020), but in the region of Chamela, the impacts on the vegetal structure and changes in the hydrological regimen were intense [47][48]. Unfortunately, post-storm surveys were made only on the mainland. So, what the effects were on the islands of Chamela Bay are unknown.
7.4. Wildfires
Wildfires are particularly catastrophic on islands since the area available for the fauna to escape is very reduced or even nonexistent (Figure 15). For example, in February of 1997, a wildfire consumed in less than 24 h the totality of the vegetation of Isla La Larga, one of the Marietas islands in Bahía de Banderas, probably caused by an uncontrolled bonfire set by tourists. That incident encouraged authorities to create stricter regulations for tourism on islands.

Figure 15. Vegetation damaged by a wildfire, Isla Pajaros, Gulf of California, near Mazatlán, Sinaloa. APFF Islas del Golfo de California (27 March 2019). Photo by Comisión Nacional de Áreas Naturales Protegidas (CONANP) obtained via Instituto Nacional de Transparencia, Acceso a la Información y Protección de Datos Personales (INAI).
Given the isolation of the sites, the logistics for containing fires are extremely difficult in many cases. A clear example of the difficulty in attending to such contingencies is the event on Isla Guadalupe on the 15th of September 2008, when an uncontrolled burning of trash (made by workers of an ONG) near a biological station grew into a wildfire. The transport of personnel and equipment onto the island to attend to this contingency required the use of airplanes and ships and required the coordination of ~90 fieldworkers from several government institutions and ONGs, among other actors. It took approximately 80 h to be controlled and more than 300 h for its total extinction. This fire resulted in the damage of ~637 ha of vegetation (mostly grasslands) [49].
As it occurs in continental areas of Quintana Roo [17], the pressure for urbanization can provide a reason to clear the terrain and gain space for developing tourist infrastructure. This could be the reason behind the wildfire on Holbox Island between the 17th and 21st September 2016, during those days, a forest fire broke out east of the small island of Holbox, in the area known as “La Ensenada.” The official report of the Federal Attorney for Environmental Protection (PROFEPA) determined that it was an arson attack, which affected a total area of 87.22 hectares of low deciduous forest and coastal scrub. Despite Holbox being near a mainland island, to extinguish the fire required an important interinstitutional effort which involved 85 people (39 brigades from CONAFOR, 7 from CONANP, 7 from SEMAR, 7 from SEDENA, 6 from PROFEPA, 6 from PRONATURA Yucatan Peninsula and 13 from the island of Holbox); 22 vehicles were also used, including trucks, boats, a helicopter, and a twin-engine plane. Despite the early warning and quick response, it took four days to extinguish the fire, and destroyed 87 has.
7.5. Tourism
According to Coccossis and Parpairis [50], the tourism-carrying capacity is “The maximum level of recreation use, in terms of visitor numbers and activities, that can be accommodated before a decline in ecological value sets in.” Thus, the carrying capacity would be the point at which the demand of infrastructure requirements and natural resources become insufficient to meet the needs of the visitors and residents before environmental hazards appear. Considering this situation, it is evident that due to their isolation, reduced size, and vulnerability, the concept of carrying capacity is highly relevant when talking about the number of tourists an island can sustain.
Tourism is the third most important economic activity in Mexico. Despite the general perception of Mexico being an insecure country to visit, the truth is that tourism demonstrates a sustained growth year by year and Mexico usually ranks among the principal tourist destinations of the world. The region of the Yucatán Peninsula (and especially Quintana Roo) stands out as the principal sun and beach destination in the country. Much of this attraction is due to what is offered by the islands in the Mexican Caribbean, such Cozumel, Isla Mujeres, and Cancún. In the case of the latter, however, the insular ecosystem has been destroyed almost entirely by the tourism megainfrastructure and the physiography of the island has been modified to such a degree that it can hardly be considered an island anymore, but rather has been converted into a peninsula [17].
In the atoll of Banco Chinchorro there is a tourist attraction which began in 2013 that consists of swimming with American crocodiles (Crocodylus acutus) in the reef lagoon. This activity seems to be a good option for the sustainable use of crocodiles, however the methodology used seems to have caused a change in the behavior of the species a few years after its beginning [51]. Indeed, between 2017 and 2020 several human–crocodile incidents (n = 6) occurred in Cayo Centro, which corresponds to a significant increase since only 2 incidents were recorded before 2017. However, the swimming with crocodile activity does not seem to be the only factor responsible for the change in crocodile behavior and the situation is currently being studied. In the meantime, the authorities of the Banco Chinchorro reserve have decided not to authorize the activity (Figure 16).

Figure 16. Tourist interacting with Crocodylus acutus in Banco Chinchorro. Insular species, due to their isolated habitats, often exhibit a sense of naïvety resulting from the absence of predatory pressures. This holds true for American crocodiles residing in the atoll, where their ethological adaptations are evident through decreased aggressiveness. As a result, Chinchorro’s crocodiles have become accustomed to interacting with tourists and fishermen, displaying a habituation to their presence. Although this activity is not currently allowed by the CONANP, few touristic agencies continue to provide opportunities for observing or swimming with these crocodiles as part of their tours. Photo by Pierre Charruau.
Another popular touristic destination are the Islas Marietas, in where the whale watching, scuba diving, snorkeling, and primarily, the “Playa del Amor” (a semi-hidden beach located inside a cave within the island) makes this place one of the most attractive Mexican islands for tourists. The high demand, chaotic management, and excessive number of visitors led in 2016 to a controversial temporary closure of the island, to carry out ecological restoration labors and to redesign the visitors’ policies, since the maximum carrying capacity of visitors for the island was calculated as 625. The average number of visitors, however, is over 1000, with peaks of more than 3000 on some days (https://www.informador.mx/Jalisco/Cierran-Islas-Marietas-por-dano-ecologico-20160414-0084.html accessed on 1 June 2020).
7.6. Invasive Species
The introduction of invasive species into insular ecosystems has occurred from prehistoric times and has been considerably accelerated during the last 50 years [52][53]. Subsequently, the impact of invasive species has been widely recognized since the 1950s [54] and is considered to be the second most important cause of global biodiversity loss [55], and the first in insular systems [4][7][52]. Usually, the species living in an isolated environment free of predators tend to become naïve and lose their anti-predatory behavior [5][56]. This is why the effects of invaders are much more accentuated in insular ecosystems, where a few mammal species are responsible for most of the insular diversity declines, including black rats (Rattus spp.), feral cats (Felis catus), goats (Capra hircus), pigs (Sus scrofa), donkeys (Equus asinus), and European rabbits (Oryctolagus cuniculus) [57]. The insular reptiles are particularly vulnerable, since populations are more affected by feral cats [58] and black rats [59]. Several declines of insular herpetofaunas due to invasive species has been documented across the world [60], and those mammals are among the most frequent insular invaders [53][58][61]. In Mexico, cat introductions are associated with the decline of the endemic U. auriculatus in Socorro [62] (Figure 17), and on Isla Isabel (prior to the eradication of cats), a survey estimated that at least 24% of cat scats contained remains of reptiles [63]. Venomous reptiles, too, are not free of cat predation; Arnaud et al. [64] reported 13% of cat scats with remains of the endemic Crotalus catalinensis (prior to the eradication of cats on that island).

Figure 17. Urosaurus auriculatus, a Socorro Island Tree Lizad, Endemic of Isla Socorro although very conspicuous due to its bright blue color; females and juveniles are most frecuently of greyish color. After the eradication of feral cats in Socorro, its populations showed a growing tendency. Distributional Status = IE, EVS = H (16), IUCN = EN, NOM-059 = NS. Photo by Juan Diego Arias-Montiel.
The impact of invasive species on islands are not limited to direct predation, but also can involve change in land cover. The introduction onto islands of ungulate mammals, such as sheep, pigs, and goats, among others, as a source of food goes back to colonial times [40] and has resulted in severe losses of vegetal cover, soil erosion, and plant species extinctions on several Mexican islands, such as Guadalupe [65][66].
As the main source of impact, it is clear that the eradication programs of invasive species carry the greatest gains for biodiversity conservation; therefore, these programs have been increasing in scope, frequency, and complexity [8]. Since the end of the 20th century, many coordinated interinstitutional efforts involving governmental agencies, NGOs, and the academic community have successfully accomplished several eradication programs of invasive mammals on Mexican islands [57][67].
Most rodent eradications are carried out through the dispersion (areal or by hand) of brodifacoum, an anticoagulant rodenticide, which is known to have impacts on non-target species of mammals and birds. Little is known of its impact on reptiles, but large lizards, such as iguanas, might be susceptible due to their consumption of the baits (frequently they are made with a mixture of rodenticide and cereal or other attractants), or by secondary exposure as a result of eating poisoned dead rats, but the mortality rate seems to be low. Thus, reptiles might have a low risk of population-level declines through brodifacoum-induced mortality after rodent eradications [68]. Therefore, in a cost–benefit relation, reptiles can benefit highly from rodent eradication. Some examples of reptile recoveries after the extirpation of invasive species are the “reappearance” of Lampropeltis californiae (listed in the report as L. getula nigrita) on San Pedro Martir Island afther two years of rat eradication. Phyllodactylus homolepidurus went from “extremely rare” to “low abundance” in Farallon de San Ignacio (also after two years of rat eradication), and Ctenosaura pectinata on Isabel [69], whereas the Socorro Tree Lizard (Urosaurus auriculatus) showed an increase in population numbers since the implementation of programs to control the feral cats [70]. Also, an increase in sightings of boas (Boa imperator) has been observed on Cayo Centro in the atoll of Banco Chinchorro since the eradication of rats (Rattus rattus) and feral cats on the island (Pierre Charruau, pers. comm.). Major challenges for future generations of conservationists, however, persist on the largest and most populated islands such as Cozumel (Figure 18) in the Caribbean and Cedros in the Pacific of Baja California.

Figure 18. A large male of Boa imperator, rescued by members of the fire department from a hotel, in Cozumel, Quintana Roo, as mentioned by González-Sánchez, et al. [52]. This species was introduced in Cozumel by filmmarkers in 1971, also, the central American Boa Constrictor may be alien for Banco Chinchorro (Mexican Caribbean), and Venados (Gulf of California), but this must be confirmed or rejected by further studies. Photo by Lizbeth E. Lara-Sánchez.
7.7. Global Climate Change
Climate change is expected to become the principal cause of extinctions in future decades [71]. Also, it has been implicated as the main driver of population decline for both reptiles [60] and for amphibians [72]. A characteristic of insular populations is their reduced genetic variability, so these species are limited in their capacity to survive changing conditions [53]. One of the main consequences of climate change will be the shift in range distribution of species in order to find more suitable conditions to survive, along with alterations in community composition and changes in the interactions among species [71][73]. Obviously, for the great majority of insular species, range distribution shift is not an option for survival. Moreover, most islands are small-sized and, generally, their topography does not have the range of altitudes found in mainland territories.
Ectotherms are especially vulnerable to global warming, since activity periods [74] and processes such as spermatogenesis and sex determination are heavily influenced by temperature [73]. Not much is known of the effects of global warming in herpetofaunal insular populations in Mexico, but the existence of sex-biased ratio in the population of Crocodylus acutus (a species with temperature-dependent sex determination) (Figure 19) in Banco Chinchorro due to incubation temperature conditions is documented [44][75]. The main threat due to climate change, however, could be the destruction of habitat by two factors: (1) increase of severity and frequency of hurricanes and tropical storms, as discussed in previous paragraphs, and (2) increase of sea level. These factors are especially important on the islands of the Yucatán Peninsula that have low elevations, such Arrecife Alacranes and Banco Chinchorro. In the latter case, most of its surface is permanently emerged. A side-effect of the sea level rising and alterations of tides is erosion. This factor could be an important threat on sandy islands or those of coralline origin. Again, the islands of Banco Chinchorro, Arrecife Alacranes, and the islands of Veracruz are particularly susceptible to being damaged by these processes. An imperative measure is to annually map the perimeters of those islands, in order to ensure early detection of any change in shape and/or size and to take measures such restoring the beaches or the mangroves in the shore.

Figure 19. Specimen of Crocodylis acutus in Banco Chinchorro. Hybridization in Mexican crocodiles is a growing phenomenon due to the frequent translocations of swamp crocodiles (Cr. Moreletti) which successfully establish new population towards their invasion front. Since Morelett’s crocodiles’ haplotype is dominant, increasing hybridization events are a menace for American crocodiles due to genetic introgression. Banco Chichorro is crucial as an outpost for preserving Cr. acutus from genetic pollution, since its remoteness from the mainland constitutes an effective barrier to colonization by Cr. moreletti. However, the resilience of this population is under threat from global warming. The increased frequency and intensity of hurricanes, rising sea levels, and a sex-biased ratio (as crocodiles exhibit temperature-dependent sex determination) pose significant challenges to their long-term survival. Photo by Israel Sánchez-Ortega.
The effects of El Niño Southern Oscillation (ENSO) are particularly pernicious in insular ecosystems, and are important throughout the trophic chain, especially among seabirds, plankton, fishes, and marine mammals [76]. Evidently, those alterations might influence reptile populations, such as those of crocodiles [77] and/or marine turtles [78][79][80]. Cerdá-Ardura (2018) hinted that this phenomenon could make food scarcer, and result in the starvation of Chukwallas on Rasa Island. Still, how the most intense ENSO regimens would affect the Mexican insular populations of reptiles and amphibians is a matter which has not yet been explored.
As explained in the description of physiographic regions, most of the islands of the Pacific and the Gulf of California lack permanent freshwater sources, so the effects of drought are more serious on islands than on the mainland. Thus, intense droughts can lead to significant fluctuations and rapid population loss, as reported by Case (1982, in Lovich, et al. [81]) for Sauromalus hispidus on Angel de la Guarda. Likely, this effect could be more accentuated in snakes, due to the decline of prey populations. Of course, droughts are even more serious among amphibians.
7.8. Oil and Gas Industries
The Gulf of Mexico is a semi-enclosed sea that connects in the east to the Atlantic Ocean through the Straits of Florida, and in the south to the Caribbean Sea through the Yucatan Channel. An important characteristic of this sea is the dominance of the Loop Current in the Yucatan Channel, and the formation swirls detached from that current (eddies) [82]. In the eventual case of hydrocarbon spill, it can be driven to several areas of the Gulf, including the insular systems. For example, the explosion in the shallow waters of the Bay of Campeche of the PeMex Ixtoc-I exploratory well occurred in 1979, resulting in the second major oil spill in the history of the Gulf of Mexico (just below the level of the 2010 Deepwater Horizon spill). Several months after the incident, the Ixtoc-I oil could be found all along the Gulf Coast from Texas to Yucatan [83]. Several insular systems such as the Arrecife Alacranes, Sistema Arrecifal Veracruzano, and the islands of Laguna de Términos are in the path of the Ixtoc-I oil well.
In 2005, Chevron-Texaco’s announcement regarding the installation of a liquefied Natural Gas Regasification Terminal just 600 m away from the Coronado islands, offshore of Tijuana, raised significant concerns (http://www.jornada.com.mx/2005/03/15/index.php?section=economia&article=025n1eco accessed on 1 June 2020) raised bitter controversy among conservationists, ONG’s, environmental authorities, and the business sector of Baja California [84]. A decisive point was the numerous inconsistences detected in the process (in fact, the government initially authorized permission to build the terminal, even though the Environmental Impact Manifestation presented by Chevron–Texaco referred to a project located in Yucatán, not Baja California). The platform would increase the risk of catastrophic explosion, oil spill, and introduction of rats to the Coronado islands, among other problems. After two years of controversy, Chevron–Texaco finally stopped the construction of the terminal (P.L.F., 2007).
As a result of the discovery in 1971 of Cantarell in the bank of Campeche, the national oil industry experienced a bonanza. A collateral effect of the successful oil industry was the development of associated infrastructure, mostly in the 1970s, when a rapid urbanization process in the Laguna de Términos region led to the subsequent habitat degradation and land-use change. This process was not limited to the mainland, but also occurred with Isla del Carmen and Isla Aguada. These islands went from being towns, whose main activities were the fisheries, farming, and forestry, to becoming important urban centers, with important processes of land-use transformation [85]. Like the case of Cancún, the infrastructure development has transformed Isla Aguada into a peninsula [17].
Because of its strategic position, Cayo Arcas (a group of three sandy cays west of the coast of Campeche) is an important seaport for the charging of oil tankers and constitutes the most important port for the national oil industry). But it is also an important nesting site for marine turtles and seabirds. By 2 November 2019, researchers from Universidad Autónoma del Carmen detected via remote sensing two potential oil spills nearby Cayo Arcas, the first on October 4th with ~200 ha of extension, while the October 7th event was larger with an estimated affected area of 4500 ha, which probably damaged seabirds and hatchlings of Chelonia mydas. Despite the existence of a public statement from the Universidad Autónoma Del Carmen (https://www.pagina66.mx/wp-content/uploads/2019/12/DERRAME-CAYO-ARCAS.pdf accessed on 7 June 2023), several communication attemps with that university from the lead author of this paper remain unanswered. Even so, researchers could obtain photographic evidence (Figure 20) and a internal presentation facilitated by a reporter from a local media.An answer from PEMEX to an information request (number; 1,857,200,483,719, de fecha 08 de enero de 2020 en la Plataforma Nacional de Transparencia (PNT)) where they acknowledge two oil spills nearby Cayo Arcas (which they catalogue as having had an impact that is of a “minor” level), which constitutes confirmation that these spills really occurred. However, the whole extension and consequences of those spills remain unknown since the Agency for Security, Energy and Environment (Agencia de Seguridad, Energía y Ambiente “ASEA”) maintains a three-year ban for the disclosure of any information (number of expedient: ASEA/USIVI/DGSIVEERC/AMB/0011/2019) related with that event.

Figure 20. Brigades of PEMEX cleaning an oil spill in the beaches of Cayo Arcas (Gulf of Mexico) in late 2019. By its strategic location, Cayo Arcas is relevant as a logistical stepping stone for oil tankers. Since the oil industry is a matter of national security in Mexico, the information regarding these impacts is classified and not easily accessible, but it´s clear that oils spills are a constant menace for Mexican islands in the Gulf of Mexico. Photo by Secretaria de Marina (SEMAR) ceded by PAGINA 66 (www.pagina66.mx).
Even more serious is that the information closure extends to any oil spill incident on the Mexican islands: researchers made an information enquiry (331002522000635) to ASEA to require the relation of oil spills that had impacted (since 1950) on Mexican islands. However, that agency declared itself as incapable of providing an answer. Afterwards researchers filed a complaint and, subsequently, ASEA was ordered to answer request. Nonetheless, they declared it would be impossible to provide an answer due to the “inexistence of records”. The consequences of this blockade could be potentially harmful for the environment, since it makes it impossible to quantify the number and extention of those impacts and constitutes a major obstacle for conservation and/or restoration efforts. Finally, it contributes to the lack of awareness on the severity of the problem, since many of the oil spills would remain unknown to academics, conservationists, and the general public.
Unfortunately, the threat that oil and gas industries represent for Mexican biodiversity is far from being mitigated: in contrast with the rest of the world, which is moving towards alternative energy sources, the Mexican government has passed several legal reforms since 2018 that discourage investment in alternative energy sources and favors the oil industry, especially the highly contaminant parastatal PEMEX. There is little hope for a short- or mid-term change in this situation, as the production of petroleum and gas remains a deeply ingrained nationalist matter that most Mexicans are reluctant to challenge. Consequently, altering the existing legal framework for energy generation carries an immense political cost that no Mexican governors are willing to undertake.
7.9. Delinquency and Organized Crime
It is of public knowledge that Mexico has faced a crisis in matters of public security since the mid-2000s. How this crisis of insecurity affects the efforts of conservationists is something that is discussed in the informal talks among Mexican conservationists, but it is a topic that, with few exceptions, such as the problems involving the endemic porpoise known as the Vaquita (Phocoena sinus) on the High Gulf of California [86], is not discussed openly. It is even less possible to find any formal study focusing on that problem (but see the compendium of Arroyo-Quiroz and Wyatt [87]), although subtle mentions of the problem can be found in few publications that discuss the many challenges of Mexican conservation [88][89]. Due to the obscure nature of these problems, their effects on biodiversity are not understood, but one of the main consequences of criminal activity is the displacement or abandonment of conservation efforts and/or research studies in sites that are considerated too risky or dangerous for researchers and conservationists. This problem might be the principal effect in some insular territories; due to their remoteness, the islands are sites of difficult survelliance, and some of them are vulnerable to being used as a refuge for illegal fishermen (called “piratas” in the North and “pachocheros” in the Caribbean), or as steppingstones for drug dealers on their routes to the USA. Although it must be noted that the Mexican Navy is cooperative with the efforts of conservationists in the insular or oceanic territories, including sites where the activity of organizated crime is intense, such as the High Gulf of California, where, otherwise, carrying out conservation programs could be much riskier [90].
7.10. Illegal Collecting
Illegal collecting is one of the activities that has had a great impact on reptile and amphibian populations [60]. In fact, reptiles are the second vertebrate group (just behind birds) most subjected to the pet trade, and only 8% of reptile species are regulated by CITES [91]. Additionally, the perceived rarity of a species increases the interest of collectors, which is reflected in an increase of collecting and an acceleration of extinction [92]. This problem also occurs with newly described species [93]. This problem is particularly worrisome with insular species, since they meet several risk factors, i.e., they are of restricted distribution, they are rare, and many of them are newly or yet to be described species; also, the characteristic naïvity of insular organisms makes them easy to collect.
Despite these considerations, the documentation of cases related to insular herpetofaunas in Mexico is scarce. The best-known example is the marine turtle, which is especially vulnerable when spawning on beaches, as they are exposed to poaching and egg harvesting [17]. This problem is especially evident on Cayo Lobos (Banco Chinchorro) in the Caribbean; this cay appears to be an important site for sea turtle nesting, but due to its remotenes and absence of surveillance, the site is frequented by illegal fisermen who collect turtle eggs [75]. In the Gulf of California, the hunting of sea turtles dates back for 12,000 years, with a peak in the mid-20th century, when this activity went from one of subsistence to one of marked economy. The regulation in the 1970s and the subsequent ban in 1990 reduced the exploitation of sea turtles [94], but it still occurs in the Baja California Peninsula [95]. researchers are unaware of the current situation of sea turtle hunting and/or egg collecting in the islands of Baja California, but certainly they are vulnerable sites for poachers.
The illegal collection of living specimens for the pet trade is one of the principal menaces for rattlesnakes [96]. Little, however, is known about this problem. Arnaud et al. [64] mentioned that illegal collecting of C. catalinensis occurred in the past, but also suggested a diminution of this activity, thanks to the environmental education of the local people. Mellink (1995, in Lovich, [81]) reported illegal collecting of Lampropeltis herrerae in Todos Santos islands by trapping (using living mice as bait), and spotted people searching for kingsnakes by turning rocks (Figure 21). He also mentioned another case of supposed scientists collecting the variety of Mexican Rosy Boas (Lichanura trivirgata) on Isla Cedros, who left the island when they were requested to show their scientific collecting permit [97]. Delibes et al. [98] found that populations of Aspidoscelis hyperythra showed a high ability to escape predators in the populations of seven islands in the south of Baja California. They considered that this conduct, uncharacteristic of isolated populations, might be the result of selection due to intense reptile harvesting.

Figure 21. Todos Santos Island Kingsnake (Lampropeltis herrerae). The beauty, uniqueness, and rarity (endemic to Todos Santos Archipielago) of this Kingsnake make it an appealing trophy for unethical herpetoculturists. Furthermore, the Todos Santos islands are not far from Ensenada’s (Baja California) port, so they are easily reachable to fishermen and boatmen. Furthermore, Todos Santos Sur is known wordwide for its tubular waves, which attract surfers from all over the world. So, the easy access, plus frequent and unregulated visitors, together with poor vigilance action, makes this Kingsnake highly vulnerable to extinction by poachers. Distributional Status = IE, EVS = H (17), IUCN = CR, NOM-059 = A. Matt Cage, 2018-Isla Todos Santos.
The damage of collecting is not limited to the individual level, but it can extend to the population and community level as well. Often collectors use their hands, crowbars, hydraulic jacks, and other tools to break rocks in an attempt to collect reptiles. This activity results in the permanent destruction of refuges or microhabitats that might have been used for decades by amphibians or reptiles. Thus, the continuous destruction of microhabitats has detrimental effects on populations of herpetofauna [99]. Certanly, this can be pernicious on small-sized islands, since the area and habitat available are limited.
8. Conservation Status
8.1. The SEMARNAT System
The SEMARNAT system was developed by the Secretaria del Medio Ambiente y Recursos Naturales and is legally established through the NOM-059-SEMARNAT-2010, which has the objective to “identify the species or populations of wild flora and fauna threatened in the Mexican Republic” [100]. This norm is the principal reference frame for Mexican biologists to assign a risk category and encompasses four levels: probably extinct in the wild (E), endangered (P), threatened (A), and under special protection (Pr).
Of the 218 native species of herpetofauna occurring in the Mexican insular systems, 119 (54.6%) are not listed in the NOM-059 in any risk category, 51 (23.4%) are under special protection (Pr), 40 (18.3%) are in the threatened level (A), and just 8 (3.7%) are in the endangered (P) status. Of these endangered species, seven are turtles, six of which are marine turtles, and the other is the river turtle Dermatemys mawii. Only one teiid lizard, A. rodecki, appears in this category. The low incidence of endangered species under the NOM-059 is striking, and the omission of particularly relevant species such the Clarion Nightsnake (Hypsiglena unaocularus) [101], believed to be extinct, or the Santa Catalina Kingsnake (Lampropeltis catalinensis), which is known only from the holotype [102], is of concern. Hence, researchers assert that the NOM-059 underestimates the actual level of risk faced by insular populations of herpetofauna. This underestimation becomes more worrisome if researchers consider the existence of subspecific taxa on islands that might be facing a higher risk of extinction than the continental lineages. Consequently, in the next revision to the NOM-059, special attention must be paid to addressing insular reptiles, since they are species with a high risk of extinction.
8.2. The IUCN System
The International Union for Conservation of Nature has been, since the 1950s, the principal institution for compiling lists of species at risk of extinction. The primary objective behind creating red lists is to heighten awareness and guide conservation efforts for various species. [103]. Initially, the red lists were heavily dependent of the opinion of experts, but since the mid-1990s the IUCN Red List has become based on a standard set of criteria in order to provide a useful listing and categorization for conservation, monitoring, and decision-making [104]. Therefore, the aims of the red list are: (1) to provide a global index of the state of degeneration of biodiversity; and (2) identify and document those species most in need of conservation attention if global extinction rates are to be reduced [103].
Although not exempt from criticism, the IUCN is the most-used system for assessing species risk; consequently, researchers use this list to provide a frame of comparison with the EVS and SEMARNAT systems. This IUCN Red List encompasses eight categories, including Extinct (EX), Extinct in the Wild (EW), Critically Endangered (CR), Endangered (EN), Vulnerable (VU), Near Threatened (NT), Least Concern (LC), and Data Deficient (DD).
Of the 218 native species of herpetofauna, only 21 (9.6%) have been allocated to the three “threat categories,” including six in the CR category, three in the EN category, and twelve in the VU category. The two “lower risk” categories include 141 species (64.7%), including 6 NT and 135 LC species, respectively. Finally, 56 species (25.7%) are placed in the DD and NE categories. No insular species is considered to be Exctinct (EX) or Extinct in the Wild (EW). Although, Lampropeltic catalinensis almost certanily is extinct.
8.3. The EVS System
The Environmental Vulnerability Score system is an algorithm developed by Wilson and McCranie [105] to assess the vulnerability of herpetofaunal species using measures such the extent of geographic range, extent of ecological distribution, and degree of specialization of reproductive mode in amphibians or, in the case of reptiles, the degree of human persecution. The utility and practicality of this system is discussed by Johnson et al. [106] and Wilson et al. [107][108]. The EVS values range from 3 to 20 and are grouped into three categories, i.e., Low (3–9), Medium (10–13), and High (14–20) vulnerability.
The highest score researchers obtained was 19 for four species: Crotalus catalinensis, C. estebanensis, C. lorenzoensis, and Trachemys venusta. The species of Crotalus are of particular concern. Besides these four species, another seven have an EVS of 18: Coleonyx gypsicolus, C. hemilopha, Crotalus angelensis, C. caliginis, C. polisi, C. thalassoporus, and C. tortuguensis. Again, species of Crotalus are of significant interest. Two other rattlesnakes have an EVS of 16 (C. cerastes and C. tigris); thus, 10 of the 16 species (62.5%) of the rattlesnakes occupying insular systems in Mexico are allocated to the high vulnerability category.
Regarding the amphibians, most of them have very low EVS values; in fact, of the eight lowest values researchers estimated (EVS = 3–4), seven were for amphibians. Only one toad, Incilius mazatlanensis, has a medium value (12) and only Eleutherodactylus pallidus has a high EVS (17). Additionally, the only two known salamanders occurring on Mexican islands, Aneides lugubris and Batrachoseps major, both have a high EVS value (14) (Figure 22).

Figure 22. Batrachoseps major, a plethodonthid salamander inhabiting the Californian region. Although this specimen is from San Diego County (U.S.A.), The presence of this species in the Coronado Archipelago suggests the possibility of it being a relic population that recalls the islands’ continental origin, but this asseveration must be proven by molecular studies. Distributional Status = NE, EVS = H (14), IUCN = LC, NOM-059 = NS. Photo from the Amphibian and Reptile Atlas of Peninsular California ([herpatlas.sdnhm.org], San Diego Natural History Museum), courtesy of Bradford Hollingsworth.
Reptiles as a group have EVS ranging from 4 to 19. The EVS for these creatures are arranged into the three categories of vulnerability as follows: low—46 species; medium—57 species; and high—84 species. Thus, it is evident that the number of species rise through the levels of vulnerability from low, through medium, to high.
In summary, of the 212 species, 56 (26.4%) have a low EVS, 62 (29.2%) have a medium EVS, and 94 (44.5%) have a high EVS. Thus, the same pattern is evident for the entire herpetofauna as for the reptiles alone, with the proportion of the low EVS more heavily represented for the total herpetofauna than for the reptile component, giving greater representation of low-EVS species among the amphibians. Clearly, this pattern of EVS representation reflects the relatively high level of endemism, especially reptilian endemism, in insular systems. This same pattern exists in Mexican states with high levels of endemism, such as in Oaxaca [23] and Puebla [24], with the inverse pattern occurring in regions with limited endemism, such as the Yucatan Peninsula [17].
When comparing the EVS and IUCN categorizations, the results indicate that only 15 belong to any of the three risk categories (Critically Endangered, Endangered, or Vulnerable) of the IUCN system. This figure is only 17.2% of the 87 species with high EVS values. Alternatively, the 135 species within the LC category of the IUCN constitute 2.4 times the number of the low vulnerability species (57 species, which are the 42.2% of the IUCN LC). As can be seen, the results of the application of the EVS and the IUCN systems do not correspond well with each other. This discrepancy is of concern if researchers consider that the IUCN Red List omits several endemic and/or conspicuous species, such as iguanas of the genera Ctenosaura, Sauromalus, and Dipsosaurus, notable endemics such Elgaria cedrosensis, and Hypsiglena unaocularis (Figure 23), abundant and common species like Oxybelis aeneus, and also has not evaluated species of recent description such as Crotalus polisi and C. thalassoporus (Figure 24).

Figure 23. Hypsiglena unaocularis believed extinct for decades, its rediscovery allowed for molecular analysis, which concluded that it is a valid species (as H. unaocularus; [109]). EVS = H (16), UICN = NE, NOM-059 = NS. Photo by Humberto Almanza.

Figure 24. Crotalus thalassoporus (Isla Piojo Speckled Rattlesnake) is a rattlesnake of recent description (2018) and only known from its type locality (Isla Piojo, Gulf of California). An appealing advantage of EVS is that it gives a quick valoration of the vulnerability degree for species of recent description like this snake which, despite it being highly vulnerable, it still has not been listed neither by IUCN, nor NOM-059. Distribution status = IE, EVS = H (18), IUCN = NE, NOM-059 = NS. Photo by Tania Pérez-Fiol.
The lack of correspondence between the EVS and IUCN systems of conservation categorization is explicable for two principal reasons: first, there are several species that are in the category of not evaluated (NE) or data deficient (DD), as determined by the IUCN. Of these 56 species, 4 are allocated to the DD category and 52 to the NE category. The four DD species include one anuran and three squamates (colubrid snakes), with EVS ranging from 16 to 17. Thus, all four are high vulnerability species and the three species with an EVS of 17 most likely should be allocated to the CR category and the single species with an EVS of 16 to the EN category. The 52 NE species are all squamates, except for two turtles. Their EVS vary from 5 (L) to 19 (H); therefore, some of these 52 species that have not been evaluated by the IUCN are allocated to each of the three risk categories in the EVS system; 15 species in the low level, 12 in the medium level, and 25 in the high vulnerability level. Remarkably, this means that 40.1% of the species unevaluated by the IUCN are of high risk according to the EVS system.
The second cause of the discrepancy is the possibility of an overuse of the LC category in the IUCN Red List. from the 212 native non-marine insular species, 135 (63.7%) correspond to the Least Concern level. Moreover, the EVS values for these 135 species ranges from L (3) to H (19), encompassing almost all the possible values for the EVS system (3–20). By looking at those values, as arranged in the three summary categories of EVS, it is interesting to see that they are distributed almost equally: 47 species in the High EVS level (38.5%), 46 in the Medium (34.1%), and 42 for Low EVS level (31.1%). It is tempting to think that this distribution, almost divided into thirds, suggests that either the IUCN or the EVS system could lead to random classifications of insular herpetofauna. An explanation could be that the IUCN might be biased by geographic scale issues, or the fact that the IUCN systems require enough knowledge of the species in order to weight a set of criteria [110][111], regarding the geographic scale, since the IUCN evaluates the risk of global extinction, this issue should not be a matter of discrepancy in endemic species, but might be in species with reduced distribution ranges [112]. With reference to the second point, insular herpetofaunal populations are comparatively less studied than their continental counterparts, mainly due to their limited accessibilityHowever, this is a primary speculation, but researchers think this is a topic worthy of exploration by a larger contrast of the IUCN against the EVS of insular populations in several countries.
Of the 212 species that can be provided with an EVS categorization, 94 species (47.2%) fall within the high vulnerability category. Of considerable significance is that of these 94 species, 77 (81.9%) are allocated to the LC, NE, and DD categories.
| Elgaria nana | Aspidoscelis cana |
| Crotaphytus dickersonae | Aspidoscelis carmenensis |
| Crotaphytus insularis | Aspidoscelis celeripes |
| Coleonyx gypsicolus | Aspidoscelis ceralbelsis |
| Ctenosaura conspicuosa | Aspidoscelis danheimae |
| Dipsosaurus catalinensis | Aspidoscelis espiritensis |
| Sauromalus klauberi | Aspidoscelis franciscensis |
| Petrosaurus slevini | Aspidoscelis pictus |
| Sceloporus angustus | Lampropeltis catalinensis |
| Sceloporus grandaevus | Masticophis barbourin |
| Sceloporus lineatulus | Masticophis slevini |
| Uta nolascensis | Pituophis insularis |
| Phyllodactylus angelensis | Rhinocheilus etheridgei |
| Phyllodactylus apricus | Hypsiglena catalinae |
| Phyllodactylus bugastrolepis | Hypsiglena unaocularis |
| Phyllodactylus cleofasensis | Crotalus angelensis |
| Phyllodactylus coronatus | Crotalus caliginis |
| Phyllodactylus isabelae | Crotalus estebanensis |
| Phyllodactylus lupitae | Crotalus lorenzoensis |
| Phyllodactylus partidus | Crotalus polisi |
| Phyllodactylus tuberculosus | Crotalus thalassoporus |
| Aspidoscelis bacata | Crotalus tortuguensis |

Figure 25. Cerralvo Island Whiptail (Aspidoscelis ceralbensis). With 22 species, Whiptal lizards contribute greatly to insular herpetofauna diversity (21 Aspidoscelis and 1 Holcosus). Eleven Aspidoscelis are insular endemics. Most of them are on the Sea of Cortes (13 species). Six are under IUCN’s Low-Concern category, despite having High EVS values. Photo: Amphibian and Reptile Atlas of Peninsular California (herpatlas.sdnhm.org), San Diego Natural History Museum), courtesy of Bradford Hollingsworth.
9. Relative Herpetofaunal Priority
The Relative Herpetofauna Priority (RHP) measure was developed with the intention to estimate the relative importance of the herpetofauna documented in a geographic area of interest for conservation, like a political entity or a physiographic region [25]. To assign conservation priority among the recognized geographical entities (in this case, the five physiographic insular regions), the RHP ranks them in two ways: one as the absolute number of regional and country endemics found in the region, and two, with higher rankings to regions with proportionally higher absolute numbers of high EVS category species.
Tropical Pacific region, with 29 endemic species of a total of 57 species (50.9%). The third region is the Pacific Baja California islands, with 13 endemics of a total of 40 species (32.5%). Thus, the three most conservation-significant regions are those on the Pacific side of the country. This area of the country is also the only one in which insular endemics (Like the Clarion Island Whipsnake, Figure 26) are found. The fourth and fifth areas of priority significance are located on the Atlantic side of Mexico. The endemic species found there are wholly country endemics and comprise three species (6.5%) of a total of 46 species in the Gulf of Mexico region and five species (7.5%) of a total of 53 species in the Mexican Caribbean region.

Figure 26. Mexican insular endemic herpetofauna species are found solely on Pacific Islands, such as the Clarion Island Whipsnake (Masticophis anthonyi). This particular snake species is exclusive to Clarion Island, which is part of the Revillagigedo Archipelago. It is one of several Mexican snakes that are uniquely confined to individual islands, making them highly vulnerable to extinction in the event of invasive mammal colonization on these islands.. EVS = H (17), IUCN = CR, NOM-059 = A. Photo by Humberto Almanza.
Based on the number of high-EVS vulnerability species per physiographic region, the islands of the Gulf of California support the largest number of such species by far, with 58, which is higher than that for the remaining four regions put together (41). Next highest is the Tropical Pacific islands group, with 16 species. The remaining three regions differ from one another by double species numbers, i.e., nine in the Pacific islands of Baja California and the islands of the Mexican Caribbean, and seven in the islands of the Gulf of Mexico.
Based on the results of the RHP analysis, it is obvious that the region of greatest conservation significance, by far, is that of the islands of the Gulf of California. This region is characterized both by the greatest number of country and insular endemic species and the most sizable number of high vulnerability species. The 52 endemics consist of all squamates, with 15 country endemics, and 39 insular endemics (like C. tortuguensis, Figure 27). The Gulf of California island physiographic region also harbors 58 high vulnerability species, all of which are squamates (41 lizards and 16 snakes), except for one turtle. These 58 species and their respective EVS values are as follows (country endemic = *, insular endemic = **):
| Crotaphytus dickersonae ** | (16) | Phyllodactylus unctus * | (15) |
| Crotaphytus insularis ** | (16) | Aspidoscelis bacata ** | (17) |
| Coleonyx gypsicolus ** | (18) | Aspidoscelis cana ** | (16) |
| Ctenosaura conspicuosa ** | (16) | Aspidoscelis carmenensis ** | (17) |
| Ctenosaura hemilopha * | (18) | Aspidoscelis catalinensis ** | (17) |
| Ctenosaura nolascensis ** | (17) | Aspidoscelis celeripes ** | (15) |
| Dipsosaurus catalinensis ** | (17) | Aspidoscelis ceralbelsis ** | (17) |
| Sauromalus hispidus * | (14) | Aspidoscelis danheimae ** | (16) |
| Sauromalus klauberi ** | (16) | Aspidoscelis espiritensis ** | (16) |
| Sauromalus slevini * | (16) | Aspidoscelis franciscensis ** | (17) |
| Sauromalus varius * | (16) | Aspidoscelis martyris ** | (17) |
| Petrosaurus slevini ** | (16) | Aspidoscelis pictus ** | (17) |
| Phrynosoma solare | (14) | Lampropeltis catalinensis ** | (17) |
| Sceloporus angustus ** | (16) | Masticophis barbourin | (17) |
| Sceloporus grandaevus ** | (16) | Masticophis slevini ** | (17) |
| Sceloporus hunsakeri * | (14) | Rhinocheilus etheridgei ** | (16) |
| Sceloporus lineatulus ** | (17) | Sonora avage * | (15) |
| Uta encantadae ** | (17) | Hypsiglena catalinae ** | (16) |
| Uta lowei ** | (17) | Micruroides euryxanthus | (15) |
| Uta nolascensis ** | (17) | Crotalus angelensis ** | (18) |
| Uta palmeri ** | (17) | Crotalus catalinensis | (19) |
| Uta squamata | (17) | Crotalus cerastes | (16) |
| Uta tumidarostra ** | (17) | Crotalus estebanensis ** | (19) |
| Phyllodactylus angelensis ** | (16) | Crotalus lorenzoensis ** | (19) |
| Phyllodactylus apricus ** | (17) | Crotalus polisi ** | (18) |
| Phyllodactylus bugastrolepis ** | (17) | Crotalus thalassoporus ** | (18) |
| Phyllodactylus coronatus ** | (16) | Crotalus tigris | (16) |
| Phyllodactylus homolepidurus * | (15) | Crotalus tortuguensis ** | (18) |
| Phyllodactylus partidus ** | (16) | Gopherus morafkai | (15) |

Figure 27. Tortuga Island Rattlesnake (Crotalus tortuguensis), endemic of Tortuga. Rattlesnakes constitute 16% of the insular herpetofaunal species with high EVS values. Status = IE, EVS = H (18), IUCN = NE, NOM-059 = Pr. Photo by Ruben A. Carbajal-Márquez.
Of these 58 species, eight (13.8%) are country endemics, forty-four (75.8%) are insular endemics, and six (10.3%) are non-endemics; their EVS values range from 14 to 19.
The next most significant islands are the Tropical Pacific islands. The 29 endemic species comprise two anurans, twenty-six squamates, and one turtle, including 21 country endemics (Like U. clarionensis Figure 27) and eight insular endemics. In addition, the Tropical Pacific islands physiographic region supports 16 high vulnerability species. These species, and their respective EVS values, are as follows (country endemic = *, insular endemic = **):
| Eleutherodactylus pallidus * | (17) | Phyllodactylus lanei * | (15) |
| Crocodylus acutus | (14) | Phyllodactylus lupitae ** | (16) |
| Ctenosaura pectinata | (15) | Phyllodactylus tuberculosus | (17) |
| Urosaurus auriculatus ** | (16) | Aspidoscelis communis * | (14) |
| Urosaurus clarionensis ** | (17) | Aspidoscelis lineatissima * | (14) |
| Phyllodactylus benedetii * | (15) | Leptophis diplotropis * | (14) |
| Phyllodactylus cleofasensis ** | (16) | Masticophis anthonyi ** | (17) |
| Phyllodactylus isabelae ** | (16) | Hypsiglena unaocularis ** | (16) |

Figure 28. Urosaurus clarionensis, Clarion Island Tree Lizad, endemic of Isla Clarion, is easy to spot perched on rocks. As occurs with many insular species, this lizard shows naïve behavior, and it is easier to catch compared with continental Urosaurus (VHGS, pers. observ.). Thus, the introduction of an invasive mammal could be catastrophic for this lizard. Distributional Status = IE, EVS = H (17), IUCN = VU, NOM-059 = NS.
Of these 16 species, six are country endemics, eight are insular endemics, and two are non-endemics; their EVS values range from 14 to 17.
The third most significant region is the Pacific islands of Baja California. Thirteen endemic species inhabit these islands, all of which are squamates, and include nine country endemics and four insular endemics (Like E. nana Figure 29). Additionally, the Pacific islands of Baja California harbors nine high vulnerability species; these nine species and their respective EVS values are as follows (country endemic = *, insular endemic = **):
| Aneides lugubris (14) | Phrynosoma cerroense * (16) |
| Batrachoseps major (14) | Lampropeltis herrerae ** (17) |
| Elgaria cedrosensis * (16) | Pituophis insulanus ** (16) |
| Elgaria nana ** (16) | Crotalus calignis ** (18) |
| Bipes biporus * (14) |

Figure 29. Los Coronados Alligator Lizard (Elgaria nana) from Coronado Norte (Archipielago Coronado). Distributional Status = IE, EVS = H (16), IUCN = LC, NOM-059 = Pr.
Of these nine species, three are country endemics, four are insular endemics, and two are non-endemics.
The fourth most significant region comprises the Mexican Caribbean islands; these islands harbor six endemic species, all of which are squamates and country endemics. The Mexican Caribbean islands also support eight high vulnerability species (nine with Boa imperator, but it is introduced in Cozumel and probably Chinchorro as well); these eight species and their respective EVS values are as follows (country endemic = *, insular endemic = **):
| Crocodylus acutus | (14) | Aspidoscelis rodecki * | (16) |
| Sceloporus cozumelae | (15) | Holcosus gaigeae * | (15) |
| Aspidoscelis cozumela * | (16) | Agkistrodon russeolus * | (15) |
| Aspidoscelis maslini | (15) | Trachemys venusta | (19) |
Of these eight species, five are country endemics and three are non-endemics. Their EVS values vary from 14 to 19.
Finally, the least significant physiographic area is the Gulf of Mexico islands. Only three endemic species occur on these islands. In addition, these islands support seven high vulnerability species; these five species and their respective EVS values are as follows (country endemic = *, insular endemic = **):
| Aspidoscelis cozumela * | (16) | Dipsas brevifacies | (15) |
| Aspidoscelis maslini | (15) | Dermatemys mawii | (17) |
| Holcosus gaigeae * | (15) | Staurotypus triporcatus | (14) |
| Boa imperator * | (15) |
Of these five species, two teiids, and one Boa imperator, all are country endemics. None are insular endemic and the other four are non-endemics. Their EVS values range from 14 to 17.
A perusal of the lists of high vulnerability species above demonstrates that insular endemic species of this status (56) are located solely on the Pacific side of Mexico, with the highest number by far found on the islands of the Gulf of California (44) and significantly fewer on the Tropical Pacific islands (8) and the Pacific islands of Baja California (4). These three regions also contain a preponderance of country endemic species with high EVS values, with eight, six, and three species, respectively. On the Atlantic side of Mexico, where insular endemics do not figure into the tally, non-endemic species of high EVS values predominate, with two in the Mexican Caribbean islands and four in the Gulf of Mexico, as compared to country endemics six and three, respectively. Clearly, the islands on the Pacific side of Mexico provide the most significant conservation challenges.
10. Protected Areas
researchers identified 28 protected areas that encompass both portions of the coastal mainland as well as insular elements. Two areas are of state jurisdiction. One of these is the Santuario del Manatí, which is a protected area in Quintana Roo, and comprises a coastal lagoon, where the island Tamalcab, along with other cays and islands of smaller sizes are found (such Cayo Venado, Cayo Violines, Dos Hermanos, Siete Mogotes, and several smaller unnamed islets). Independently of the jurisdiction of the protected area, Tamalcab Island is administrated by the Mexican federation [113]. The other of these state areas is the Reserva Estatal Selvas y Humedales de Cozumel. On this island several protected areas overlap, which is explained by González-Sánchez et al. [17].
There are four world heritages sites (WHSs) with insular elements, but three of them are not concordant with the boundaries of the Mexican-protected areas, notably the Islands and Protected Areas of the Gulf of California. This WHS ranges from the Rio Colorado delta in the upper Gulf of California, through all the Sea of Cortes and far south of the Los Cabos region to the islands of Nayarit. This site includes all the protected areas researchers list for the Gulf of California (also those in that region researchers do not list due to their lack of insular elements, such as Cabo Pulmo) and the Marietas and Isabel islands, which researchers list here as located in the tropical Pacific region. The second site is the Whale Sanctuary of El Vizcaíno, which is considerably smaller than the Biosphere Reserve; the WHS only includes the Ojo de Liebre and San Ignacio lagoons with their respective interior islands. Thus, other islands from El Vizcaíno, such as Asunción and San Roque, and the ones in the Gulf of California, are not included in this WHS. The third WHS is the Sian Ka’an region; it includes a few islets and cays inside the Espiritu Santo and Ascension Bays (in Quintana Roo). Although Sian Ka’an is a site well-studied by herpetologists, few records exist for the insular elements in those bays. The fourth WHS is the Revillagigedo Archipelago, concordant with the homonymous biosphere reserve; this is the most recently established WHS site (2016). Banco Chinchorro is on the tentative list of proposals as a WHS site but has yet to be declared.
Of the federal administrated protected areas, nine are placed in the Biosphere Reserve category. Of these, Isla Guadalupe can be considered as the flagship ecological restoration program in Mexico. Amphibians and reptiles, however, are absent from Guadalupe and its associated islets. The islands of the Pacific region of Baja California constitute the most recently created biosphere reserve in Mexico (December 2016). The Gran Caribe Mexicano Biosphere Reserve is the largest protected area in the Mexican Caribbean, but it preserves mostly marine surface, and the polygon surrounds the extant protected areas in the region (such as Cozumel, Chinchorro, and Sian Ka’an). researchers do not include these as part of the Gran Caribe Mexicano, since those protected areas are still administratively independent.
Eight national parks exist that encompass insular elements. Especially important is the Revillagigedo Archipielago, also a WHS, because of its geological characteristics, its level of endemism, and its remoteness, which make this archipelago crucially important in extending the Mexican Exclusive Economic Zone. Decreed originally as a Biosphere Reserve in 1994, it was recategorized as a National Park in 2017. Importantly, however, this redesignation does not imply a downgrade in its level of protection, rather a change in what regulations apply and what land uses are allowed in the interior of the protected area. There are fewer activities, in fact, allowed inside a national park than in a biosphere reserve, which has more flexible land use regulations.
Another six sites comprise the categories of Area de Proteccion de Flora y Fauna and Sanctuary. Of importance is the APFF Islas del Golfo de California. This sole reserve includes an estimated ~900 islands and islets, with their surface area equaling 50% of the national insular territory. This area is so extensive and complex that it was necessary to create interior reserves that operate as components of the larger one; an example is the Componente del Complejo Insular Espíritu Santo (which researchers list). In the Parque Nacional Huatulco there are some islands in the bays, such as Cacaluta, San Agustín, and Blanca with a surface area of approximately 16, 12, and 2 has, respectively, and another eleven islands with an area of less than 1 ha. Despite the fact that the Huatulco Bays are a well-known place for tourism, researchers did not find any herpetofaunal records for any of these islands. Only one reserve has the Sanctuary categorization, i.e., “Islas La Pajarera, Cocinas, Mamut, Colorada, San Pedro, San Agustin, San Andrés y Negrita y los Islotes Los Anegados, Novillas, Mosca y Submarino, situated in la Bahía de Chamela” in the Chamela region of Nayarit.
The effort expended in protecting the Mexican insular systems is astonishing, since almost all of the Mexican islands have some degree of protection. The only exception researchers could identify is one in the Pacific coast of Baja California, i.e., Santa Margarita Island, which was not included in the Islas del Pacífico de Baja California decree [114]. This island, however, is relatively safe since there is a Mexican Navy base located there, which imposes restrictions on accessibility and development on that island. The other examples are on the coast of Guerrero, i.e., the islands of Coral (peña), Ixtapa, and La Roqueta. Because of these efforts, of the 220 native Mexican insular amphibian and reptile species, only 3 are not protected within a Natural Protected Area. Aspidoscelis guttata, and Epictia bakewelli, from Isla Ixtapa, are both species whose insular population is not found within a Protected Area. Aspidoscelis guttata is a country endemic, but not exclusive to islands, since it is a common whiptail in southwestern Mexico, especially on the coastal regions of Guerrero, Oaxaca, and Chiapas.Same case for Phyllodatylus lanei, who inhabites islands on the coast of the southern Pacific, but it lives coastal areas of Pacific versant. Thus, researchers conclude that P. lupitae is the only insular endemic reptile not protected by any Natural Protected Area.
Unfortunately, researchers are not optimistic that the goal of protecting 100% of the offshore insular elements of the country will be reached in the short- or the mid-term, since Isla Ixtapa and La Roqueta are important touristic attractions at the Zihuatanejo and Acapulco ports, and any limitation on the development of tourism will face strong resistance. Also, the outgoing federal administration failed in implementing strong environmental policies, expressing only verbal support for the conservation of natural resources, but in reality enacted severe budget cuts to environmental and scientific institutions since its beginning [115][116][117]. In fact, before this administration started, it was very likely that two new federal reserves would be created in the Yucatan Peninsula, the APFF of Bacalar and the APFF Sayab Ha’ [17], but, since then, that process has entered limbo. It was not until the second half of this presidential period that three federal protected areas were decreed [118][119][120], none of those were located in marine or coastal regions. Whether the current environmental policies approach will prevail in the following administration (which will begin in late 2024), is unclear and remains to be seen.
The region with the greatest number of protected areas is the Mexican Caribbean, with eight, six of federal administration and two at the state level. The Gulf of California has six, with the Componente del Complejo Insular Espíritu Santo as an integral part of the APFF Islas del Golfo de California, with its own management program. The Tropical islands of the Gulf have six protected areas, whereas the Gulf of Mexico has five and the islands of the Pacific of Baja California have only three, including Isla Guadalupe which, as mentioned before, has no herpetofaunal species. The Vizcaíno Biosphere Reserve is one of the largest of Mexico’s protected areas, but its insular surface is limited to a few rocky islands of small size.
The oldest protected area is the APFF Islas del Golfo de California, which was decreed in December 1978, followed by the Islas Marías and Isla Isabel national parks, both decreed in 1980. Most protected areas for insular systems were created between the mid-1900s and 2011. The Islas del Pacífico de la Península de Baja California and the Gran Caribe Mexicano Biosphere Reserves were the last decreed in December 2016.
Herpetofaunal surveys are not complete in at least 14 of these protected areas. Significantly, the herpetofaunal listings of records are scarce or even lacking for some sites that, at a first glance, one might think are well known, such as the islands in the Bahias de Huatulco, Isla de Sacrificios (Sistema Arrecifal Veracruzano) of the islands on the Delta del Rio Colorado. Sian Ka’an is a region extensively surveyed by biologists, but this is not the case for its insular elements, whose herpetofauna is practically unknown. Only the Cuban anole (Norops sagrei) is known from its cays. The herpetofauna of the islands of the Gulf of California have been surveyed several times in the past and the knowledge of their herpetofauna is outstanding, but due to the complexity and number of insular elements in that region, there are still many islands that have not been surveyed as of yet. In addition, limited information exists about the herpetofauna of the islands of the Tamaulipecan coast.
In general, due to federal jurisdiction, the remoteness and inaccessibility of the islands, most of them are unpopulated, as mentioned earlier, so few important settlements exist. This is why 13 of the protected areas with insular systems have no landowners within them. In nine cases, however, such is the case; examples are Cozumel, Isla Mujeres, Cedros, and Isla del Carmen. In most cases, the settlements consist only of settlements of fishermen, and almost always are occupied only seasonally, as occurs in the San Benito Archipelago.
Of the 28 protected areas, 26 have management plans. The APFF Islas del Golfo de California has two plans, including the specific management plan for the Espiritu Santo Component (thus, 27 management plans are available). Just two protected areas lack such plans, i.e., the APFF Yum Balam and the Islas del Pacífico de la Península de Baja California Biosphere Reserve. The latter, as mentioned previously, is of recent creation. Yum Balam is a reserve which has been in existence since 1994, and the lack of a management plan is of concern since the main island within that reserve (Holbox) is subject to pressure from tourist development.
Finally, the reserve or protected area that hosts the greatest number of species of reptiles and amphibians is the APFF Islas del Golfo de California, with 98. This single reserve protects 43.4% of the known insular species of Mexico; 43 of these 98 are insular endemics. This number represents 76.8% of the 56 insular endemics existing in the entire country. The next most important reserve in species richness is the APFF Laguna de Términos with 42 species, but it hosts only one country with endemic species, no insular endemics, 38 non-endemic species, and 3 exotic species. The Complex of Cozumel, the Islas del Pacífico de la Península de Baja California, The Bahía de los Ángeles, Islas Marías Biosphere Reserves, and the Archipielago de Revillagigedo National Park are also important, given the number of their country and/or insular endemics. The Sian Ka’an and El Vizcaíno Biosphere Reserves have low herpetofaunal species richness in their cays or islands, respectively.
References
- Whittaker, R.J.; Fernández-Palacios, J.M. Island Biogeography: Ecology, Evolution, and Conservation; Oxford University Press: Oxford, UK, 2007.
- May, R.M. Foreword. In The Theory of Island Biogeography Revisited; Losos, J.B., Ricklefs, R.E., Eds.; Princeton University Press: Princeton, NJ, USA, 2010; pp. vii–x.
- Itescu, Y. Are island-like systems biologically similar to islands? A review of the evidence. Ecography 2018, 42, 1298–1314.
- Donlan, C.J.; Tershy, B.R.; Keitt, B.S.; Wood, B.; Sanchez, J.; Weinstein, A.; Croll, D.A.; Alguilar, J. Island conservation action in northwest Mexico. In Proceedings of the Fifth California Islands Symposium, Santa Barbara, CA, USA, 29 March–1 April 1999; pp. 330–338.
- Paulay, G. Biodiversity on oceanic islands: Its origin and extinction1. Am. Zool. 1994, 34, 134–144.
- Enderson, E.F.; Quijada-Mascareñas, A.; Turner, D.S.; Rosen, P.C.; Bezy, R.L. The herpetofauna of Sonora, Mexico, with comparisons to adjoining states. Check List 2009, 5, 632–672.
- Tershy, B.R.; Shen, K.-W.; Newton, K.M.; Holmes, N.D.; Croll, D.A. The Importance of Islands for the Protection of Biological and Linguistic Diversity. BioScience 2015, 65, 592–597.
- Keitt, B.; Campbell, K.; Saunders, A.; Clout, M.; Wang, Y.; Heinz, R.; Newton, K.; Tershy, B. The global islands invasive vertebrate eradication database: A tool to improve and facilitate restoration of island ecosystems. In Island Invasives: Eradication and Management; IUCN: Gland, Switzerland, 2011; pp. 74–77.
- Lara-Lara, J.; Arreola-Lizárraga, J.; Calderón-Aguilera, L.; Camacho-Ibar, V.; De la Lanza-Espino, G.; Escofet-Giansone, A.; Espejel-Carbajal, M.; Guzmán-Arroyo, M.; Ladah, L.; López-Hernández, M. Los ecosistemas costeros, insulares y epicontinentales. Cap. Nat. De México 2008, 1, 109–134.
- Salgado-Sánchez, K.; Dávila-Acosta, L.; Pérez-Rocha, A.M. Mexico y Sus Estados; Ediciones Culturales Internacionales S., A.: Ciudad de México, Mexico, 2011.
- Ochoa-Ochoa, L.M.; Flores-Villela, O. Áreas de Diversidad y Endemismo de la Herpetofauna Mexicana; CONABIO, Universidad Nacional Autónoma de México: Ciudad de México, Mexico, 2006; p. 211.
- Méndez Buenos Aires, M. Las islas mexicanas: Importancia económica, régimen jurídico y proyecciones internacionales. Rev. Mex. De Política Exter. 1990, 28, 33–39.
- González-Avelar, M. El territorio insular como frontera. Front. Norte 2017, 9, 161–169.
- INEGI. Catalogo del Territorio Insular Mexicano; Instituto Nacional de Estadística y Geografía: Aguascalientes, México, 2015; p. 252.
- Ramírez-Bautista, A.; Torres-Hernández, L.A.; Cruz-Elizalde, R.; Berriozabal-Islas, C.; Hernández-Salinas, U.; Wilson, L.D.; Johnson, J.D.; Porras, L.W.; Balderas-Valdivia, C.J.; González-Hernández, A.J. An updated list of the Mexican herpetofauna: With a summary of historical and contemporary studies. ZooKeys 2023, 1166, 287.
- Cruz-Sáenz, D.; Muñoz-Nolasco, F.J.; Mata-Silva, V.; Johnson, J.D.; García-Padilla, E.; Wilson, L.D. The herpetofauna of Jalisco, Mexico: Composition, distribution, and conservation status. Mesoamerican Herpetol. 2017, 4, 22–118.
- González-Sánchez, V.; Johnson, J.; García-Padilla, E.; Mata-Silva, V.; DeSantis, D.; Wilson, L. The herpetofauna of the Mexican Yucatan Peninsula: Composition, distribution, and conservation. Mesoamerican Herpetol. 2017, 4, 264–380.
- Díaz-Gamboa, L.; May-Herrera, D.; Gallardo-Torres, A.; Cedeño-Vázquez, R.; González-Sánchez, V.H.; Chiappa-Carrara, X.; Yáñez-Arenas, C. Catálogo de Reptiles de la Península de Yucatán; Escuela Nacional de Estudios Superiores (ENES) Campus Mérida: Merida, Yucatan, 2020; p. 315.
- Martínez, J.I.B.; Pineda, C.S. Cedros: Origin and permanence of the placenames in a Mexican Pacific island. Isl. Stud. J. 2016, 11, 399–416.
- Duellman, W.E. Herpetofaunas in Neotropical rainforests: Comparative composition, history, and resource use. In Four Neotropical Rainforests; Yale University Press: New Haven, CT, USA, 1990; pp. 455–505.
- Alvarado-Díaz, J.; Suazo-Ortuño, I.; Wilson, L.; Medina-Aguilar, O. Patterns of physiographic distribution and conservation status of the herpetofauna of Michoacán, Mexico. Amphib. Reptile Conserv. 2013, 7, 128–170.
- Woolrich-Piña, G.; Ponce-Campos, P.; Loc-Barragán, J.; Ramírez-Silva, J.; Mata-Silva, V.; Johnson, J.; García-Padilla, E.; Wilson, L. The herpetofauna of Nayarit, Mexico: Composition, distribution, and conservation. Mesoamerican Herpetol. 2016, 3, 376–448.
- Mata-Silva, V.; Johnson, J.; Wilson, L.; García-Padilla, E. The herpetofauna of Oaxaca, Mexico: Composition, physiographic distribution, and conservation status. Mesoamerican Herpetol. 2015, 2, 6–62.
- Woolrich-Peña, G.A.; García-Padilla, E.; DeSantis, D.L.; Johnson, D.J.; Mata-Silva, V.; Wilson, L.D. The herpetofauna of Puebla, Mexico: Composition, distribution, and Conservation status. Mesoamerican Herpetology. Mesoamerican Herpetol. 2017, 4, 2–95.
- Johnson, J.D.; Mata-Silva, V.; Padilla, E.G.; Wilson, L.D. The Herpetofauna of Chiapas, Mexico: Composition, distribution, and conservation. Mesoamerican Herpetol. 2015, 2, 272–329.
- Lazcano, D.; Nevarez-de los Reyes, M.; Garcia-Padilla, E.; Johnson, J.D.; Mata-Silva, V.; DeSantis, D.L.; Wilson, L. The herpetofauna of Coahuila, Mexico: Composition, distribution, and conservation status. Amphib. Reptile Conserv. 2019, 13, 31–94.
- Nevárez-de-los-Reyes, M.; Lazcano, D.; García-Padilla, E.; Mata-Silva, V.; Johnson, J.D.; Wilson, L.D. The herpetofauna of Nuevo León, Mexico: Composition, distribution, and conservation. Mesoamerican Herpetol. 2016, 3, 558–638.
- Terán-Juárez, S.; García-Padilla, E.; Mata-Silva, V.; Johnson, J.; Wilson, L. The herpetofauna of Tamaulipas, Mexico: Composition, distribution, and conservation. Mesoamerican Herpetol. 2016, 3, 43–113.
- Nava-Landeros, R.A. Changes in Morphology and Behavior of the Coronado Island Rattlesnake (Crotalus Oreganus Caliginis), An Insular Population of the Western Rattlesnake (C. oreganus); San Diego State University: San Diego, CA, USA, 2022.
- Bowen, T. Archaeology, biology and conservation on islands in the Gulf of California. Environ. Conserv. 2004, 31, 199–206.
- Luque, D.; Doode, S. Sacralidad, territorialidad y biodiversidad comcáac (seri). Los sitios sagrados indígenas como categorías de conservación ambiental. Relaciones. Estud. De Hist. Y Soc. 2007, 28, 157–184.
- Landeros, A.T. Baja California: Uso y Abuso de su Biodeiversidad; Editorial Miguel Ángel Porrúa: Ciudad de México, México, 2006; p. 306.
- Santos del Prado-Gasca, K.; Negrete-Fernández, G.; Gabriel-Morales, J. Diagnóstico ambiental participativo en la Colonia Penal Federal Islas Marías. Hacia la conservación y el manejo sustentable de su territorio. Gac. Ecológica 2006, 81, 5–18.
- Escudero, M.; Silva, R.; Mendoza, E. Beach Erosion Driven by Natural and Human Activity at Isla del Carmen Barrier Island, Mexico. J. Coast. Res. 2014, 71, 62–74.
- Hernández, M.G.D.A.; Gutiérrez, M.J.G. El reto de la política fiscal ante una industria petrolera inestable. El caso de Ciudad del Carmen, México (The challenge of fiscal policy in the face of an oil industry unstable. The case of Ciudad del Carmen, Mexico). EN-CONTEXTO 2017, 6, 233–258.
- Day, J.W.; Conner, W.H.; Ley-Lou, F.; Day, R.H.; Navarro, A.M. The productivity and composition of mangrove forests, Laguna de Términos, Mexico. Aquat. Bot. 1987, 27, 267–284.
- INE. Programa de Manejo del Área de Protección de Flora y Fauna” Laguna de Términos”; Instituto Nacional de Ecología: Ciudad de México, Mexico, 1997; p. 167.
- Rodríguez, L.G.; Gracia, M.A.; Baltazar, E.B.; Maya, E.M.A. Social metabolism and ecotourism: The problem of waste in Holbox island, Quintana Roo, Mexico. Nova Sci. 2018, 10, 779–822.
- Rubio-Cisneros, N.T.; Herrera-Silveira, J.; Morales-Ojeda, S.; Moreno-Báez, M.; Montero, J.; Pech-Cárdenas, M. Water quality of inlets’ water bodies in a growing touristic barrier reef Island “Isla Holbox” at the Yucatan Peninsula. Reg. Stud. Mar. Sci. 2018, 22, 112–124.
- Álvarez-Romero, J.G.; Medellín, R.A.; Oliveras de Ita, A.; Gómez de Silva, H.; Sánchez, O. Animales Exóticos en México: Una Amenaza Para la Biodiversidad; Comisión Nacional para el Conocimiento y Uso de la Biodiversidad, Instituto de Ecología, UNAM, Secretaría del Medio Ambiente y Recursos Naturales: Ciudad de México, Mexico, 2008; p. 502.
- Reyes-Gómez, H.G.; Vázquez-Lule, A.D. Caracterización del sitio de manglar Isla del Carmen. In Sitios de Manglar con Relevancia Biológica y con Necesidades de Rehabilitación Ecológica; Comisión Nacional para el Conocimiento y Uso de la Biodiversidad (CONABIO): Ciudad de México, Mexico, 2009.
- Frutos-Cortés, M. La violencia social en el sureste mexicano. Un acercamiento al estudio de la descomposición social en una ciudad petrolera: Ciudad del Carmen, Campeche. Rev. De La Univ. Cris. Colón 2011, 26, 58–92.
- Labarre, D.; Charruau, P.; Parsons, W.; Larocque-Desroches, S.; Gallardo-Cruz, J. Major hurricanes affect body condition of American crocodile Crocodylus acutus inhabiting Mexican Caribbean islands. Mar. Ecol. Prog. Ser. 2020, 651, 145–162.
- Charruau, P. Microclimate of American crocodile nests in Banco Chinchorro biosphere reserve, Mexico: Effect on incubation length, embryos survival and hatchlings sex. J. Therm. Biol. 2012, 37, 6–14.
- Charruau, P.; Thorbjarnarson, J.B.; Hénaut, Y. Tropical cyclones and reproductive ecology of Crocodylus acutus Cuvier, 1807 (Reptilia: Crocodilia: Crocodylidae) on a Caribbean atoll in Mexico. J. Nat. Hist. 2010, 44, 741–761.
- Rogers, R.F.; Aberson, S.; Bell, M.M.; Cecil, D.J.; Doyle, J.D.; Kimberlain, T.B.; Morgerman, J.; Shay, L.K.; Velden, C. Rewriting the Tropical Record Books: The Extraordinary Intensification of Hurricane Patricia (2015). Bull. Am. Meteorol. Soc. 2017, 98, 2091–2112.
- Maass, M.; Ahedo-Hernández, R.; Araiza, S.; Verduzco, A.; Martínez-Yrízar, A.; Jaramillo, V.J.; Parker, G.; Pascual, F.; García-Méndez, G.; Sarukhán, J. Long-term (33 years) rainfall and runoff dynamics in a tropical dry forest ecosystem in western Mexico: Management implications under extreme hydrometeorological events. For. Ecol. Manag. 2017, 426, 7–17.
- Parker, G.; Martínez-Yrízar, A.; Álvarez-Yépiz, J.C.; Maass, M.; Araiza, S. Effects of hurricane disturbance on a tropical dry forest canopy in western Mexico. For. Ecol. Manag. 2017, 426, 39–52.
- Olivares Bañuelos, N.; Ángel Vargas-Tellez, M.A. Atención de un Incendio Forestal en la Reserva de la Biósfera Isla Guadalupe. In Informe Final. SNIB-CONABIO Proyecto No. GR012; PRONATURA NOROESTE, A. C.: México D. F, Mexico, 2009.
- Coccossis, H.; Parpairis, A. Tourism and the Environment—Some Observations on the Concept of Carrying Capacity. In Tourism and the Environment: Regional, Economic and Policy Issues; Briassoulis, H., van der Straaten, J., Eds.; Springer: Dordrecht, The Netherlands, 1992; pp. 23–33.
- Charruau, P.; Macías-Díaz, D.A. A recent change in crocodile’s behavior in a Mexican Caribbean atoll: Possible causes and short-term actions. In Proceedings of the Program and Abstracts of the Twenty-Sixth Working Meeting of the Crocodile Specialist Group of the International Union for Conservation of Nature, Chetumal, Qroo, Mexico, 3–9 July 2022; p. 19.
- González-Sánchez, V.H.; Johnson, J.D.; González-Solís, D.; Fucsko, L.A.; Wilson, L.D. A review of the introduced herpetofauna of Mexico and Central America, with comments on the effects of invasive species and biosecurity methodology. ZooKeys 2021, 1022, 79–154.
- Hofman, C.A.; Rick, T.C. Ancient Biological Invasions and Island Ecosystems: Tracking Translocations of Wild Plants and Animals. J. Archaeol. Res. 2018, 26, 65–115.
- Richardson, D.M.; Pyšek, P.; Carlton, J.T. A compendium of essential concepts and terminology in invasion ecology. In Fifty Years of Invasion Ecology. The Legacy of Charles Elton; Richardson, D.M., Ed.; Blackwell Publishing Ltd.: Hoboken, NJ, USA, 2011; pp. 409–420.
- Brook, B.W.; Sodhi, N.S.; Bradshaw, C.J.A. Synergies among extinction drivers under global change. Trends Ecol. Evol. 2008, 23, 453–460.
- Blumstein, D.T.; Daniel, J.C. The loss of anti-predator behaviour following isolation on islands. Proc. R. Soc. B Biol. Sci. 2005, 272, 1663–1668.
- Aguirre-Muñoz, A.; Croll, D.A.; Donlan, C.J.; Henry, R.W.; Hermosillo, M.A.; Howald, G.R.; Keitt, B.S.; Luna-Mendoza, L.; Rodríguez-Malagón, M.; Salas-Flores, L.M.; et al. High-impact Conservation: Invasive Mammal Eradications from the Islands of Western México. AMBIO A J. Hum. Environ. 2008, 37, 101–107.
- Medina, F.M.; Bonnaud, E.; Vidal, E.; Tershy, B.R.; Zavaleta, E.S.; Josh Donlan, C.; Keitt, B.S.; Le Corre, M.; Horwath, S.V.; Nogales, M. A global review of the impacts of invasive cats on island endangered vertebrates. Glob. Change Biol. 2011, 17, 3503–3510.
- Smith, M.J.; Cogger, H.; Tiernan, B.; Maple, D.; Boland, C.; Napier, F.; Detto, T.; Smith, P. An oceanic island reptile community under threat: The decline of reptiles on Christmas Island, Indian Ocean. Herpetol. Conserv. Biol. 2012, 7, 206–218.
- Gibbon, J.W.; Scott, D.E.; Ryan, T.J.; Buhlmann, K.A.; Tuberville, T.D.; Metts, B.S.; Greene, J.L.; Mills, T.; Leiden, Y.; Poppy, S.; et al. The Global Decline of Reptiles, Déjà Vu Amphibians. BioScience 2000, 50, 653–666.
- Drake, D.R.; Hunt, T.L. Invasive rodents on islands: Integrating historical and contemporary ecology. Biol. Invasions 2009, 11, 1483–1487.
- Arnaud, G.; Rodríguez, A.; Ortega-Rubio, A.; Alvarez-Cárdenas, S. Predation by Cats on the Unique Endemic Lizard of Socorro Island (Urosaurus auriculatus), Revillagigedo, Mexico. Ohio J. Sci. 1993, 93, 101–104.
- Osorio-Beristain, M.; Torres, R. Depredación de reptiles por gatos domésticos introducidos en Isla Isabel, Nayarit. Boletín De La Soc. Herpetológica Mex. 1992, 4, 10–12.
- Arnaud, G.; Martins, M.R.C.; Burguete-Trujillo, L.; Hernández-Rodríguez, I.; Avila-Villegas, H.; Murillo-Quero, R.; Quijada-Mascareñas, A. Historia natural de la serpiente de cascabel Crotalus catalinensis, endémica de las isla Santa Catalina, Golfo de California, México. In Estudios de las Islas del Golfo de California; Universidad Autónoma de Sinaloa: Cluliacán, México, 2008.
- Donlan, C.J.; Croll, D.A.; Tershy, B.R. Islands, Exotic Herbivores, and Invasive Plants: Their Roles in Coastal California Restoration. Restor. Ecol. 2003, 11, 524–530.
- Oberbauer, T.A. La vegetación de isla Guadalupe. Entonces y ahora. Gac. Ecológica 2006, 81, 46–58.
- Samaniego-Herrera, A.; Aguirre-Muñoz, A.; Bedolla-Guzmán, Y.; Cárdenas-Tapia, A.; Félix-Lizárraga, M.; Méndez-Sánchez, F.; Reina-Ponce, O.; Rojas-Mayoral, E.; Torres-García, F. Eradicating invasive rodents from wet and dry tropical islands in Mexico. Oryx 2017, 1–12.
- Harper, G.; Zabala, J.; Carrion, V. Monitoring of a Population of Galápagos Land Iguanas (Conolophus Subcristatus) during a Rat Eradication Using Brodifacoum; Veitch, C.R., Clout, M.N., Towns, D.R., Eds.; Island Invasives: Eradication and Management; IUCN: Gland, Switz, 2011; pp. 309–312.
- Latofski-Robles, M. Restoration Priorities for Mexican Islands. Master Thesis, Universidad Autónoma de San Luis Potosí, San Luis Potosí, Mexico, July 2012.
- Ortiz-Alcaraz, A.; Arnaud, G.; Galina-Tessaro, P.; Rojas-Mayoral, E.; Méndez-Sánchez, F.; Ortega-Rubio, A. Progress in the eradication of the feral cat (Felis catus) and recovery of the native fauna on Socorro Island, Revillagigedo Archipelago, Mexico. Therya 2017, 8, 3–9.
- Thomas, C.D.; Cameron, A.; Green, R.E.; Bakkenes, M.; Beaumont, L.J.; Collingham, Y.C.; Erasmus, B.F.N.; de Siqueira, M.F.; Grainger, A.; Hannah, L.; et al. Extinction risk from climate change. Nature 2004, 427, 145–148.
- Kiesecker, J.M.; Blaustein, A.R.; Belden, L.K. Complex causes of amphibian population declines. Nature 2001, 410, 681–684.
- Walther, G.-R.; Post, E.; Convey, P.; Menzel, A.; Parmesan, C.; Beebee, T.J.C.; Fromentin, J.-M.; Hoegh-Guldberg, O.; Bairlein, F. Ecological responses to recent climate change. Nature 2002, 416, 389–395.
- Sinervo, B.; Méndez-de-la-Cruz, F.; Miles, D.B.; Heulin, B.; Bastiaans, E.; Villagrán-Santa Cruz, M.; Lara-Resendiz, R.; Martínez-Méndez, N.; Calderón-Espinosa, M.L.; Meza-Lázaro, R.N.; et al. Erosion of Lizard Diversity by Climate Change and Altered Thermal Niches. Science 2010, 328, 894–899.
- Charruau, P.; Díaz de la Vega Pérez, A.H.; Méndez de la Cruz, F.R. Reptiles of Banco Chinchorro: Updated List, Life History Data, and Conservation. Southwest. Nat. 2015, 60, 299–312.
- Tershy, B.R.; Breese, D.; Alvarez-Borrego, S. Increase in cetacean and seabird numbers in the Canal de Ballenas during an El Niño-Southern Oscillation event. Mar. Ecol. Prog. Ser. 1991, 69, 299–302.
- Pérez, O.; Escobedo-Galván, A.H. Potential effects of El Niño-South Oscillation (ENSO) on growth of the American crocodile, Crocodylus acutus (Crocodylia: Crocodilydae) in captivity. J. Therm. Biol. 2009, 34, 14–16.
- Limpus, C.; Nicholls, N. ENSO Regulation of Indo-Pacific Green Turtle Populations. In Applications of Seasonal Climate Forecasting in Agricultural and Natural Ecosystems; Hammer, G.L., Nicholls, N., Mitchell, C., Eds.; Springer: Dordrecht, The Netherlands, 2000; pp. 399–408.
- Saba, V.S.; Santidrián-Tomillo, P.; Reina, R.D.; Spotila, J.R.; Musick, J.A.; Evans, D.A.; Paladino, F.V. The effect of the El Niño Southern Oscillation on the reproductive frequency of eastern Pacific leatherback turtles. J. Appl. Ecol. 2007, 44, 395–404.
- Cerdá-Ardura, A.; Langarica-Andonegui, E. On the presence of a spiny chuckwalla Sauromalus hispidus in Rasa island, Mexico. Rev. Latinoam. De Herpetol. 2018, 1, 17–28.
- Lovich, R.E.; Grismer, L.L.; Danemann, G. Conservation status of the herpetofauna of Baja California, México and associated islands in the Sea of Cortez and Pacific Ocean. Herpetol. Conserv. Biol. 2009, 4, 358–378.
- Oey, L.; Ezer, T.; Lee, H. Loop Current, Rings and Related Circulation in the Gulf of Mexico: A Review of Numerical Models and Future Challenges. In Circulation in the Gulf of Mexico: Observations and Models; Sturges, W., Lugo, A., Eds.; American Geophysical Union: Whasington, DC, USA, 2013; pp. 31–56.
- Sun, S.; Hu, C.; Tunnell, J.W. Surface oil footprint and trajectory of the Ixtoc-I oil spill determined from Landsat/MSS and CZCS observations. Mar. Pollut. Bull. 2015, 101, 632–641.
- Dedina, S. Wild Sea: Eco-Wars and Surf Stories from the Coast of the Californias; University of Arizona Press: Tucson, AZ, USA, 2011.
- Soto-Galera, E.; Piera, J.; López, P. Spatial and temporal land cover changes in Terminos Lagoon Reserve, Mexico. Rev. De Biol. Trop. 2010, 58, 565–575.
- Bessesen, B. Vaquita: Science, Politics, and Crime in the Sea of Cortez; Island Press: Washington, DC, USA, 2018.
- Arroyo-Quiroz, I.; Wyatt, T. Green Crime in Mexico: A Collection of Case Studies; Springer: Berlin/Heidelberg, Germany, 2018.
- Ceballos, G.; García, A. Challenges and Opportunities for Conservation of Mexican Biodiversity. In Conservation Biology: Voices from the Tropics; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2013; pp. 105–112.
- Bunge, V.; Reyes, J.A. Características sociales de las Áreas Naturales Protegidas federales y su relación con la conservación ambiental. In Crisis Civilizatoria En El México Rural; Ayala-Ortiz, D.A., Camacho-Velazquez, D., Castañeda-Zavala, Y., López-León, A., Eds.; Asociación Mexicana de Estudios Rurales: Ciudad de México, Mexico, 2015; pp. 23–40.
- Alvarado Martínez, I.; Martínez, E.R. Trafficking of Totoaba Maw. In Green Crime in Mexico: A Collection of Case Studies; Arroyo-Quiroz, I., Wyatt, T., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 149–170.
- Auliya, M.; Altherr, S.; Ariano-Sanchez, D.; Baard, E.H.; Brown, C.; Brown, R.M.; Cantu, J.-C.; Gentile, G.; Gildenhuys, P.; Henningheim, E.; et al. Trade in live reptiles, its impact on wild populations, and the role of the European market. Biol. Conserv. 2016, 204, 103–119.
- Angulo, E.; Courchamp, F. Rare Species Are Valued Big Time. PLoS ONE 2009, 4, e5215.
- Stuart, B.L.; Rhodin, A.G.; Grismer, L.L.; Hansel, T. Scientific description can imperil species. Science 2006, 312, 1137.
- Early-Capistrán, M.-M.; Sáenz-Arroyo, A.; Cardoso-Mohedano, J.-G.; Garibay-Melo, G.; Peckham, S.H.; Koch, V. Reconstructing 290 years of a data-poor fishery through ethnographic and archival research: The East Pacific green turtle (Chelonia mydas) in Baja California, Mexico. Fish Fish. 2018, 19, 57–77.
- Mancini, A.; Koch, V. Sea turtle consumption and black market trade in Baja California Sur, Mexico. Endanger. Species Res. 2009, 7, 1–10.
- SEMARNAT. Programa de Acción para la Conservación de las Especies: Serpientes de Cascabel (Crotalus spp.); SEMARNAT/CONANP: Ciudad de México, Mexico, 2018.
- Mellink, E. Biological conservation of Isla de Cedros, Baja California, Mexico: Assessing multiple threats. Biodivers. Conserv. 1993, 2, 62–69.
- Delibes, M.; Blázquez, M.d.C.; Soriano, L.; Revilla, E.; Godoy, J.A. High Antipredatory Efficiency of Insular Lizards: A Warning Signal of Excessive Specimen Collection? PLoS ONE 2011, 6, e29312.
- Goode, M.J.; Horrace, W.C.; Sredl, M.J.; Howland, J.M. Habitat destruction by collectors associated with decreased abundance of rock-dwelling lizards. Biol. Conserv. 2005, 125, 47–54.
- SEMARNAT. Norma Oficial Mexicana-059-SEMARNAT-2010, Protección Ambiental- Especies Nativas de México de Flora y Fauna Silvestres-Categorías de Riesgo y Especificaciones su Inclusión, Exclusión o Cambio-Lista de Especies en Riesgo; Diario Oficial de la Federación: Ciudad de México, Mexico, 2010.
- Mulcahy, D.G.; Martínez-Gómez, J.E.; Aguirre-León, G.; Cervantes-Pasqualli, J.A.; Zug, G.R. Rediscovery of an Endemic Vertebrate from the Remote Islas Revillagigedo in the Eastern Pacific Ocean: The Clarión Nightsnake Lost and Found. PLoS ONE 2014, 9, e97682.
- Grismer, L.L. Amphibians and Reptiles of Baja California, including Its Pacific islands and the islands in the Sea of Cortés; University of California Press: Berkeley, CA, USA, 2002; Volume 4.
- Mace, G.M.; Collar, N.J.; Gaston, K.J.; Hilton-Taylor, C.; Akçakaya, H.R.; Leader-Williams, N.; Milner-Gulland, E.J.; Stuart, S.N. Quantification of Extinction Risk: IUCN’s System for Classifying Threatened Species. Conserv. Biol. 2008, 22, 1424–1442.
- Rodrigues, A.S.L.; Pilgrim, J.D.; Lamoreux, J.F.; Hoffmann, M.; Brooks, T.M. The value of the IUCN Red List for conservation. Trends Ecol. Evol. 2006, 21, 71–76.
- Wilson, L.D.; McCranie, J.R. The conservation status of the herpetofauna of Honduras. Amphib. Reptile Conserv. 2004, 3, 6–33.
- Johnson, J.D.; Mata-Silva, V.; Wilson, L.D. A conservation reassessment of the Central American herpetofauna based on the EVS measure. Amphib. Reptile Conserv. 2015, 9, 1–94.
- Wilson, L.; Johnson, J.; Mata-Silva, V. A conservation reassessment of the amphibians of Mexico based on the EVS measure. Amphib. Reptile Conserv. 2013, 7, 97–127.
- Wilson, L.D.; Mata-Silva, V.; Johnson, J.D. A conservation reassessment of the reptiles of Mexico based on the EVS measure. Amphib. Reptile Conserv. 2013, 7, 1–47.
- Smith, H.M. Summary of the collections of snakes and crocodilians made in Mexico under the Walter Rathbone Bacon Traveling Scholarship. Proc. United States Natl. Mus. 1943, 93, 393–504.
- Gärdenfors, U.; Hilton-Taylor, C.; Mace, G.M.; Rodríguez, J.P. The Application of IUCN Red List Criteria at Regional Levels. Conserv. Biol. 2001, 15, 1206–1212.
- Gärdenfors, U. Classifying threatened species at national versus global levels. Trends Ecol. Evol. 2001, 16, 511–516.
- García-Aguilar, M.C.; Luévano-Esparza, J.; Cueva, H.D.L. La fauna nativa de México en riesgo y la NOM-059: ¿Están todos los que son y son todos los que están? Acta Zoológica Mex. 2017, 33, 188–198.
- Garmendia-Cedillo, X. Patrimonio Nacional Islas: Patrimonio Insular Mexicano. Prax. De La Justicia Fisc. Y Adm. 2010, 2, 106–197.
- D.O.F. DECRETO por el que se declara Área Natural Protegida, con el carácter de reserva de la biosfera, la región conocida como Islas del Pacífico de la Península de Baja California. D. Of. Federación 2016, DCCLIX, 52–84.
- Lazcano, A. Quo vadis, Mexican science? Science 2019, 365, 301.
- Uglielmi, G. Mexican science suffers under debilitating budget cuts. Nature 2019.
- Wade, L. Mexico’s new president shocks scientists with budget cuts and disparaging remarks. Science 2019.
- D.O.F. DECRETO por el que se Declara área Natural Protegida, con el Carácter de área de Protección de Recursos Naturales, la Superficie de 4,843-59-73.12 Hectáreas de la Zona Conocida Como Peña Colorada, en los Municipios de Querétaro y El Marqués, en el Estado de Querétaro; Diario Oficial de la Federación: Ciudad de México, Mexico, 2023.
- D.O.F. DECRETO por el que se Declara área Natural Protegida con el carácter de área de Protección de Flora y Fauna, la Zona Conocida como Sierra de San Miguelito, en los Municipios de Mexquitic de Carmona, San Luis Potosí, Villa de Arriaga y Villa de Reyes, en el Estado de San Luis Potosí; Diario Oficial de la Federación: Ciudad de México, Mexico, 2021.
- D.O.F. DECRETO por el que se Declara área Natural Protegida con el carácter de área de Protección de Flora y Fauna, la región Conocida Como Jaguar, en el Municipio de Tulum en el Estado de Quintana Roo; Diario Oficial de la Federación: Ciudad de México, Mexico, 2022.
More
Information
Subjects:
Biodiversity Conservation
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
967
Revisions:
2 times
(View History)
Update Date:
22 Aug 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




